Abstract
The rare form of retinal dystrophy, Bietti crystalline dystrophy, is associated with variations in CYP4V2, a member of the cytochrome P450 family. This study reports patients affected by typical and atypical Bietti crystalline dystrophy, expanding the spectrum of this disease. This is an observational case series of patients with a clinical and molecular diagnosis of Bietti crystalline dystrophy that underwent multimodal imaging. Four unrelated patients are described with two known variants, c.802-8_810del17insGC and c.518T > G (p.Leu173Trp), and one novel missense variant, c.1169G > T (p.Arg390Leu). The patient with the novel homozygous variant had the most severe phenotype resulting in macular hole formation and retinal detachment in both eyes. To the best of our knowledge, there is no association of these features with Bietti crystalline dystrophy. Patient 1 was the youngest patient and had the mildest phenotype with crystals in the retina without chorioretinal atrophy and visual complaints. Patients 2 and 3 presented with fewer crystals and chorioretinal atrophy. These three patients presented a classic phenotype. The fourth patient presented with an atypical and severe phenotype. This study reveals a new genotype and new phenotype associated with this disorder.
1. Introduction
Bietti crystalline dystrophy (BCD) (OMIM210370) is an inherited recessive disorder characterized by crystalline deposits in the retina and sometimes at the corneoscleral limbus [1,2]. Pathogenic variants in the CYP4V2 gene (OMIM#608614) were identified as disease-causing [3]. The protein encoded by this gene is a member of the cytochrome P450 family. It has been suggested that CYP4V2 proteins are implicated in the lipid recycling system between the retinal pigment epithelium (RPE) and outer photoreceptor segments, which is essential for maintaining visual acuity [1,4]. This gene is expressed in the human heart, brain, lung, liver, kidney, placenta, retina, and lymphocytes [1,3,4]. Histopathology showed lipid inclusions in lymphocytes, skin fibroblasts; however, clinically significant abnormalities remain only in the eye [3,5,6].
BCD causes nyctalopia, decreased visual acuity, and visual field constriction, similar to other forms of retinal degeneration [1,2,3,4]. The crystalline deposits seen early in the retina are the hallmark of BCD. However, these crystalline deposits can also appear in other retinal diseases such as reticular pseudodrusen, retinitis punctata albescens, cystinosis, and Sjögren-Larsson syndrome [7]. Perimacular yellow-white dots are also seen in Alport syndrome [8]. With disease progression, the yellow-white crystals disappear [7]. The advanced stage [9] of BCD is characterized by extensive chorioretinal atrophy that can be similar to other inherited retinal diseases such as choroideremia, retinitis pigmentosa, fundus albipunctatus, and gyrate atrophy [1]. The overlap of clinical phenotype, in both early and late stages of the disease, emphasizes the importance of molecular testing in establishing a precise diagnosis and has an impact on disease management [1].
The current study describes four patients with BCD from the Federal University of São Paulo and Instituto de Genética Ocular in Brazil. One of them with an atypical phenotype associated with a novel homozygous missense variant c.1169G > T (p.Arg390Leu) in the CYP4V2 gene.
2. Materials and Methods
This study was performed in accordance with the Declaration of Helsinki and protection of the patient’s identity and was approved by the Research Ethics Committee of the Federal University of São Paulo (number 1191.0071.10). This is a case series of patients with a molecular diagnosis of BCD. The medical records were reviewed. The patients underwent detailed ophthalmic exams including best-corrected visual acuity (BCVA), slit-lamp exam, and multimodal retinal imaging: fundus images (VISUCAM 500, Zeiss, Oberkochen, Germany), FAF (HRA2, Heidelberg Engineering, Heidelberg, Germany), OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany). Electroretinogram was performed in patient 1 in accordance with the International Society for Clinical Electrophysiology of Vision (ISCEV) [10]. The next-generation sequencing panel, targeting inherited retinal diseases, including genes that cause chorioretinal dystrophies such as CHM, OAT, RPGR, CYP4V2, and more than 200 genes, was performed to establish a genetic diagnosis in patients 1, 3, and 4. CYP4V2 gene sequencing was performed by using Sanger sequencing in patient 2.
Nucleotide and protein changes were described as recommended by the Human Genome Variation Society (HGVS) and based on NM_207352.4 and NP_997235.3 reference sequences. All variants found were compared with variants listed in the Human Gene Mutation Database (HGMD) [11] and ClinVar [12]. VarSome Software (Saphetor, Lausanne, Switzerland) was also used [13].
3. Results
The findings of the four patients are summarized in Table 1.

Table 1.
Molecular and clinical features of patients with Bietti crystalline dystrophy.
3.1. Case 1
A 19-year-old woman without complaints was referred because of fundus findings observed at age 12 years. Her BCVA was 20/20 in both eyes (OU). The patient was the only affected member of her family; her maternal grandparents were from China, and her paternal grandparents were Japanese. The slit-lamp exam was unremarkable. Fundus exam showed crystalline deposits in the retina; optic disc and retinal vessels were normal. Fundus autofluorescence showed hypoautofluorescent dots throughout the posterior pole. Spectral-domain optical coherence tomography (OCT) showed spherical intraretinal hyperreflective lesions, which confirmed the presence of intraretinal crystals (Figure 1A–C). The full-field electroretinogram was normal at age 12. Molecular testing identified a homozygous indel variant c.802-8_810delinsGC found more frequently in Asian patients [7].
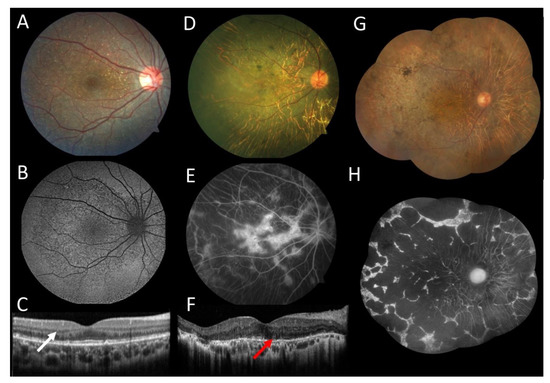
Figure 1.
Multimodal imaging of female patients with Bietii crystalline dystrophy. (A–C) Patient 1 at age 19: (A) Color fundus photograph of the right eye showed crystalline deposits throughout the central retina. (B) Autofluorescence showed hypoautofluorescent dots representing the areas of atrophy. (C) The horizontal line scan from the optical coherence tomography (OCT) in the right eye showed spherical intraretinal hyperreflective crystals (white arrow). (D–F) Patient 2 at age 54: (D) photography of the right eye exhibited chorioretinal atrophy and pigment clumps with few crystalline deposits. (E) Fluorescein angiography showed multiple patchy coalescent hypofluorescent areas located throughout the degenerative lesions. (F) The horizontal line scan from the OCT in the right eye showed extensive outer retinal atrophy with few intraretinal crystals and outer retinal tubulations (red arrow). (G,H) Patient 3 at age 69: (G) color fundus montage of the right eye showed crystalline deposits, diffuse chorioretinal atrophy, and pigment clumps. (H) Fundus autofluorescence exhibited nummular anthropic centered.
3.2. Case 2
A female patient, 54 years old, with a Japanese background and a history of decreased visual acuity since she was 12 years old. Her BCVA was 20/50 in the right eye and 20/400 in the left eye. The slit-lamp exam showed intraocular lenses OU. Fundus exam showed normal optic disc, normal retinal vessels, crystalline deposits in the central retina, diffuse chorioretinal atrophy, and pigment clumps in both eyes. Fluorescein angiography showed a central hyperfluorescent island surrounded by multiple patchy coalescent hypofluorescent areas located throughout the degenerative lesions. OCT showed extensive outer retinal atrophy with few intraretinal crystals and outer retinal tubulations in both eyes (Figure 1D–F). Molecular testing found a missense variant c.518T > G (p.Leu173Trp) described by Lin et al. [14] and a deletion c.802-8_806del described by Li et al. [3].
3.3. Case 3
A 69-year-old female patient diagnosed first with retinitis pigmentosa was referred for genetic testing. She was referred to parental consanguinity. Her BCVA was 20/150 in both eyes. The slit-lamp exam showed intraocular lenses OU. Fundus exam showed normal optic disc, cup/disc 0.4, mild arterioles attenuation, crystalline deposits in the central retina, diffuse chorioretinal atrophy, and pigment clumps in both eyes. Fundus autofluorescence showed nummular areas of atrophy (Figure 1G,H). Molecular testing identified a known homozygous missense variant c.518T > G (p.Leu173Trp), the same variant found in patient 2.
3.4. Case 4
A Portuguese 59-year-old man who moved to Brazil at 9 years of age was diagnosed with retinitis pigmentosa when he was 34 years old. He denied family history. He underwent posterior vitrectomy and cataract surgery due to a retinal detachment in the right eye at the age of 40 years. He was complaining of progressively decreasing vision during the previous five years. His BCVA was light perception in the right eye and hand movements in the left eye. The slit-lamp exam showed an intraocular lens in the right eye and a sclerotic nuclear cataract in the left eye without crystalline deposits at the corneal limbus. Fundus exam showed extensive areas of retinal and choroidal atrophy in both eyes, silicone oil in the right eye, and a macular hole with retinal detachment in the posterior pole of the left eye (Figure 2A,B). OCT showed extensive atrophy and diffused thinning of all retinal and choroidal layers OU (Figure 2C,D) and a retinal detachment in the left eye (Figure 2E). Because he had no fixation, OCT was not focused on the macula in the left eye, and exams such as microperimetry and fundus autofluorescence could not be performed. The clinical features were inconclusive for establishing a correct diagnosis. Crystals were absent from his exam. The differential diagnosis was retinitis pigmentosa, choroideremia, and BCD. Molecular testing identified only a homozygous variant c.1169G > T (p.Arg390Leu) in CYP4V2 that had not been described previously.
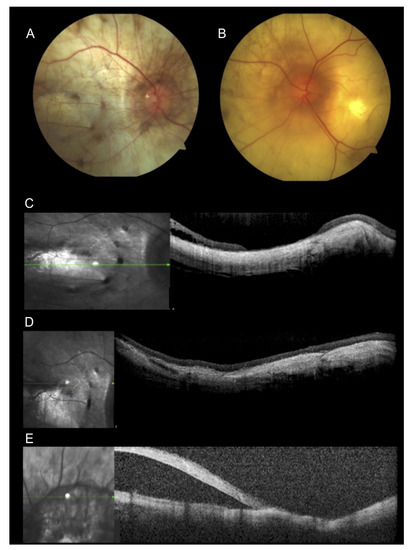
Figure 2.
Multimodal imaging of a 59-year-old patient. (A,B) Fundus image presented extensive chorioretinal atrophy. (A) Fundus photography of the right eye with silicone oil and retinal attached at the central retina. (B) Fundus photography of the left eye showing macular hole and retinal detachment in the posterior pole. (C,D) Optical coherence tomography (OCT) scans of the right eye showed retinal and choroidal atrophy. (E) Horizontal OCT scan showed atrophy and retinal detachment in the left eye.
4. Discussion
Bietti crystalline dystrophy mainly affects Asians [1,4]. The Brazilian population is ethnically very heterogeneous [15]. The largest Japanese population outside of Japan lives in Brazil, mainly in São Paulo state [16]. The estimated prevalence of rare BCD is up to 1 in 67,000 individuals [4]. The pathogenic variant in patient 1 (c.802-8_810delinsGC) has been reported previously [17]. This is the most common pathogenic variant in East Asian patients due to a founder effect [7,18,19,20]. This pathogenic variant is the result of a combination of the insertion of two nucleotides and deletion of 62 amino acid-encoding exon 7 [18]. The absence of exon 7 eliminates the essential parts of the protein structure and disrupts enzyme activity [1,3]. A more severe phenotype based on electrophysiological testing when patients had splice site mutation causing skipped exons on both alleles compared to patients without biallelic splice was reported [18]. Further, a statistically significant early age of onset between the patients with compound heterozygous c.802-8_810delinsGC mutation compared to those without c.802-8_810delinsGC was also documented [19]. However, the majority of authors did not establish any clear genotype-phenotype correlation [7,21,22].
Patients 2 and 3 presented with the same missense variant c.518T > G (p.Leu173Trp) located at exon 4. This variant was described in the literature for the first time in a 54-year-old homozygous Japanese female patient presenting suitable visual acuity in a homozygote state [14]. The Leu173 amino acid is highly conserved among eukaryotic homologs. The leucine (Leu) change at position 173 to tryptophan (Trp) may result in a structural change affecting the proper folding of the CYP4V2 protein [14]. Patient 2 and patient 3 presented a classic phenotype of tiny yellow crystals and chorioretinal atrophy in the fundus exam. Both patients also presented with pigmented spicules in both eyes. According to chart review, there were no crystals deposits at corneal limbus different from the patient previously described [14]. However, as limbal corneal crystals deposits are very subtle, they can be unnoticed even by experienced ophthalmologists [1]. They can also be absent because CYP4V2 protein is less expressed in the cornea than in the retina [19,23].
The missense variant c.1169G > T (p.Arg390Leu) found in patient 4 has never been reported. It is absent in all populations of the gnomAD database and was classified as disease-causing by nine pathogenicity predictors (DANN, GERP, FATHMM, LRT, MutationAssessor, Mutation Taster, PROVEANS, FATHMM-MKL, and SIFT) [24,25,26]. Furthermore, the Arg390 amino acid is highly conserved, and other missense variants have already been reported at the same codon (p.Arg390His and p.Arg390Cys) [17,27]. In addition, more than 35 pathogenic and likely pathogenic missense variants have been identified throughout all 11 exons of CYP4V2 [11]. More than 80% of the mutant alleles are located in exons 7, 8, and 9 [27]. Based on ACMG [13] criteria and aforementioned information, this novel variant in exon 9 of the CYP4V2 gene was classified as likely pathogenic.
To the best of our knowledge, there is no report of macular hole or retinal detachment in patients with BCD, as shown in the OCT of our patient 4. A giant macular hole has already been described in another coalescing fleck retinopathy, Alport syndrome [8]. The OCT findings evaluate the severity of BCD progression. In the early stage, there is a loss of the interdigitation zone and disruption in the ellipsoid zone. Outer retinal atrophy and RPE loss occurs as the severity increases. It is known that there is a relationship between age and the progressive loss of the chorioretinal layers and function [4]. The younger patient had a milder phenotype, as expected. It is also expected that the disease progresses to posterior chorioretinal atrophy, diminution of the yellow-white crystals, and slowly deteriorating vision leading to legal blindness in the fifth or sixth decades of life [1]. However, there is a well-documented high phenotypic variability [1,21]. Factors such as diet [1,11,13] may affect this variable phenotype because mutations in the CYP4V2 gene affect lipid metabolism [3,17,21,28]. The patient with novel missense mutation presented with the worst vision reinforcing the pathogenicity of the p.Arg390Leu. It was not the older patient that had the most severe phenotype in our cohort.
There are limitations in the present study. The retrospective analysis of the Federal University of São Paulo and Instituto de Genética Ocular database from 1998 until 2020 found only four Brazilian patients with variants in CYP4V2 of a total of 2299 individuals with inherited retinal dystrophies. The fourth patient underwent a retinal dystrophy panel that included genes that cause other crystalline retinopathies such as retinitis punctata albescens (RLBP1), and fundus albipunctatus (RDH5), and genes that cause other chorioretinal diseases such as choroideremia (CHM), gyrate atrophy (OAT), and retinitis pigmentosa (RPGR). CTNS, ALDH3A2, COL4A5 that can cause cystinosis, Sjogren-Larson syndrome, and Alport syndrome were not tested. Only the novel variant c.1169G > T was identified. Additional cases with the same variant are missing to compare with our findings. Functional assessment of the novel missense variant may further elucidate its pathogenicity. Segregation analysis is not available in our cohort of patients. However, our results expand the phenotypic and genotypic spectrum of this ultra-rare disease.
Author Contributions
Conceptualization, J.M.F.S.; methodology, J.M.F.S. and M.M.d.P.; software, F.L.M.; validation, M.M.d.P., F.L.M., M.V.S., C.H.M.T., A.V.G., R.C.-M., and J.M.F.S.; formal analysis, M.M.d.P., F.L.M., M.V.S., C.H.M.T., A.V.G., R.C.-M., and J.M.F.S.; investigation, M.M.d.P., F.L.M., and J.M.F.S.; resources, J.M.F.S.; data curation, M.M.d.P., F.L.M., M.V.S., C.H.M.T., A.V.G., R.C.-M., and J.M.F.S.; writing—original draft preparation, M.M.d.P.; writing—review and editing, M.M.d.P., F.L.M., M.V.S., C.H.M.T., A.V.G., R.C.-M., and J.M.F.S.; visualization, J.M.F.S.; supervision, J.M.F.S. and R.C.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the FEDERAL UNIVERSITY OF SÃO PAULO (protocol code 1191.0071.10/2018).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garcia-Garcia, G.P.; Martinez-Rubio, M.; Moya-Moya, M.A.; Perez-Santonja, J.J.; Escribano, J. Identification of novel CYP4V2 genotypes associated with Bietti crystalline dystrophy and atypical anterior segment phenotypes in Spanish patients. Acta Ophthalmol. 2018, 96, e865–e873. [Google Scholar] [CrossRef]
- Lockhart, C.M.; Smith, T.B.; Yang, P.; Naidu, M.; Rettie, A.E.; Nath, A.; Weleber, R.; Kelly, E.J. Longitudinal characterization of function and structure of Bietti crystalline dystrophy: Report on a novel homozygous mutation in CYP4V2. Br. J. Ophthalmol. 2018, 102, 187–194. [Google Scholar] [CrossRef]
- Li, A.; Jiao, X.; Munier, F.L.; Schorderet, D.F.; Yao, W.; Iwata, F.; Hayakawa, M.; Kanai, A.; Chen, M.S.; Lewis, R.A.; et al. Bietti crystalline dystrophy is caused by mutations in the novel gene CYP4V2. Am. J. Hum. Genet. 2004, 74, 817–826. [Google Scholar] [CrossRef]
- Ng, D.S.C.; Lai, T.Y.Y.; Ng, T.K.; Pang, C.P. Genetics of Bietti crystalline dystrophy. Asia Pac. J. Ophthalmol. 2016, 5, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.J.; Weleber, R.G.; Klein, M.L.; Welch, R.B.; Green, W.R. Bietti’s crystalline dystrophy. A clinicopathologic correlative study. Arch. Ophthalmol. 1989, 107, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kaiser-Kupfer, M.I.; Chan, C.C.; Markello, T.C.; Crawford, M.A.; Caruso, R.C.; Csaky, K.G.; Guo, J.; Gahl, W.A. Clinical biochemical and pathologic correlations in Bietti’s crystalline dystrophy. Am. J. Ophthalmol. 1994, 118, 569–582. [Google Scholar] [CrossRef]
- Oishi, A.; Oishi, M.; Miyata, M.; Hirashima, T.; Hasegawa, T.; Numa, S.; Tsujikawa, A. Multimodal imaging for differential diagnosis of Bietti crystalline dystrophy. Ophthalmol. Retin. 2018, 2, 1071–1077. [Google Scholar] [CrossRef]
- Savige, J.; Sheth, S.; Leys, A.; Nicholson, A.; Mack, H.G.; Colville, D. Ocular features in Alport syndrome: Pathogenesis and clinical significance. Clin. J. Am. Soc. Nephrol. 2015, 10, 703–709. [Google Scholar] [CrossRef]
- Yuzawa, M.; Mae, Y.; Matsui, M. Bietti’s crystalline retinopathy. Ophthalmic Paediatr. Genet. 1986, 7, 9–20. [Google Scholar] [CrossRef]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Evans, K.; Hayden, M.; Heywood, S.; Hussain, M.; Phillips, A.D.; Cooper, D.N. The Human Gene Mutation Database: Towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 2017, 136, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant Interpret and supporting evidence. Nucleic Acids Res. 2018, 4, D1062–D1067. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Lin, J.; Nishiguchi, K.M.; Nakamura, M.; Dryja, T.P.; Berson, E.L.; Miyake, Y. Recessive mutations in the CYP4V2 gene in East Asian and Middle Eastern patients with Bietti crystalline corneoretinal dystrophy. J. Med. Genet. 2005, 42, e38. [Google Scholar] [CrossRef] [PubMed]
- Salzano, F.M.; Sans, M. Interethnic admixture and the evolution of Latin American populations. Genet. Mol. Biol. 2014, 37, 151–170. [Google Scholar] [CrossRef]
- Brazilian Citizens with Japanese Ancestry Is Now at 1.5 Million. Available online: http://www.brazil.gov.br/about-brazil/news/2017/06/brazil-has-1-5-million-citizens-of-japanese-origin (accessed on 7 March 2021).
- Xiao, X.; Mai, G.; Li, S.; Guo, X.; Zhang, Q. Identification of CYP4V2 mutation in 21 families and overview of mutation spectrum in Bietti crystalline corneoretinal dystrophy. Biochem. Biophys. Res. Commun. 2011, 409, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Ng, T.K.; Tam, P.O.; Yam, G.H.; Ngai, J.W.; Chan, W.M.; Liu, D.T.L.; Lam, D.S.C.; Pang, C.P. Genotype-phenotype analysis of Bietti’s crystalline dystrophy in patients with CYP4V2 mutations. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5212–5220. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, K.; Dong, B.; Peng, X.; Li, Q.; Jiang, F.; Xie, Y.; Tian, L.; Li, Y. Comprehensive screening of CYP4V2 in a cohort of Chinese patients with Bietti crystalline dystrophy. Mol. Vis. 2018, 24, 700–711. [Google Scholar]
- Jiao, X.; Li, A.; Jin, Z.B.; Wang, X.; Iannaccone, A.; Traboulsi, E.I.; Gorin, M.B.; Simonelli, F.; Hejtmancik, J.F. Identification and population history of CYP4V2 mutations in patients with Bietti crystalline corneoretinal dystrophy. Eur. J. Hum. Genet. 2017, 25, 461–471. [Google Scholar] [CrossRef]
- Astuti, G.D.N.; Sun, V.; Bauwens, M.; Zobor, D.; Leroy, B.P.; Omar, A.; Jurklies, B.; Lopez, I.; Ren, H.; Yazar, V.; et al. Novel insights into the molecular pathogenesis of CYP4V2-associated Bietti’s retinal dystrophy. Mol. Genet. Genom. Med. 2015, 3, 14–29. [Google Scholar] [CrossRef]
- Rossi, S.; Testa, F.; Li, A.; Yaylacioğlu, F.; Gesualdo, C.; Hejtmancik, J.F.; Simonelli, F. Clinical and genetic features in Italian Bietti crystalline dystrophy patients. Br. J. Ophthalmol. 2013, 97, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.C.; Koh, A.H.C.; Aung, T.; Yong, V.H.K.; Yeung, K.; Ang, C.-L.; Vithana, E.N. Characterization of Bietti crystalline dystrophy patients with CYP4V2 mutations. Investig. Opthalmol. Vis. Sci. 2005, 46, 3812–3816. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Cooper, G.M.; Goode, D.L.; Ng, S.B.; Sidow, A.; Bamshad, M.J.; Shendure, J.; Nickerson, D.A. Single-nucleotide evolutionary constraint scores highlight disease-causing mutations. Nat. Methods 2010, 7, 250–251. [Google Scholar] [CrossRef]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Yokoi, Y.; Sato, K.; Aoyagi, H.; Takahashi, Y.; Yamagami, M.; Nakazawa, M. A novel compound heterozygous mutation in the CYP4V2 gene in a Japanese patient with Bietti’s crystalline corneoretinal dystrophy. Case Rep. Ophthalmol. 2011, 2, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Halford, S.; Liew, G.; Mackay, D.S.; Sergouniotis, P.I.; Holt, R.; Broadgate, S.; Volpi, E.V.; Ocaka, L.; Robson, A.G.; Holder, G.E.; et al. Detailed phenotypic and genotypic characterization of Bietti crystalline dystrophy. Ophthalmology 2014, 121, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).