Abstract
Thermophilic Campylobacter species of poultry origin have been associated with up to 80% of human campylobacteriosis cases. Layer chickens have received less attention as possible reservoirs of Campylobacter species. Initially, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of two archived Campylobacter isolates (Campylobacter jejuni strain 200605 and Campylobacter coli strain 200606) from layer chickens to five antimicrobials (ciprofloxacin, nalidixic acid, erythromycin, tetracycline, and gentamicin) were determined using broth microdilution while the presence of selected antimicrobial resistance genes was performed by polymerase chain reaction (PCR) using specific primers. Whole-genome sequencing (WGS) was performed by the Illumina HiSeq X platform. The analysis involved antimicrobial resistance genes, virulome, multilocus sequence typing (MLST), and phylogeny. Both isolates were phenotypically resistant to ciprofloxacin (MIC: 32 vs. 32 µg/mL), nalidixic acid (MIC: 128 vs. 64 µg/mL), and tetracycline (MIC: 64 vs. 64 µg/mL), but sensitive to erythromycin (MIC: 1 vs. 2 µg/mL) and gentamicin (MIC: 0.25 vs. 1 µg/mL) for C. jejuni strain 200605 and C. coli strain 200606, respectively. WGS confirmed C257T mutation in the gyrA gene and the presence of cmeABC complex conferring resistance to FQs in both strains. Both strains also exhibited tet(O) genes associated with tetracycline resistance. Various virulence genes associated with motility, chemotaxis, and capsule formation were found in both isolates. However, the analysis of virulence genes showed that C. jejuni strain 200605 is more virulent than C. coli strain 200606. The MLST showed that C. jejuni strain 200605 belongs to sequence type ST-5229 while C. coli strain 200606 belongs to ST-5935, and both STs are less common. The phylogenetic analysis clustered C. jejuni strain 200605 along with other strains reported in Korea (CP028933 from chicken and CP014344 from human) while C. coli strain 200606 formed a separate cluster with C. coli (CP007181) from turkey. The WGS confirmed FQ-resistance in both strains and showed potential virulence of both strains. Further studies are recommended to understand the reasons behind the regional distribution (Korea, China, and Vietnam) of such rare STs.
1. Introduction
Worldwide, C. jejuni and C. coli are considered the leading etiologies of human campylobacteriosis [1,2]. Currently, most of the studies have focused on C. jejuni, which is associated with 85% of human infections [1]. However, C. coli has not received the same attention, but it is second to C. jejuni in causing human campylobacteriosis [2,3]. The major reservoirs include chickens and cattle, but other farm animals or food products and wild birds have been implicated in disease transmission [4,5,6]. Chicken ceca are colonized by high levels of Campylobacter which may persist in feces that are used as biofertilizers [7]. Human campylobacteriosis is of public health concern due to the increased number of Campylobacter strains that are resistant to both drugs of choice (macrolides and fluoroquinolones) and alternative therapies (aminoglycosides and tetracyclines) [8]. The missense mutation (C257T) in the quinolone resistance-determining region (QRDR) of gyrA has been associated with high-level resistance to quinolones [9]. The widespread FQ-resistant C. jejuni lineages via food and travel need urgent monitoring and mitigation strategies [10].
To control Campylobacter-related infections, it is necessary to understand virulence factors and molecular mechanisms contributing to pathogenesis [11,12]. WGS data from different pathogenic and non-pathogenic mutant strains have been used to classify virulence gene clusters linked to pathogenicity [13]. Although there are gaps in understanding the pathogenesis of Campylobacter [14], the roles played by several virulence factors involved in adhesion, invasion, chemotaxis, and motility are known [12,15]. However, there are various genes coding for other virulence factors, like the lipopolysaccharide (LPS), lipooligosaccharide (LOS), and capsule, which need to be well elucidated [12]. Several studies have confirmed the roles of some of the virulence genes by observing the limited capacities of mutants to attach to, colonize, and invade eukaryotic cells [15,16]. Mutant strains lacking flaA and flaB were unable to complete the colonization process in chickens [13,17]. Also, cadF and ciaB mutant strains showed a reduced ability to adhere to and invade cell lines [17].
Multilocus sequence typing (MLST) has been the gold standard method used for epidemiological surveillance and source-attribution studies [18,19]. However, MLST does not include clinically important information, like the virulence or antibiotic resistance determinants, mobile genetic elements, nucleotide polymorphism, and other recombination events [20]. Campylobacter species can be well characterized based on their virulomes often acquired via horizontal gene transfer [21]. For instance, there are C. coli hybrid strains with DNA segments from C. jejuni, and MLST failed to genotype such strains [22].
Currently, WGS is considered the most informative and discriminative typing method of bacterial pathogens [2,23]. For instance, the WGS led to the creation of the core genome (cgMLST), a novel typing method encompassing hundreds of loci from the traditional seven loci [24]. Additionally, studies using single nucleotide polymorphism (SNP) allow the establishment of the best phylogenetic relationship among different pathogens [25]. The WGS is used for various purposes including novel antibiotic and diagnostic test development, studying the emergence of antibiotic resistance, disease surveillance, and direct infection control measures in both clinical settings and communities [26]. Next-generation sequencing (NGS) technologies are preferred in pathogen typing due to affordable cost and reduced turnaround time [27]. The NGS systems available include Illumina Genome Analyzer (HiSeq, MiSeq), Life Technologies Ion Torrent, and the PacBio RX system [28]. However, the use of WGS daily in genotyping and pathogen characterization faces hurdles related to bioinformatics, like resources, lack of validated workflows, and expertise, which are all required for data analysis [25]. This makes the efficient use of WGS data in public health investigations very hard [29]. It is important to note that some countries like the US have incorporated the WGS in routine checking of human pathogens from clinical samples and food.
Despite the progress in understanding the complicated and multifactorial pathogenesis of Campylobacter as an enteric pathogen, there is a gap regarding the combination of phenotypic and genotypic characteristics [30]. Furthermore, several epidemiological studies have been carried out on Campylobacter species from broiler chickens [31], but there is a dearth of information on Campylobacter from layer chickens [7]. Layer chickens have been reported to be the source of antimicrobial-resistant Campylobacter strains [7,32]. The WGS allows for comprehensive phylogenetic analyses of several factors associated with virulence or antibiotic resistance [20]. Based on findings of the partial characterization of layer chicken-derived Campylobacter isolates, we hypothesize that the WGS-characterized isolates harbor various antimicrobial and virulence-related genes contributing to their pathogenicity. To the best of our knowledge, there are no previous reports of WGS data of Campylobacter from layers in South Korea. Hence, the objectives of this study were to genomically characterize two FQ-resistant C. jejuni and C. coli of layer chicken origin by WGS and to establish phylogenetic relationships of the two isolates to the existing ones.
2. Materials and Methods
2.1. Campylobacter Strains and Culture Conditions
The two Campylobacter strains used in this study were selected from our previously published research work [8]. For this experiment, preserved strains were revived by inoculating them onto Mueller Hinton Agar as previously described [33]. Subculturing was performed to get colonies free from glycerol.
2.2. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing (AST) against five antimicrobials, including FQs, namely ciprofloxacin (CIP) and nalidixic acid (NAL) (0.25–512 µg/mL), macrolide (erythromycin or ERY) (0.06–64 µg/mL), aminoglycoside (gentamicin or GEN) (0.06–64 µg/mL) and tetracycline (TET) (0.125–1024 µg/mL) was performed by two-fold broth microdilution [34]. The optical density was recorded spectrophotometrically at 600 nm (Synergy HT; BioTek Instruments Inc., Winooski, VT, USA). The same protocol used in our previous study was followed for minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) determination [8]. The AST procedure was done in six replicates for reproducibility. The MIC was measured spectrophotometrically with a microplate reader (Synergy HT; BioTek Instruments Inc., Winooski, VT, USA) and confirmed by the addition of iodonitrotetrazolium chloride.
2.3. DNA Extraction, Species Confirmation, and Antimicrobial Resistance (AMR) Genes Detection
The genomic DNA was extracted from pure colonies using the Qiagen QIAamp® PowerFecal® Kit (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. For genes specific for Campylobacter genus and species or genes associated with antimicrobial resistance [tet(O), gyrA, and cmeB], PCR was performed using specific primers (Table 1). After electrophoresis, bands of PCR products were observed on a Dual UV Transilluminator (Core Bio System, Huntington Beach, CA, USA) under ultraviolet (UV) light. Bands were compared to the 100 bp marker (Dyne bio, Seongnam-si, Korea). PCR products were purified with AMPure XP beads (Beckman Coulter, Fullerton, CA, USA) and sequenced by the Sanger method at SolGent (Solutions for Genetic Technologies, Daejeon, South Korea). The presence of resistance genes, as well as point mutations in the 23S rRNA and quinolone resistance-determining region (QRDR) of the gyrA, rpsL, and cmeR genes, was determined using ResFinder (Center for Genomic Epidemiology) with settings of a threshold of 85% identity and a minimum length of 60% [35].

Table 1.
Primers used for species and antimicrobial resistance confirmation.
2.4. Whole-Genome Sequencing
The extraction of genomic DNA was performed as above and the sequencing library was prepared with the Illumina TruSeq Nano DNA Kit, as per the manufacturer’s instructions with a library size of 350 bp. WGS was performed by Illumina HiSeq X technology at Macrogen (Seoul, South Korea) with a read length of 151 bp. The pair-ended reads passed the quality control check, followed by adapter trimming and quality filtering using Trimmomatic (v0.36) [36].
2.5. Construction of Phylogenetic Tree
The genome sequences (from our study) and those collected from public databases (Table 2 were uploaded to the Type (Strain) Genome Server (TYGS), a free bioinformatics platform available online: https://tygs.dsmz.de (accessed on 19 February 2021), for a whole genome-based taxonomic analysis [37]. TYGS employs the Genome-BLAST Distance Phylogeny method (GBDP) [38] to compare whole-genome sequences at the nucleotide level, allowing to calculate the digital DNA-DNA hybridization (dDDH) value and construct the phylogram. Submitted genomes were compared against all type strain genomes available in the TYGS database via the MASH algorithm, a fast approximation of intergenomic relatedness [39], and the 10 type strains with the smallest MASH distances were chosen per submitted genomes. An additional 10 closely related type strains selected by RNAmmer [40] were determined via the 16S rDNA gene sequences, and each sequence was subsequently BLASTed against the 16S rDNA gene sequences of type strains available in the TYGS database [41]. Intergenomic distances were used to infer a balanced minimum evolution tree with branch support via FASTME 2.1.4 including SPR post-processing [42]. Branch support was inferred from 100 pseudo-bootstrap replicates each. The trees were rooted at the midpoint [43] and visualized with PhyD3 [44]. The type-based species clustering using a 70% dDDH radius around each of the 13 type strains was done as previously described [37]. Subspecies clustering was done using a 79% dDDH threshold as previously introduced [45].

Table 2.
Genomic features of strains submitted to the TYGS Database.
2.6. Data Analysis
The MIC values were interpreted using epidemiological cut-off values of the European Committee for Antimicrobial Susceptibility Testing (EUCAST, http://www.eucast.org, accessed on 28 November 2020).
BioEdit software (version 7.2.6.1) (http://www.mbio.ncsu.edu/BioEdit/bioedit.html, accessed on 19 February 2021) was used to edit, align, and analyze the DNA chromatograms [46]. A BLAST search was performed to compare consensus sequences (gyrA and tet(O)) with those from the GenBank database. Standard sensitive strains (L04566.1 and U63413.1) and resistant strains (KX982339.1 and MT176401.1) for gyrA were used for comparison. For the gyrA gene, the comparison was performed with Clustal Omega [47]. Amino acid sequences were deduced from the DNA sequences using the ExPASyTranslate tool [48].
For bioinformatics analysis, the filtered reads were mapped to reference genomes (NCTC11168 and NCTC11366) using Burrows-Wheeler Aligner (BWA-MEM), followed by variants identification and annotation. Produced mass sequence data were used to search for genetic variation based on the NCBI reference genome. After removing duplicates with Sambamba (v0.6.7) [49] and identifying variants with SAMTools [50], information on each variant was gathered and classified. SnpEff [51] was used to predict the variant effect at the protein level. Data was paired and assembled using SKESA assembler [52] while Quality Assessment Tool for Genome Assemblies QUAST [53] was used for assembly statistics and the genomes were annotated using Prokka [54]. Acquired AMR genes and point mutations conferring resistance to antimicrobials were searched using Abricate (https://github.com/tseemann/abricate, accessed on 22 January 2021) and NCBI’s AMRFinderPlus database [55]. Virulence genes were screened with VFDB [56]. The genomes deposited in GenBank were further annotated with PGAP version 5.1 [57]. GenBank accession numbers JAFETJ000000000 and JAFETK000000000 for C. jejuni and C. coli, respectively, were given after submission.
3. Results
3.1. Antimicrobial Resistance Profiles
The phenotypic AMR results revealed high-level resistance of C. jejuni strain 200605 and C. coli strain 200606 to ciprofloxacin (CIP), nalidixic acid (NAL), and tetracycline (TET) with MIC values ranging between 32 μg/mL and 128 μg/mL. Also, C. jejuni strain 200605 and C. coli strain 200606 were sensitive to erythromycin (MIC: 1 vs. 2 µg/mL), and gentamicin (MIC: 0.25 vs. 1 µg/mL), respectively.
PCR confirmed the presence of DNA of gyrA and tet(O) genes, but no band was seen for cmeB. WGS confirmed the presence of the C257T point mutation in the quinolone resistance-determining region of the gyrA gene of both strains. Abricate and Resfinder [35] confirmed the phenotypic data related to FQ-resistance (C257T mutation). Furthermore, tet(O/32/O) and tet(O) genes associated with resistance to doxycycline, tetracycline, and minocycline were found in both isolates by the WGS. Apart from blaOXA-452 found in both isolates, C. jejuni strain 200605 also showed the blaOXA-521 and blaOXA-193 genes. The detection of PointFinder genes returned mutations in gyrA and 23S rRNA genes, but no mutations were found in cmeR and rpsL for C. jejuni strain 200605. Conversely, cmeR was not detected, while rpsL was found but without a mutation for C. coli strain 200606. The latter also showed 12 point mutations in 23S rRNA. Mass screening of contigs of both isolates using ABRicate also showed resistance to cephalosporin, penam, and the presence of cmeB (efflux pump) conferring resistance to different antimicrobials.
3.2. Whole-Genome Sequencing Data
The annotation of the C. jejuni strain 200605 genome with PGAP returned 116 contigs: 1808 genes, of which 1688 were CDSs (with protein), 41 were RNAs (35 tRNAs, 3 ncRNAs, 1 rRNA), and 79 were pseudogenes (67 frame-shifted genes, 11 incompletes, 15 internal stops, and 13 multiple problems).
The annotation of the C. coli strain 200606 genome returned 29 contigs: 1,865 genes, of which 1743 were CDSs (with protein), 42 were RNAs (36 tRNAs, 3 ncRNAs, and 1 rRNA), and 80 were pseudogenes (62 frame-shifted genes, 16 incompletes, 12 internal stops, and 7 multiple problems). Additional details of both strains are given in Table 3 and were made publicly available on BioProject PRJNA694501.

Table 3.
Genome characteristics and accession numbers of C. jejuni and C. coli strains.
Of the called variants (Table 3), SNPs, insertions, deletions, transitions, and transversions were 21,816; 231; 219; 18,333; and 3483, and 45,561; 284; 257; 32,766; and 12,795 for C. jejuni strain 200605 and C. coli strain 200606, respectively.
3.3. Virulence Genes
C. jejuni strain 200605 and C. coli strain 200606 showed 87 and 57 virulence genes, respectively (Supplementary File S1). Adhesion factors (cadF, pebA, and jlpA), a cytolethal distending toxin (cdtABC), invasion genes (ciaB, ciaC), and a biofilm formation gene (eptC) were only found in C. jejuni strain 200605 and not in C. coli strain 200606. However, both strains harbor genes coding for lipooligosaccharides (LOS), lipopolysaccharides (LPS), capsular (gmh, waa, and kps genes), chemotaxis (cheA, cheV, cheW), and motility (flh, fla, flg, ptm) factors.
3.4. Phylogenetic Analysis
The Genome BLAST Distance Phylogeny (GBDP) approach used to generate a phylogenomic tree (Figure 1) shows that C. jejuni strain 200605 forms a cluster with CP014344, which was isolated from a human in South Africa. It is also closely related to other strains of chicken origin from several countries including South Korea (CP028933), the USA (CP023866, CP017863), and China (CP059968, CP059970). However, it is separated from another cluster of CP059964 (chicken) and CP048756 (duck), both from China (Figure 1). There were no differences among the species, subspecies, and percent G+C data of all C. jejuni strains used to generate the tree except for the C. jejuni (CP010502) strain that was isolated from human blood in Finland. The genome size was slightly higher compared to isolates from Type (Strain) Genome Server (TYGS), and it varied from 1.48–1.94 Mbp.
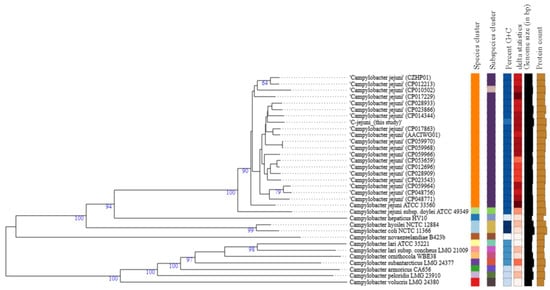
Figure 1.
Type (Strain) Genome Server (TYGS) result for C. jejuni strain 200605 dataset. Tree inferred with FastME 2.1.4 [42] from GBDP distances calculated from genome sequences. Branch lengths are scaled in terms of GBDP distance formula d5; numbers above branches are GBDP pseudo-bootstrap support values from 100 replications. Percent G+C (27.39–30.98); δ statistics (0.138–0.286); protein content (1379–2041).
The GBDP phylogenomic tree (Figure 2) shows that C. coli strain 200606 formed a separate cluster (species and subspecies) along with C. coli (CP007181) that was isolated from turkey and belongs to the same ST-1150 as the isolate of this study. Also, δ values were lower (0.181–0.175) than values for the cluster at the top of the tree (>0.2) (Figure 2). The overall treelikeness of the data set appeared to be high (low δ values). Briefly, δ statistics calculated using distance matrices allow for assessing the impact of individual operational taxonomic units (OTUs) on overall treelikeness (the lower the δ values, the better the treelikeness) [37].
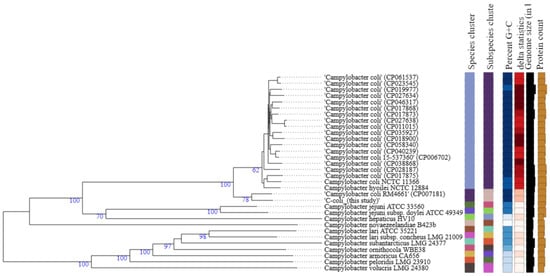
Figure 2.
Type (Strain) Genome Server (TYGS) result for C. coli strain 200606 dataset. Tree was generated as for C. jejuni. Percent G+C (27.39–31.5); δ statistics (0.137–0.295); protein content (1379–2162).
3.5. Multilocus Sequence Typing (MLST)
C. jejuni strain 200605 belongs to ST-5229. So far, ST-5229 has not been assigned to a given clonal complex (CC). C. coli strain 200606 belongs to ST-5935, which belongs to CC-1150.
4. Discussion
Although the prevalence of Campylobacter spp. in table eggs is low, there is limited knowledge of their prevalence and ecology in layer chickens. Also, studies on the antimicrobial resistance profiles of layer chicken-derived Campylobacter isolates are limited [7,32]. This implies that the available data on whole-genome sequences of Campylobacter from layers important for epidemiological studies are also scanty.
This study highlights the genomic characterization and phylogenetic analysis of two FQ-resistant strains from layers in Gangneung. The isolates showed increased resistance to FQs. The resistance to ciprofloxacin has been attributed to two loci that were found in our isolates. The first one is the C257T point mutation in the gyrA gene, while the second factor is the cmeABC operon coding for an efflux pump [9,58]. Increased resistance of Campylobacter strains to FQs has been previously reported in Korea [59,60] and worldwide [9,61], but these strains are known to be highly persistent, even in the absence of the use of FQs [62,63]. The wide use of some FQs (enrofloxacin) in poultry farming has been associated with the spread of resistant Campylobacter strains and may explain the increasing resistance trend [60,64,65]. FQ-resistant Campylobacter strains have been classified by the World Health Organization (WHO) as high-priority antibiotic-resistant pathogens for which new drugs are required [66,67].
Ciprofloxacin and erythromycin have been used as the drugs of choice for treating Campylobacter infections [68]. The global distribution of ciprofloxacin-resistant strains has led to the adoption of erythromycin as the appropriate drug for campylobacteriosis therapy due to a limited number of macrolide-resistant strains [61]. Both strains of this study were sensitive to erythromycin and the WGS confirmed the results due to a lack of responsible point mutations (2074 and 2075) in the V domain of the 23S rRNA gene [69]. The reduced resistance to macrolides in Campylobacter strains from poultry may be associated with the limited use of macrolides in poultry production. Tylosin is used in swine or cattle, but not in poultry [70,71]. However, Sub-Saharan Africa (SSA) has recorded a lower prevalence of Campylobacter strains that are ciprofloxacin-resistant compared to erythromycin-resistant ones [68,72].
Phenotypic and genomic data showed resistance to tetracycline, which concurs with previous findings all over the world [59,73,74]. Higher resistance to tetracycline has been associated with the tet(O) gene coding for the ribosomal protection protein TetO [19] found in various Gram-positive and Gram-negative bacteria [63]. Moreover, tetracycline is overused in chicken and swine industries due to its affordability, and simple administration via drinking water [75]. It is worth noting that the chicken body temperature (42 °C) favors the conjugation process and thus contributes to the sharing of plasmids carrying various antimicrobial-resistant genes [76].
Campylobacter spp. are known to be inherently resistant to β-lactams including ampicillin [70], and we did not test for ampicillin resistance by broth microdilution. However, the WGS showed the presence of blaOXA-452, 521, and 193 genes which are inherent to Campylobacter. Ampicillin resistance is mainly due to enzymatic inactivation by blaOXA-61, but other factors like porins and reduced affinity to penicillin-binding protein (PBP) have also been reported [70,77]. The isolates of the current study were sensitive to gentamicin, which corroborates previous reports [78,79,80]. However, higher resistance was reported in China for C. coli strains [74]. The limited resistance to gentamicin has been associated with its limited use to only systemic infections [81,82] and it is not used in poultry production [79]. Both ABRicate and ResFinder did not yield any resistance to streptomycin, as the rpsL was found but without mutation. Surveillance of gentamicin-resistant strains should be performed in response to the increasing number of resistant strains as reported in the USA and China [61].
This study revealed that adhesion (cadF, pebA, and jlpA), invasion (ciaBC), toxin (cdtABC), flgSR two-component system, and biofilm formation (eptC) factors were only found in the C. jejuni strain 200605 genome and not in the C. coli strain 200606 genome. These factors highlight the virulent nature of the C. jejuni strain compared to C. coli which concurs with the literature [83]. Both strains expressed various other virulence factors involved in pathogenesis, like chemotaxis (cheA, V, W), LOS, LPS, and capsule formation (gmh, waa, and kps genes). The mentioned genes contribute to the pathogenicity of Campylobacter strains while infecting humans, as they are all required for successful colonization and survival [15,84] of the bacteria within the host. Studies demonstrated that mutant Campylobacter strains were negatively affected in absence of some important genes [13,66]. For instance, Campylobacter strains lacking cdtB and cdtC were not cytotoxic, had reduced colonization, and had extra-intestinal invasiveness [15,85]. Flagellar genes (flaA, flaB, flgB, flgE, and flaC) are involved in various cell functions, like motility and biofilm formation [86,87]. The presence of capsular genes (kps D, E, F, C, S, T) and LPS associated gene (hldE) in both strains underline their virulence potential. The role of the capsule in the pathogenesis of Campylobacter has not been well defined, but it is suspected to interact with the mucus layer during adhesion, and it helps with intracellular survival [12]. HldE is involved in protein glycosylation and correct LPS configuration [88]. Surprisingly, the C. coli strain 200606 harbored additional genes (cj1420c; cj1419c, cj1417c, cj1416c) involved in capsule biosynthesis [89] for C. jejuni, suggesting an exchange of some genes between C. jejuni and C. coli species. However, the introgression of C. coli by C. jejuni is not new [90]. Taken together, WGS data highlights the virulence profiles of study strains, which may give a clue to their respective pathogenicity.
The GBDP phylogenomic tree showed that C. jejuni strain 200605 clustered together with another isolate previously found in chicken meat in Korea (CP028933), but it was distantly related to another strain of human origin also reported in Korea (CP017229). This suggests some host preference and adaptation in Campylobacter. A study in Japan highlighted a distant relationship between C. jejuni from wild crows and poultry, showing the possibility of divergence due to host adaptation [91]. On the contrary, C. jejuni strain 200605 clustered with CP014344 collected from humans in South Africa, which could not be justified by the current study. We speculate that travel may be a predisposing factor in the occurrence of such a phenomenon. However, the phylogenetic tree (Figure 1) shows that other factors like the species, subspecies, percent G+C, and δ statistics were comparable for most of the C. jejuni strains used to build the tree. C. coli strain 200606 clustered with C. coli (CP007181) isolated from turkey, and this cluster was distantly related to other C. coli strains used to build the tree. Both chickens and turkeys are domestic poultry, and it seems common to find both strains clustering together. Also, introgression of CP007181 by C. jejuni would explain the clustering together with C. coli strain 200606 of this study in which some C. jejuni genes were found. Furthermore, the analysis showed that other factors like the species, subspecies, percent G+C, and δ statistics were different from the values of other C. coli strains used to build the tree (Figure 2). Campylobacter is evolving at high speed due to many recombination events that could lead to specific niche adaptation and thus justifying the obtained diversity [71]. Differential responses to environmental factors and/or management practices have also been suggested to contribute to strain distribution among various niches [92].
C. jejuni strain 200605 belongs to ST-5229 which so far has not been assigned to any clonal complex. This ST may be specific to the region, as other isolates (n = 4) of the same ST have been previously collected from chickens in Korea [60], while one isolate was isolated from swine in China, as shown by the pubMLST website. There is a shortage of information on this ST and why it has not been reported in other parts of the globe. C. coli strain 200606 belongs to ST-5935, which is part of the CC-1150. This ST is not common, but it has been reported in C. coli of chicken origin in Vietnam [93]. The CC-1150 has also been reported as the predominant clonal complex among C. coli from chickens in China [94]. Further studies are needed to understand the particularities of both STs and why they are not widely distributed. We also recommend studies on the roles played by indoor and cage-free laying hens along with their environment in disseminating Campylobacter species to the environment.
A limitation in our study was a low number of sequenced strains due to limited resources. However, the phylogenetic trees included Campylobacter strains from various hosts and countries to indicate the taxonomic features of isolates used in this study.
5. Conclusions
The current study describes the WGS of C. jejuni strain 200605 and C. coli strain 200606 from layer chickens in Korea. Both strains showed C257T point mutation in gyrA and cmeABC operon often associated with quinolone resistance. The two strains also carry tet(O) genes associated with tetracycline resistance. The presence of various virulence factors involved in motility, adhesion, invasion, toxin production, and chemotaxis shows the pathogenic potential of the studied strains. Phylogenomics revealed that the two strains resemble other strains of poultry and human origins. C. jejuni strain 200605 and C. coli strain 200606 belong to less common STs and this warrants further investigation. To the best of our knowledge, this is the first report of WGS data from Campylobacter species from layer chickens in Korea. Special attention should be paid to FQ-resistant strains due to a limited number of available alternative treatments.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12081131/s1, Supplementary File S1: Virulence genes.
Author Contributions
N.G., C.-H.P., R.G.A. and E.V.G.K. conceived the study. N.G. and D.-G.S. collected samples. N.G. and K.-Y.Y. carried out the experiments, analyzed, interpreted the data, and wrote the manuscript. R.G.A. and D.M. substantially contributed to the analysis of the results. L.E.G.M. and M.I.M. substantially revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was financially supported by the Ministry of Oceans and Fisheries, Korea (Grant No: 20170488) and the Partnership for Skills in Applied Sciences, Engineering and Technology-Regional Scholarship and Innovation Fund (PASET-RSIF) in collaboration with the Government of the Republic of Korea.
Institutional Review Board Statement
Not applicable as no treatment was given to chicken.
Informed Consent Statement
Not applicable.
Data Availability Statement
Datasets generated and/or analysed during the current study are available in GenBank repository under the BioProject number PRJNA694501 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA694501/). C. jejuni strain 200605 sequence accession number is JAFETJ000000000 (https://www.ncbi.nlm.nih.gov/nuccore/JAFETJ000000000.1/) while C. coli strain 200606 accession number is JAFETK000000000 (https://www.ncbi.nlm.nih.gov/nuccore/JAFETK000000000.1/).
Acknowledgments
We gratefully acknowledge the World Bank, the Partnership for Skills in Applied Sciences, Engineering and Technology (PASET), the Government of the Republic of Korea, and the Korea Institute for Science and Technology (KIST) for their contribution to this study and its publication (KIST intramural fund 2Z06482 and 2Z06483).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Ghatak, S.; He, Y.; Reed, S.; Irwin, P. Comparative Genomic Analysis of a Multidrug-Resistant Campylobacter jejuni Strain YH002 Isolated from Retail Beef Liver. Foodborne Pathog. Dis. 2020, 17, 576–584. [Google Scholar] [CrossRef]
- Elhadidy, M.; Miller, W.G.; Arguello, H.; Alvarez-Ordóñez, A.; Dierick, K.; Botteldoorn, N. Molecular epidemiology and antimicrobial resistance mechanisms of Campylobacter coli from diarrhoeal patients and broiler carcasses in Belgium. Transbound. Emerg. Dis. 2019, 66, 463–475. [Google Scholar] [CrossRef]
- Humphrey, T.; O’Brien, S.J.; Madsen, M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007, 117, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Shyaka, A.; Kusumoto, A.; Asakura, H.; Kawamoto, K. Whole-Genome Sequences of Eight Campylobacter jejuni Isolates from Wild Birds. Genome Announc. 2015, 3, e00315-15. [Google Scholar] [CrossRef]
- Epping, L.; Golz, J.C.; Knüver, M.-T.; Huber, C.; Thürmer, A.; Wieler, L.H.; Stingl, K.; Semmler, T. Comparison of different technologies for the decipherment of the whole genome sequence of Campylobacter jejuni BfR-CA-14430. Gut Pathog. 2019, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kassem, I.I.; Kehinde, O.; Kumar, A.; Rajashekara, G. Antimicrobial-Resistant Campylobacter in Organically and Conventionally Raised Layer Chickens. Foodborne Pathog. Dis. 2017, 14, 29–34. [Google Scholar] [CrossRef]
- Gahamanyi, N.; Song, D.-G.; Cha, K.H.; Yoon, K.-Y.; Mboera, L.E.; Matee, M.I.; Mutangana, D.; Amachawadi, R.G.; Komba, E.V.; Pan, C.-H. Susceptibility of Campylobacter Strains to Selected Natural Products and Frontline Antibiotics. Antibiotics 2020, 9, 790. [Google Scholar] [CrossRef]
- Sproston, E.L.; Wimalarathna, H.M.L.; Sheppard, S.K. Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom. 2018, 4, e000198. [Google Scholar] [CrossRef] [PubMed]
- Cha, W.; Emosci, R.; Wengert, S.L.; Singh, P.; Newton, D.W.; Esalimnia, H.; Lephart, P.; Ekhalife, W.; Mansfield, L.S.; Rudrik, J.T.; et al. Antimicrobial Susceptibility Profiles of Human Campylobacter jejuni Isolates and Association with Phylogenetic Lineages. Front. Microbiol. 2016, 7, 589. [Google Scholar] [CrossRef] [PubMed]
- Kovács, J.K.; Cox, A.; Schweitzer, B.; Maróti, G.; Kovács, T.; Fenyvesi, H.; Emődy, L.; Schneider, G. Virulence Traits of Inpatient Campylobacter jejuni Isolates, and a Transcriptomic Approach to Identify Potential Genes Maintaining Intracellular Survival. Microorganisms 2020, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Rokney, A.; Valinsky, L.; Moran-Gilad, J.; Vranckx, K.; Agmon, V.; Weinberger, M. Genomic Epidemiology of Campylobacter jejuni Transmission in Israel. Front. Microbiol. 2018, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Kreling, V.; Falcone, F.H.; Kehrenberg, C.; Hensel, A. Campylobacter sp.: Pathogenicity factors and prevention methods—new molecular targets for innovative antivirulence drugs? Appl. Microbiol. Biotechnol. 2020, 104, 10409–10436. [Google Scholar] [CrossRef]
- Nguyen, T.N.M.; Hotzel, H.; El-Adawy, H.; Tran, H.T.; Le, M.T.H.; Tomaso, H.; Neubauer, H.; Hafez, H.M. Genotyping and antibiotic resistance of thermophilic Campylobacter isolated from chicken and pig meat in Vietnam. Gut Pathog. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Bolton, D.J. Campylobacter virulence and survival factors. Food Microbiol. 2015, 48, 99–108. [Google Scholar] [CrossRef]
- Yamasaki, S.; Asakura, M.; Tsukamoto, T.; Faruque, S.M.; Deb, R.; Ramamurthy, T. Cytolethal Distending Toxin (CDT): Genetic Diversity, Structure and Role In Diarrheal Disease. Toxin Rev. 2006, 25, 61–88. [Google Scholar] [CrossRef]
- Ramires, T.; de Oliveira, M.G.; Kleinubing, N.R.; Würfel, S.D.F.R.; Mata, M.M.; Iglesias, M.A.; Lopes, G.V.; Dellagostin, O.A.; da Silva, W.P. Genetic diversity, antimicrobial resistance, and virulence genes of thermophilic Campylobacter isolated from broiler production chain. Braz. J. Microbiol. 2020, 51, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.; Moran, L.; Ridley, A.; Newell, D.; Madden, R. Inter-laboratory evaluation of three flagellin PCR/RFLP methods for typing Campylobacter jejuni and C. coli: The CAMPYNET experience. J. Appl. Microbiol. 2003, 95, 1321–1333. [Google Scholar] [CrossRef]
- Elhadidy, M.; Miller, W.G.; Arguello, H.; Alvarez-Ordóñez, A.; Duarte, A.; Dierick, K.; Botteldoorn, N. Genetic Basis and Clonal Population Structure of Antibiotic Resistance in Campylobacter jejuni Isolated From Broiler Carcasses in Belgium. Front. Microbiol. 2018, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Fiedoruk, K.; Daniluk, T.; Rozkiewicz, D.; Oldak, E.; Prasad, S.; Swiecicka, I. Whole-genome comparative analysis of Campylobacter jejuni strains isolated from patients with diarrhea in northeastern Poland. Gut Pathog. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Iraola, G.; Pérez, R.; Naya, H.; Paolicchi, F.; Pastor, E.; Valenzuela, S.; Calleros, L.; Velilla, A.; Hernández, M.; Morsella, C. Genomic Evidence for the Emergence and Evolution of Pathogenicity and Niche Preferences in the Genus Campylobacter. Genome Biol. Evol. 2014, 6, 2392–2405. [Google Scholar] [CrossRef]
- Golz, J.C.; Epping, L.; Knüver, M.-T.; Borowiak, M.; Hartkopf, F.; Deneke, C.; Malorny, B.; Semmler, T.; Stingl, K. Whole genome sequencing reveals extended natural transformation in Campylobacter impacting diagnostics and the pathogens adaptive potential. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Llarena, A.-K.; Taboada, E.; Rossi, M. Whole-Genome Sequencing in Epidemiology of Campylobacter jejuni Infections. J. Clin. Microbiol. 2017, 55, 1269–1275. [Google Scholar] [CrossRef]
- Maiden, M.C.J.; Van Rensburg, M.J.J.; Bray, J.; Earle, S.G.; Ford, S.A.; Jolley, K.; McCarthy, N.D. MLST revisited: The gene-by-gene approach to bacterial genomics. Nat. Rev. Genet. 2013, 11, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, B.; Winand, R.; Fu, Q.; Van Braekel, J.; Ceyssens, P.-J.; Mattheus, W.; Bertrand, S.; De Keersmaecker, S.C.J.; Roosens, N.H.C.; Vanneste, K. Validation of a Bioinformatics Workflow for Routine Analysis of Whole-Genome Sequencing Data and Related Challenges for Pathogen Typing in a European National Reference Center: Neisseria meningitidis as a Proof-of-Concept. Front. Microbiol. 2019, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Köser, C.U.; Ellington, M.J.; Peacock, S.J. Whole-genome sequencing to control antimicrobial resistance. Trends Genet. 2014, 30, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Oakeson, K.F.; Wagner, J.M.; Rohrwasser, A.; Atkinson-Dunn, R. Whole-Genome Sequencing and Bioinformatic Analysis of Isolates from Foodborne Illness Outbreaks of Campylobacter jejuni and Salmonella enterica. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- Quail, M.A.; Smith, M.E.; Coupland, P.; Otto, T.D.; Harris, S.R.; Connor, T.R.; Bertoni, A.; Swerdlow, H.P.; Gu, Y. A tale of three next generation sequencing platforms: Comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genom. 2012, 13, 341. [Google Scholar] [CrossRef]
- Fricke, W.F.; Rasko, D.A. Bacterial genome sequencing in the clinic: Bioinformatic challenges and solutions. Nat. Rev. Genet. 2014, 15, 49–55. [Google Scholar] [CrossRef]
- García-Sánchez, L.; Melero, B.; Jaime, I.; Rossi, M.; Ortega, I.; Rovira, J. Biofilm formation, virulence and antimicrobial resistance of different Campylobacter jejuni isolates from a poultry slaughterhouse. Food Microbiol. 2019, 83, 193–199. [Google Scholar] [CrossRef]
- Agunos, A.; Waddell, L.; Léger, D.; Taboada, E. A Systematic Review Characterizing On-Farm Sources of Campylobacter spp. for Broiler Chickens. PLoS ONE 2014, 9, e104905. [Google Scholar] [CrossRef]
- Rama, E.N.; Bailey, M.; Jones, D.R.; Gast, R.; Anderson, K.; Brar, J.; Taylor, R.; Oliver, H.F.; Singh, M. Prevalence, Persistence, and Antimicrobial Resistance of Campylobacter spp. from Eggs and Laying Hens Housed in Five Commercial Housing Systems. Foodborne Pathog. Dis. 2018, 15, 506–516. [Google Scholar] [CrossRef]
- Kurekci, C.; Bishop-Hurley, S.L.; Vercoe, P.; Durmic, Z.; Al Jassim, R.A.M.; McSweeney, C.S. Screening of Australian Plants for Antimicrobial Activity against Campylobacter jejuni. Phytother. Res. 2011, 26, 186–190. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Staerfeldt, H.-H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.S.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Comprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program: Table 1. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef]
- Farris, J.S. Estimating Phylogenetic Trees from Distance Matrices. Am. Nat. 1972, 106, 645–668. [Google Scholar] [CrossRef]
- Kreft, Ł.; Botzki, A.; Coppens, F.; Vandepoele, K.; Van Bel, M. PhyD3: A phylogenetic tree viewer with extended phyloXML support for functional genomics data visualization. Bioinformatics 2017, 33, 2946–2947. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete genome sequence of DSM 30083T, the type strain (U5/41T) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genom. Sci. 2014, 9, 1–19. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Vilella, A.J.; Cuppen, E.; Nijman, I.J.; Prins, P. Sambamba: Fast processing of NGS alignment formats. Bioinformatics 2015, 31, 2032–2034. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 genome project data processing subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Souvorov, A.; Agarwala, R.; Lipman, D.J. SKESA: Strategic k-mer extension for scrupulous assemblies. Genome Biol. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Feldgarden, M. Using the NCBI AMRFinder Tool to Determine Antimicrobial Resistance Genotype-Phenotype Correlations Within a Collection of NARMS Isolates. bioRxiv 2019, 550707. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Sproston, E.; Ogden, I.; Macrae, M.; Forbes, K.; Dallas, J.; Sheppard, S.; Cody, A.; Colles, F.; Wilson, M.; Strachan, N. Multi-locus sequence types of Campylobacter carried by flies and slugs acquired from local ruminant faeces. J. Appl. Microbiol. 2010, 109, 829–838. [Google Scholar] [CrossRef]
- Wei, B.; Cha, S.-Y.; Kang, M.; Roh, J.-H.; Seo, H.-S.; Yoon, R.-H.; Jang, H.-K. Antimicrobial Susceptibility Profiles and Molecular Typing of Campylobacter jejuni and Campylobacter coli Isolates from Ducks in South Korea. Appl. Environ. Microbiol. 2014, 80, 7604–7610. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-Y.; Kwon, Y.-K.; Wei, B.; Jang, H.-K.; Lim, S.-K.; Kim, C.-H.; Jung, S.-C.; Kang, M.-S. Epidemiological relationships of Campylobacter jejuni strains isolated from humans and chickens in South Korea. J. Microbiol. 2016, 55, 13–20. [Google Scholar] [CrossRef]
- Tang, Y.; Fang, L.; Xu, C.; Zhang, Q. Antibiotic resistance trends and mechanisms in the foodborne pathogen, Campylobacter. Anim. Health Res. Rev. 2017, 18, 87–98. [Google Scholar] [CrossRef]
- Abraham, S.; Sahibzada, S.; Hewson, K.; Laird, T.; Abraham, R.; Pavic, A.; Truswell, A.; Lee, T.; O’Dea, M.; Jordan, D. Emergence of Fluoroquinolone-Resistant Campylobacter jejuni and Campylobacter coli among Australian Chickens in the Absence of Fluoroquinolone Use. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.T.; Lynch, H.; Burke, S.; Hawkins, K.; Buttimer, C.; Mc Carthy, C.; Egan, J.; Whyte, P.; Bolton, D.; Coffey, A.; et al. Antimicrobial Resistance Determinants Circulating among Thermophilic Campylobacter Isolates Recovered from Broilers in Ireland Over a One-Year Period. Antibiotics 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-S.; Jeong, H.-J.; Yun, K.-W. Antimicrobial activity and chemical components of two plants, Artemisia capillaris and Artemisia iwayomogi, used as Korean herbal Injin. J. Ecol. Environ. 2010, 33, 141–147. [Google Scholar] [CrossRef]
- Frazão, M.R.; Cao, G.; Medeiros, M.I.C.; Duque, S.D.S.; Allard, M.W.; Falcão, J.P. Antimicrobial Resistance Profiles and Phylogenetic Analysis of Campylobacter jejuni Strains Isolated in Brazil by Whole Genome Sequencing. Microb. Drug Resist. 2021, 27, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Kim, J.; Kim, J.H.; Jung, J.I.; Cho, S.; Ryu, S.; Jeon, B. Comparative Analysis of Aerotolerance, Antibiotic Resistance, and Virulence Gene Prevalence in Campylobacter jejuni Isolates from Retail Raw Chicken and Duck Meat in South Korea. Microorganisms 2019, 7, 433. [Google Scholar] [CrossRef] [PubMed]
- Hlashwayo, D.F.; Barbosa, F.; Langa, S.; Sigaúque, B.; Bila, C.G. A Systematic Review of In Vitro Activity of Medicinal Plants from Sub-Saharan Africa against Campylobacter Spp. Evid.-Based Complementary Altern. Med. 2020, 1–13. [Google Scholar] [CrossRef]
- Gahamanyi, N.; Mboera, L.E.G.; Matee, M.I.; Mutangana, D.; Komba, E.V.G. Prevalence, Risk Factors, and Antimicrobial Resistance Profiles of Thermophilic Campylobacter Species in Humans and Animals in Sub-Saharan Africa: A Systematic Review. Int. J. Microbiol. 2020, 1–12. [Google Scholar] [CrossRef]
- Iovine, N.M. Resistance mechanisms in Campylobacter jejuni. Virulence 2013, 4, 230–240. [Google Scholar] [CrossRef]
- Kashoma, I.P.; Kassem, I.; John, J.; Kessy, B.M.; Gebreyes, W.; Kazwala, R.R.; Rajashekara, G. Prevalence and Antimicrobial Resistance of Campylobacter Isolated from Dressed Beef Carcasses and Raw Milk in Tanzania. Microb. Drug Resist. 2016, 22, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Sakata, J.; Nakamura, H.; Yamamoto, S.; Murakami, S. Phylogenetic Diversity and Antimicrobial Resistance of Campylobacter coli from Humans and Animals in Japan. Microbes Environ. 2019, 34, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hlashwayo, D.F.; Sigaúque, B.; Noormahomed, E.V.; Afonso, S.M.S.; Mandomando, I.M.; Bila, C.G. A systematic review and meta-analysis reveal that Campylobacter spp. and antibiotic resistance are widespread in humans in sub-Saharan Africa. PLoS ONE 2021, 16, e0245951. [Google Scholar] [CrossRef]
- Kabir, S.L.; Asakura, M.; Shiramaru, S.; Pal, A.; Hinenoya, A.; Yamasaki, S. Molecular identification and antimicrobial resistance profiles of Campylobacter strains of poultry origin in India with special emphasis on fluoroquinolone resistance. Asian J. Med. Biol. Res. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Shao, Y.; Hu, Y.; Lou, H.; Chen, X.; Wu, Y.; Mei, L.; Zhou, B.; Zhang, X.; et al. Molecular Characterization and Antibiotic Resistant Profiles of Campylobacter Species Isolated From Poultry and Diarrheal Patients in Southeastern China 2017–2019. Front. Microbiol. 2020, 11, 1244. [Google Scholar] [CrossRef]
- Jonker, A.; Picard, J. Antimicrobial susceptibility in thermophilic Campylobacter species isolated from pigs and chickens in South Africa. J. S. Afr. Vet. Assoc. 2010, 81, 228–236. [Google Scholar] [CrossRef]
- Cuevas, E.; Guirado, P.; Miro, E.; Iglesias-Torrens, Y.; Navarro, F.; Alioto, T.; Gómez-Garrido, J.; Madrid, C.; Balsalobre, C. Tetracycline resistance transmission in Campylobacter is promoted at temperatures resembling the avian reservoir. Vet. Microbiol. 2020, 244, 108652. [Google Scholar] [CrossRef]
- De Vries, S.P.W.; Vurayai, M.; Holmes, M.; Gupta, S.; Bateman, M.; Goldfarb, D.; Maskell, D.J.; Matsheka, M.I.; Grant, A.J. Phylogenetic analyses and antimicrobial resistance profiles of Campylobacter spp. from diarrhoeal patients and chickens in Botswana. PLoS ONE 2018, 13, e0194481. [Google Scholar] [CrossRef]
- Mäesaar, M.; Kramarenko, T.; Meremäe, K.; Sõgel, J.; Lillenberg, M.; Häkkinen, L.; Ivanova, M.; Kovalenko, K.; Hörman, A.; Hanninen, M.-L.; et al. Antimicrobial Resistance Profiles of Campylobacter spp. Isolated from Broiler Chicken Meat of Estonian, Latvian and Lithuanian Origin at Estonian Retail Level and from Patients with Severe Enteric Infections in Estonia. Zoonoses Public Health 2016, 63, 89–96. [Google Scholar] [CrossRef]
- Cantero, G.; Correa-Fiz, F.; Ronco, T.; Strube, M.L.; Cerdà-Cuéllar, M.; Pedersen, K. Characterization of Campylobacter jejuni and Campylobacter coli Broiler Isolates by Whole-Genome Sequencing. Foodborne Pathog. Dis. 2018, 15, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Amoako, D.G.; Abia, A.L.K.; Somboro, A.M.; Shobo, C.O.; Perrett, K.; Bester, L.A.; Essack, S.Y. Characterisation of Campylobacter spp. Isolated from Poultry in KwaZulu-Natal, South Africa. Antibiotics 2020, 9, 42. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Futur. Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Long, C.; Xu, J.; Guo, Q.; Zhang, W.; Zhang, Z.; Bater. Are dominant plant species more susceptible to leaf-mining insects? A case study at Saihanwula Nature Reserve, China. Ecol. Evol. 2018, 8, 7633–7648. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.; Gatica, M.A.; Riquelme, V.; Vergara, C.; Yañez, J.M.; Martín, B.S.; Sáenz, L.; Vidal, M.; Martínez, M.C.; Araya, P.; et al. Characterization of Antimicrobial Susceptibility and Its Association with Virulence Genes Related to Adherence, Invasion, and Cytotoxicity in Campylobacter jejuni and Campylobacter coli Isolates from Animals, Meat, and Humans. Microb. Drug Resist. 2016, 22, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.D.; Scheutz, F.; Pedersen, K.; Handberg, K.; Madsen, M. PCR detection of seven virulence and toxin genes of Campylobacter jejuni and Campylobacter coli isolates from Danish pigs and cattle and cytolethal distending toxin production of the isolates. J. Appl. Microbiol. 2003, 94, 1003–1014. [Google Scholar] [CrossRef]
- Wysok, B.; Wojtacka, J.; Kivistö, R. Pathogenicity of Campylobacter strains of poultry and human origin from Poland. Int. J. Food Microbiol. 2020, 334, 108830. [Google Scholar] [CrossRef]
- Konkel, M.E.; Klena, J.D.; Rivera-Amill, V.; Monteville, M.R.; Biswas, D.; Raphael, B.; Mickelson, J. Secretion of Virulence Proteins from Campylobacter jejuni Is Dependent on a Functional Flagellar Export Apparatus. J. Bacteriol. 2004, 186, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Guerry, P.; Ewing, C.P.; Schirm, M.; Lorenzo, M.; Kelly, J.; Pattarini, D.; Majam, G.; Thibault, P.; Logan, S. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 2006, 60, 299–311. [Google Scholar] [CrossRef]
- Hermansen, G.; Boysen, A.; Krogh, T.; Nawrocki, A.; Jelsbak, L.; Møller-Jensen, J. HldE Is Important for Virulence Phenotypes in Enterotoxigenic Escherichia coli. Front. Cell. Infect. Microbiol. 2018, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Elbediwi, M.; Zhou, X.; Shuai, H.; Lou, X.; Wang, H.; Li, Y.; Yue, M. Epidemiological and Genomic Characterization of Campylobacter jejuni Isolates from a Foodborne Outbreak at Hangzhou, China. Int. J. Mol. Sci. 2020, 21, 3001. [Google Scholar] [CrossRef]
- Sheppard, S.K.; McCarthy, N.D.; Jolley, K.; Maiden, M. Introgression in the genus Campylobacter: Generation and spread of mosaic alleles. Microbiology 2011, 157, 1066–1074. [Google Scholar] [CrossRef]
- Okamura, M.; Kaneko, M.; Ojima, S.; Sano, H.; Shindo, J.; Shirafuji, H.; Yamamoto, S.; Tanabe, T.; Yoshikawa, Y.; Hu, D.-L. Differential Distribution of Salmonella Serovars and Campylobacter spp. Isolates in Free-Living Crows and Broiler Chickens in Aomori, Japan. Microbes Environ. 2018, 33, 77–82. [Google Scholar] [CrossRef] [PubMed]
- El-Hamid, M.I.A.; El-Aziz, N.K.A.; Samir, M.; El-Naenaeey, E.-S.Y.; Remela, E.M.A.; Mosbah, R.A.; Bendary, M.M. Genetic Diversity of Campylobacter jejuni Isolated From Avian and Human Sources in Egypt. Front. Microbiol. 2019, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Carrique-Mas, J.J.; Bryant, J.; Cuong, N.V.; Hoang, N.V.M.; Campbell, J.; Dung, T.T.N.; Duy, D.; Hoa, N.T.; Thompson, C.; Hien, V.V.; et al. An epidemiological investigation of Campylobacter in pig and poultry farms in the Mekong delta of Vietnam. Epidemiol. Infect. 2013, 142, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Zhang, X.; Xue, F.; Wang, Y.; Jiang, L.; Jiang, Y. Phenotypic Characters and Molecular Epidemiology of Campylobacter Jejuniin East China. J. Food Sci. 2015, 81, M106–M113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).