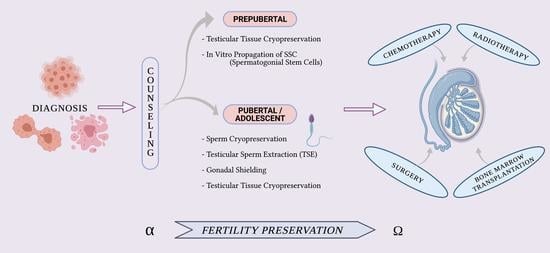
Pediatric and Adolescent Oncofertility in Male Patients—From Alpha to Omega
Abstract
1. Introduction
2. Testicular Function—Fertility Reservoir
3. Influence of Cancer Treatment on Fertility
3.1. Chemotherapy
3.2. Radiotherapy
3.3. Surgery
3.4. Bone Marrow Transplantation
4. Fertility Preservation—Promising Times
4.1. From Alpha—FP Counseling
4.2. Oncofertility—A Bridge between Science and Biotechnology
4.3. Fertility Preservation—Beyond Oncology
5. Fertility Preservation in Pubertal or Adolescent Boys
5.1. Sperm Cryopreservation
5.2. Testicular Sperm Extraction (TESE)
- -
- Penile vibratory stimulation (PVS): this is typically tried first and it is considered to be a non-invasive technique, as it can be used in the privacy of a patient’s home and does not require general anesthesia. These devices are usually quite affordable and typically have settings for variable amplitude and frequency. The vibratory pad of the device is placed on the ventral part of the penis near the frenulum, helping to trigger seminal emission and the ejaculation reflex [67].
- -
- Electroejaculation (EEJ): a more invasive option, normally involving general anesthesia. This requires coordination with operating room and laboratory staff. It is more often used in patients who have failed PVS and is considered next-line therapy [68]. It can often be performed in the same anesthetic setting as additionally required oncologic procedures with minimal morbidity [69]. Hovav [70] reported that electroejaculation in six 15 to 18-year-old boys resulted in the patients successfully obtaining sperm in all cases. A study of 30 adolescents treated with electro-ejaculation demonstrated a sperm recovery rate of 60% [71].
- -
- Surgical sperm extraction (from the epididymis or the testis): this is used when patients are unable to produce a specimen or present with azoospermia. This can be performed concurrently with other procedures such as central line placement, orchiectomy and bone marrow biopsy. Sperm can be potentially retrieved by percutaneous epididymal sperm aspiration, testicular sperm aspiration and micro-epididymal sperm aspiration [72].
- -
- Micro-TESE: the use of an operating microscope with microsurgical testicular sperm extraction (micro-TESE) can assist in the identification of focal areas with active spermatogenesis. With this procedure, the risks of testicular damage (such as scrotal hematoma and skin discoloration, infection, persistent pain, and swelling) are minimal. For these reasons, TESE has become an emerging option for azoospermic patients with cancer and has been termed ”onco-TESE” [73,74].
5.3. Gonadal Shielding
5.4. Testicular Tissue Cryopreservation (TTC) and Experimental Procedures: A Great Challenge for Prepubertal Male Patients
5.4.1. Spermatogonia Stem Cell Transplantation
5.4.2. De Novo Testicular Morphogenesis with the Introduction of SSC and Supporting Testicular Cells into a Decellularized Testicular Scaffold
5.4.3. Autologous Grafting and Xenografting of Testicular Tissue
5.4.4. Maturation of Testicular Tissue in Culture (Testicular Tissue Organ Culture)
5.4.5. Induced Pluripotent Stem Cell
6. In Vitro Propagation of SSCs—A Necessary Step before Transplantation
6.1. 2D and 3 Dimensional Culture Systems
6.2. 3D Bioprinted Scaffold
6.3. Testicular Organoids
6.4. Microfluid System and Organ-on-Chip Technology
7. Malignant Contamination and In Vitro Spermatogenesis
8. To Omega—Oncogenetic Phenomena
9. Ethical Issues
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Phillips, S.M.; Padgett, L.S.; Leisenring, W.M.; Stratton, K.K.; Bishop, K.; Krull, K.R.; Alfano, C.M.; Gibson, T.M.; deMoor, J.S.; Hartigan, D.B.; et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol. Biomark. Prev. 2015, 24, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Landier, W.; Hudson, M.M.; Robison, L.L.; Bhatia, S.; on behalf of the COG Survivorship and Outcomes Committee. Children’s Oncology Group’s 2013 blueprint for research: Survivorship and outcomes. Pediatr. Blood Cancer 2013, 60, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.L.; Armstrong, G.T.; Boice, J.D.; Chow, E.J.; Davies, S.M.; Donaldson, S.S.; Green, D.M.; Hammond, S.; Meadows, A.T.; Mertens, A.C.; et al. The Childhood Cancer Survivor Study: A National Cancer Institute–Supported Resource for Outcome and Intervention Research. J. Clin. Oncol. 2009, 27, 2308–2318. [Google Scholar] [CrossRef]
- E Barton, S.; Najita, J.S.; Ginsburg, E.S.; Leisenring, W.M.; Stovall, M.; E Weathers, R.; A Sklar, C.; Robison, L.L.; Diller, L. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013, 14, 873–881. [Google Scholar] [CrossRef]
- Crawshaw, M. Psychosocial oncofertility issues faced by adolescents and young adults over their lifetime: A review of the research. Hum. Fertil. 2013, 16, 59–63. [Google Scholar] [CrossRef]
- Urbanski, H.F.; Kohama, S.G.; Garyfallou, V.T. Mechanisms mediating the response of GnRH neurones to excitatory amino acids. Rev. Reprod. 1996, 1, 173–181. [Google Scholar] [CrossRef]
- Kaiser, U.B.; Jakubowiak, A.; Steinberger, A.; Chin, W.W. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology 1993, 133, 931–934. [Google Scholar] [CrossRef]
- Stahl, O.; Eberhard, J.; Cavallin-Stahl, E.; Jepson, K.; Friberg, B.; Tingsmark, C.; Spano, M.; Giwercman, A. Sperm DNA integrity in cancer patients: The effect of disease and treatment. Int. J. Androl. 2008, 32, 695–703. [Google Scholar] [CrossRef]
- Antal, Z.; Sklar, C.A. Gonadal function and fertility among survivors of childhood cancer. Endocrinol. Metab. Clin. N. Am. 2015, 44, 739e49. [Google Scholar] [CrossRef]
- Howell, S.; Shalet, S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. N. Am. 1998, 27, 927e43. [Google Scholar] [CrossRef]
- Bahadur, G.; Ozturk, O.; Muneer, A.; Wafa, R.; Ashraf, A.; Jaman, N.; Patel, S.; Oyede, A.W.; Ralph, D.J. Semen quality before and after gonadotoxic treatment. Hum. Reprod. 2005, 20, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Ragni, G.; Somigliana, E.; Restelli, L.; Salvi, R.; Arnoldi, M.; Paffoni, A. Sperm banking and rate of assisted reproduction treatment: Insights from a 15- year cryopreservation program for male cancer patients. Cancer 2003, 97, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.H.; Karpman, E.; Sander, J.C.; Spiess, P.E.; Pisters, L.L.; Lipshultz, L.I. Pretreatment semen parameters in men with cancer. J. Urol. 2009, 181, 736–740. [Google Scholar] [CrossRef]
- van Alphen, M.M.; van de Kant, H.J.; de Rooij, D.G. Depletion of the spermatogonia from the seminiferous epithelium of the rhesus monkey after X irradiation. Radiat. Res. 1988, 3, 473–486. [Google Scholar] [CrossRef]
- Meistrich, M.L. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil. Steril. 2013, 100, 1180–1186. [Google Scholar] [CrossRef]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. Fertility Preservation for Patients with Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Fields, E.; Chard, J.; James, D.; Treasure, T.; on behalf of the Guideline Development Group. Fertility (update): Summary of NICE guidance. BMJ 2013, 346, f650. [Google Scholar] [CrossRef] [PubMed]
- Jeruss, J.S.; Woodruff, T.K. Preservation of fertility in patients with cancer. NEJM 2009, 360, 902e11. [Google Scholar] [CrossRef]
- Reinmuth, S.; Liebeskind, A.K.; Wickmann, L.; Bockelbrink, A.; Keil, T.; Henze, G.; Borgmann, A. Having children after surviving cancer in childhood or adolescence—Results of a Berlin survey. Klin. Padiatr. 2008, 220, 159–165. [Google Scholar] [CrossRef]
- Fenn, J.E.; Udelsman, R. First use of intravenous chemotherapy cancer treatment: Rectifying the record. J. Am. Coll. Surg. 2011, 212, 413–417. [Google Scholar] [CrossRef]
- Spitz, S. The histological effects of nitrogen mustards on human tumors and tissues. Cancer 1948, 1, 383–398. [Google Scholar] [CrossRef]
- Sprauten, M.; Brydoy, M.; Haugnes, H.S.; Cvancarova, M.; Bjoro, T.; Bjerner, J.; Fossa, S.D.; Oldenburg, J. Longitudinal serum testosterone, luteinizing hormone, and follicle-stimulating hormone levels in a population-based sample of long-term testicular cancer survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 571–578. [Google Scholar] [CrossRef]
- Meistrich, M.L. Male gonadal toxicity. Pediatr. Blood Cancer. 2009, 53, 261–266. [Google Scholar] [CrossRef]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Liu, W.; Kutteh, W.H.; Ke, R.W.; Shelton, K.C.; A Sklar, C.; Chemaitilly, W.; Pui, C.-H.; Klosky, J.L.; Spunt, S.L.; et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014, 15, 1215–1223. [Google Scholar] [CrossRef]
- Hansen, P.V.; Hansen, S.W. Gonadal function in men with testicular germ cell cancer: The influence of cisplatin-based chemotherapy. Eur. Urol. 1993, 23, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L.; Wilson, G.; Mathur, K.; Fuller, L.M.; Rodriguez, M.A.; McLaughlin, P.; Romaguera, J.E.; Cabanillas, F.F.; Ha, C.S.; Lipshultz, L.I.; et al. Rapid recovery of spermatogenesis after mitoxantrone, vincristine, vinblastine, and prednisone chemotherapy for hodgkin’s disease. J. Clin. Oncol. 1997, 15, 3488–3495. [Google Scholar] [CrossRef]
- Kreuser, E.D.; Xiros, N.; Hetzel, W.D.; Heimpel, H. Reproductive and endocrine gonadal capacity in patients treated with COPP chemotherapy for Hodgkin’s disease. J. Cancer Res. Clin. Oncol. 1987, 113, 260–266. [Google Scholar] [CrossRef]
- Brawig, J.H.; Heimes, U.; Heiermann, E.; Schlegel, W.; Nieschlag, E.; Schellong, G. The effects of different cumulative doses ofchemotherapy on testicular function. Results in 75 patients treated for Hodgkin’s disease during childhood or adolescence. Cancer 1990, 65, 1298–1302. [Google Scholar] [CrossRef]
- Ntali, G.; Karavitaki, N. Efficacy and complications of pituitary irradiation. Endocrinol. Metab. Clin. N. Am. 2015, 44, 117–126. [Google Scholar] [CrossRef]
- Shalet, S.M. Effect of irradiation on gonadal function in men treated for germ cell cancer. Eur. Urol. 1993, 23, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Pettus, J.A.; Carver, B.S.; Masterson, T.; Stasi, J.; Sheinfeld, J. Preservation of ejaculation in patients undergoing nerve-sparing postchemotherapy retroperitoneal lymph node dissection for metastatic testicular cancer. Urology 2009, 73, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.S.; Bennett, R.; Bihrle, R.; Donohue, J.P. A preliminary report: Postoperative fertility assessment in nerve-sparing RPLND patients. Eur. Urol. 1993, 23, 165–167, Discussion 168. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.J.; Leach, D.R.; Warner, G.A.; Heller, C.G. Effect of Graded Doses of Ionizing Radiation on the Human Testis. Radiat. Res. 1974, 59, 665. [Google Scholar] [CrossRef] [PubMed]
- Ash, P. The influence of radiation on fertility in man. Br. J. Radiol. 1980, 53, 271–278. [Google Scholar] [CrossRef]
- Djaladat, H. Organ-sparing surgery for testicular tumours. Curr. Opin. Urol. 2015, 25, 116–120. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Boulad, F.; Gillio, A.; Sklar, C. Gonadal function after bone marrow transplantation for acute leukemia dur-ing childhood. J. Pediatr. 1997, 130, 210–216. [Google Scholar] [CrossRef]
- Anserini, P.; Chiodi, S.; Spinelli, S.; Costa, M.; Conte, N.; Copello, F.; Bacigalupo, A. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transpl. 2002, 30, 447–451. [Google Scholar] [CrossRef]
- Sanders, J.E. Growth and development after hematopoietic cell transplant in children. Bone Marrow Transpl. 2008, 41, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Rendtorff, R.; Hohmann, C.; Reinmuth, S.; Müller, A.; Dittrich, R.; Beyer, M.; Wickmann, L.; Keil, T.; Henze, G.; Borgmann-Staudt, A. Hormone and Sperm Analyses after Chemo- and Radiotherapy in Childhood and Adolescence. Klinische Pädiatrie 2010, 222, 145–149. [Google Scholar] [CrossRef]
- Anazodo, A.; Ataman-Millhouse, L.; Jayasinghe, Y.; Woodruff, T.K. Oncofertility-An emerging discipline rather than a special consideration. Pediatr. Blood Cancer 2018, 65, e27297. [Google Scholar] [CrossRef] [PubMed]
- Klosky, J.L.; Simmons, J.L.; Russell, K.M.; Foster, R.H.; Sabbatini, G.M.; Canavera, K.E.; Hodges, J.R.; Schover, L.R.; McDermott, M.J. Fertility as a priority among at-risk adolescent males newly diagnosed with cancer and their parents. Support Care Cancer 2015, 23, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.F.; Ott, M.A. Fertility preservation after a cancer diagnosis: A systematic review of adolescents’, parents’, and providers’ perspectives, experiences, and preferences. J. Pediatr. Adolesc. Gynecol. 2016, 29, 585e98. [Google Scholar] [CrossRef]
- The Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil. Steril. 2005, 83, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Raviv, L.; Applegarth, L.; Ford, J.S.; Josephs, L.; Grill, E.; Sklar, C.; Sonoda, Y.; Baser, R.E.; Barakat, R.R. A cross-sectional study of the psychosexual impact of cancer-related infertility in women: Third-party reproductive assistance. J. Cancer Surviv. 2010, 4, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Chappel, A.; Salinas, M.; Ziebland, S.; McPherson, A.; Macfarlane, A. Fertility issues: The perceptions and experiences of young men recently diagnosed and treated for cancer. J. Adolesc. Health 2007, 40, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rotker, K.; Vigneswaran, H.; Omil-Lima, D.; Sigman, M.; Hwang, K. Efficacy of Standardized Nursing Fertility Counseling on Sperm Banking Rates in Cancer Patients. Urology 2017, 104, 90–96. [Google Scholar] [CrossRef]
- Runco, D.V.; Taylor, J.F.; Helft, P.R. Ethical Barriers in Adolescent Oncofertility Counseling. J. Pediatr. Hematol. Oncol. 2017, 39, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K. The Oncofertility Consortium–addressing fertility in young people with cancer. Nat. Rev. Clin. Oncol. 2010, 7, 466–475. [Google Scholar] [CrossRef]
- Silva, C.A.; Brunner, H.I. Gonadal functioning and preservation of reproductive fitness with juvenile systemic lupus erythematosus. Lupus 2007, 16, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Mersereau, J.; Dooley, M.A. Gonadal Failure with Cyclophosphamide Therapy for Lupus Nephritis: Advances in Fertility Preservation. Rheum. Dis. Clin. N. Am. 2010, 36, 99–108. [Google Scholar] [CrossRef]
- Finlayson, C.; Johnson, E.K.; Chen, D.; Dabrowski, E.; Gosiengfiao, Y.; Campo-Engelstein, L.; Rosoklija, I.; Jacobson, J.; Shnorhavorian, M.; Pavone, M.E.; et al. Proceedings of the working group session on fertility preservation for individuals with gender and sex diversity. Transgend. Health 2016, 1, 99e107. [Google Scholar] [CrossRef] [PubMed]
- Van Batavia, J.P.; Kolon, T.F. Fertility in disorders of sex development: A review. J. Pediatr. Urol. 2016, 12, 418e25. [Google Scholar] [CrossRef]
- Carlson, C.A.; Kolon, T.F.; Mattei, P.; Hobbie, W.; Gracia, C.R.; Ogle, S.; Ginsberg, J.P. Developing a Hospital-Wide Fertility Preservation Service for Pediatric and Young Adult Patients. J. Adolesc. Heal. 2017, 61, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Pegg, D.E. Principles of cryopreservation. Methods Mol. Biol. 2015, 1257, 3–19. [Google Scholar]
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef]
- Borges, E.; Rossi, L.M.; de Freitas, C.V.L.; Guilherme, P.; Bonetti, T.C.S.; Iaconelli, A.; Pasqualotto, F.F. Fertilization and pregnancy outcome after intracytoplasmic injection with fresh or cryopreserved ejaculated spermatozoa. Fertil. Steril. 2007, 87, 316–320. [Google Scholar] [CrossRef]
- Dinofia, A.M.; Wang, X.; Yannekis, G.; Ogle, S.; Hobbie, W.L.; Carlson, C.A.; Ginsberg, J.P. Analysis of semen parameters in a young cohort of cancer patients. Pediatr. Blood Cancer 2016, 64, 381–386. [Google Scholar] [CrossRef]
- Picton, H.M.; Wyns, C.; Anderson, R.A.; Goossens, E.; Jahnukainen, K.; Kliesch, S.; Mitchell, R.T.; Pennings, G.; Rives, N.; Tournaye, H.; et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum. Reprod. 2015, 30, 2463–2475. [Google Scholar] [CrossRef]
- Hagenas, I.; Jorgensen, N.; Rechnitzer, C.; Sommer, P.; Holm, M.; Schmiegelow, K.; Daugaard, G.; Jacobsen, N.; Juul, A. Clinical and biochemical correlates of successful semen collection for cryopreservation from 12–18-year-old patients: A single-center study of 86 adolescents. Hum. Reprod. 2010, 25, 2031e8. [Google Scholar] [CrossRef] [PubMed]
- Szell, A.Z.; Bierbaum, R.C.; Hazelrigg, W.B.; Chetkowski, R.J. Live births from frozen human semen stored for 40 years. J. Assist. Reprod. Genet. 2013, 30, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.J.; Kolon, T.F.; Feldman, D.R.; Mulhall, J.P. Fertility preservation strategies for male patients with cancer. Nat. Rev. Urol. 2013, 10, 463–472. [Google Scholar] [CrossRef]
- Sandhu, R.S.; Wong, T.H.; Kling, C.A.; Chohan, K.R. In vitro effects of coital lubricants and synthetic and natural oils on sperm motility. Fertil. Steril. 2014, 101, 941–944. [Google Scholar] [CrossRef]
- Ethics Committee of American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: A committee opinion. Fertil. Steril. 2013, 100, 1224–1231. [Google Scholar] [CrossRef]
- Tur-Kaspa, I.; Segal, S.; Moffa, F.; Massobrio, M.; Meltzer, S. Viagra for temporary erectile dysfunction during treatments with assisted reproductive technologies. Hum. Reprod. 1999, 14, 1783–1784. [Google Scholar] [CrossRef]
- Meng, X.; Fan, L.; Wang, T.; Wang, S.; Wang, Z.; Liu, J. Electroejaculation combined with assisted reproductive technology in psychogenic anejaculation patients refractory to penile vibratory stimulation. Transl. Androl. Urol. 2018, 7 (Suppl. 1), S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Gat, I.; Toren, A.; Hourvitz, A.; Raviv, G.; Band, G.; Baum, M.; Inbar, R.; Madgar, I.; Lerner-Geva, L. Sperm preservation by electroejaculation in adolescent cancer patients. Pediatr. Blood Cancer 2013, 61, 286–290. [Google Scholar] [CrossRef]
- Adank, M.C.; van Dorp, W.; Smit, M.; van Casteren, N.J.; Laven, J.S.; Pieters, R.; Heuvel-Eibrink, M.M.V.D. Electroejaculation as a method of fertility preservation in boys diagnosed with cancer: A single-center experience and review of the literature. Fertil. Steril. 2014, 102, 199–205.e1. [Google Scholar] [CrossRef]
- Hovav, Y.; Dan-Goor, M.; Yaffe, H.; Almagor, M. Electroejaculation before chemotherapy in adolescents and young men with cancer. Fertil. Steril. 2001, 75, 811–813. [Google Scholar] [CrossRef]
- Berookhim, B.M.; Mulhall, J.P. Outcomes of operative sperm retrieval strategies for fertility preservation among males scheduled to undergo cancer treatment. Fertil. Steril. 2014, 101, 805–811. [Google Scholar] [CrossRef]
- Practice Committee of American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2013, 100, 1214–1223. [Google Scholar] [CrossRef]
- Schrader, M.; Müller, M.; Sofikitis, N.; Straub, B.; Krause, H.; Miller, K. “Onco-tese”: Testicular sperm extraction in azoospermic cancer patients before chemotherapy—New guidelines? Urology 2003, 61, 421–425. [Google Scholar] [CrossRef]
- Furuhashi, K.; Ishikawa, T.; Hashimoto, H.; Yamada, S.; Ogata, S.; Mizusawa, Y.; Matsumoto, Y.; Okamoto, E.; Kokeguchi, S.; Shiotani, M. Onco-testicular sperm extraction: Testicular sperm extraction in azoospermic and very severely oligozoospermic cancer patients. Andrologia 2012, 45, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Nicopoullos, J.D.; Gilling-Smith, C.; Almeida, P.A.; Ramsay, J.W. The results of 154 ICSI cycles using surgically retrieved sperm from azoospermic men. Hum. Reprod. 2004, 19, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Levron, J.; Madgar, I.; Shefi, S.; Meirow, D.; Wiser, A.; Bider, D.; Dor, J.; Raviv, G. IVF outcome with cryopreserved testicular sperm. Andrologia 2011, 43, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Fraass, B.A.; Kinsella, T.J.; Harrington, F.S.; Glatstein, E. Peripheral dose to the testes: The design and clinical use of a practical and effective gonadal shield. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 609–615. [Google Scholar] [CrossRef]
- Yadav, P.; Kozak, K.; Tolakanahalli, R.; Ramasubramanian, V.; Paliwal, B.R.; Welsh, J.S.; Rong, Y. Adaptive planning using megavoltage fan-beam CT for radiation therapy with testicular shielding. Med. Dosim. 2012, 37, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, P.R.; Kaurin, D.G.; McDonald, T.L.; Holland, J.M. Testicular shielding in low-dose total body irradiation. Bone Marrow Transpl. 2007, 39, 247–248. [Google Scholar] [CrossRef]
- Mccook, A. A future, on ice. Nat. Med. 2013, 19, 1078. [Google Scholar] [CrossRef]
- Wallace, W.H.; Anderson, R.A.; Irvine, D.S. Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005, 6, 209–218. [Google Scholar] [CrossRef]
- Tournaye, H.; Dohle, G.R.; Barratt, C.L. Fertility preservation in men with cancer. Lancet 2014, 384, 1295–1301. [Google Scholar] [CrossRef]
- Goossens, E.; Van Saen, D.; Tournaye, H. Spermatogonial stem cell preservation and transplantation: From research to clinic. Hum. Reprod. 2013, 28, 897–907. [Google Scholar] [CrossRef]
- Rashedi, A.S.; de Roo, S.F.; Ataman, L.M.; Edmonds, M.E.; Silva, A.A.; Scarella, A.; Horbaczewska, A.; Anazodo, A.; Arvas, A.; de Carvalho, B.R.; et al. Survey of Fertility Preservation Options Available to Patients with Cancer Around the Globe. JCO Glob. Oncol. 2020, 6, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Poels, J.; Van Langendonckt, A.; Many, M.-C.; Wese, F.-X.; Wyns, C. Vitrification preserves proliferation capacity in human spermatogonia. Hum. Reprod. 2013, 28, 578–589. [Google Scholar] [CrossRef]
- Baert, Y.; Van Saen, D.; Haentjens, P.; Veld, P.I.; Tournaye, H.; Goossens, E. What is the best cryopreservation protocol for human testicular tissue banking? Hum. Reprod. 2013, 28, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Medrano, J.V.; Martínez-Arroyo, A.M.; Sukhwani, M.; Noguera, I.; Quiñonero, A.; Martínez-Jabaloyas, J.M.; Pellicer, A.; Remohí, J.; Orwig, K.E.; Simón, C. Germ cell transplantation into mouse testes procedure. Fertil. Steril. 2014, 102, e11–e12. [Google Scholar] [CrossRef]
- De Rooij, D.G.; Griswold, M.D. Questions about spermatogonia posed and answered since 2000. J. Androl. 2012, 33, 1085–1095. [Google Scholar] [CrossRef]
- Mei, X.X.; Wang, J.; Wu, J. Extrinsic and intrinsic factors controlling spermatogonial stem cell self-renewal and differentiation. Asian J. Androl. 2015, 17, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, J.; Zou, K. Advances in isolation methods for spermatogonial stem cells. Stem Cell Rev. 2016, 12, 15–25. [Google Scholar] [CrossRef]
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar] [CrossRef]
- Brinster, R.L.; Avarbock, M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar] [CrossRef] [PubMed]
- Brook, P.F.; Radford, J.A.; Shalet, S.M.; Joyce, A.D.; Gosden, R.G. solation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil. Steril. 2001, 75, 269–274. [Google Scholar] [CrossRef]
- Radford, J. Restoration of fertility after treatment for cancer. Horm. Res. 2003, 59 (Suppl. 1), 21–23. [Google Scholar] [CrossRef]
- Dufour, J.M.; Rajotte, R.V.; Korbutt, G.S. Development of an in vivo model to study testicular morphogenesis. J. Androl. 2002, 23, 635–644. [Google Scholar] [PubMed]
- Kita, K.; Watanabe, T.; Ohsaka, K.; Hayashi, H.; Kubota, Y.; Nagashima, Y.; Aoki, I.; Taniguchi, H.; Noce, T.; Inoue, K.; et al. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biol. Reprod. 2007, 76, 211–217. [Google Scholar] [CrossRef]
- Gassei, K.; Schlatt, S.; Ehmcke, J. De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J. Androl. 2006, 27, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Honaramooz, A.; Snedaker, A.K.; Boiani, M.; Scholer, H.R.; Dobrinski, I.; Schlatt, S. Sperm from neonatal mammalian testes grafted in mice. Nat. Cell Biol. 2002, 418, 778–781. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Ehmcke, J.; Nurmio, M.; Schlatt, S. Autologous Ectopic Grafting of Cryopreserved Testicular Tissue Preserves the Fertility of Prepubescent Monkeys That Receive Sterilizing Cytotoxic Therapy. Cancer Res. 2012, 72, 5174–5178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nie, Y.H.; Zhang, C.C.; Cai, Y.J.; Wang, Y.; Lu, H.P.; Li, Y.Z.; Cheng, C.; Qiu, Z.L.; Sun, Q. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res. 2016, 26, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Shlush, E.; Maghen, L.; Swanson, S.; Kenigsberg, S.; Moskovtsev, S.; Barretto, T.; Gauthier-Fisher, A.; Librach, C.L. In vitro generation of Sertoli-like and haploid spermatid-like cells from human umbilical cord perivascular cells. Stem Cell Res. Ther. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Easley, C.A.; Phillips, B.T.; McGuire, M.M.; Barringer, J.M.; Valli, H.; Hermann, B.P.; Simerly, C.R.; Rajkovic, A.; Miki, T.; Orwig, K.E.; et al. Direct Differentiation of Human Pluripotent Stem Cells into Haploid Spermatogenic Cells. Cell Rep. 2012, 2, 440–446. [Google Scholar] [CrossRef]
- Arregui, L.; Rathi, R.; Zeng, W.; Honaramooz, A.; Gomendio, M.; Roldan, E.R.; Dobrinski, I. Xenografting of adult mammalian testis tissue. Anim. Reprod. Sci. 2008, 106, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Van Langendonckt, A.; Wese, F.-X.; Donnez, J.; Curaba, M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum. Reprod. 2008, 23, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Patrizio, P.; Brinster, R.L. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil. Steril. 2002, 78, 1225–1233. [Google Scholar] [CrossRef]
- Mirzapour, T.; Movahedin, M.; Koruji, M.; Nowroozi, M. Xeno-transplantation assessment: Morphometric study of human spermatogonial stem cells in recipient mouse testes. Andrologia 2015, 47, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Honaramooz, A.; Ehmcke, J.; Goebell, P.J.; Rubben, H.; Dhir, R.; Dobrinski, I.; Patrizio, P. Limited survival of adult human testicular tissue asectopic xenograft. Hum. Reprod. 2005, 21, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H. Spermatogenesis in artificial three-dimensional system. Stem Cells 2012, 30, 2355–2360. [Google Scholar] [CrossRef]
- Steinberger, A.; Steinberger, E.; Perloff, W.H. Mammalian testes in organ culture. Exp. Cell Res. 1964, 36, 19–27. [Google Scholar] [CrossRef]
- Steinberger, A.; Steinberger, E. Factors affecting spermatogenesis in organ cultures of mammalian testes. J. Reprod. Fertil. 1967, 2, 117–124. [Google Scholar]
- Ogawa, T. In vitro spermatogenesis: The dawn of a new era in the study of male infertility. Int. J. Urol. 2012, 19, 282–283. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–508. [Google Scholar] [CrossRef]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Irie, N.; Weinberger, L.; Tang, W.W.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Kubota, Y.; Ogawa, T. In vitro sperm production from mouse spermatogonial stem cell lines using anorgan culture method. Nat. Protoc. 2013, 8, 2098–2104. [Google Scholar] [CrossRef]
- Oatley, J.M.; de Avila, D.M.; Reeves, J.J.; McLean, D.J. Spermatogenesis and germ cell transgene expression in xenografted bovine testicular tissue. Biol. Reprod. 2004, 71, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Ryu, B.Y.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214. [Google Scholar] [CrossRef]
- Helsel, A.R.; Oatley, M.J.; Oatley, J.M. Glycolysis-optimized conditions enhance maintenance of regenerative integrity in mouse spermatogonial stem cells during long-term culture. Stem Cell Rep. 2017, 8, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972–980. [Google Scholar] [CrossRef]
- Abu Elhija, M.; Lunenfeld, E.; Schlatt, S.; Huleihel, M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J. Androl. 2012, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- AbuMadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of spermatogenesis in vitro in three-dimensional culture from spermatogonial cells of busulfan-treated immature mice. Int. J. Mol. Sci. 2018, 19, 3804. [Google Scholar] [CrossRef]
- Huleihel, N.; Azab, M.; Levitas, E.; Fisch, B.; Pinkas, H.; Stein, A.; Younis, J.S.; Bar-Ami, S.; Orvieto, R.; Ziadna, A.; et al. The capacity to induce generation of premeiotic and meiotic cells from biopsies without sperm from non-obstructive azoospermic and Klinefelter syndrome patients were different under in vitro culture conditions. Fertil. Steril. 2015, 104, e91. [Google Scholar] [CrossRef]
- Azab, M.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Fertility reservation of pre-pubertal cancer patient boys before aggressive chemotherapy. Preliminary results from in vitro cultures of fresh testicular tissue from three pre-pubertal patients. Fertil. Steril. 2013, 100, S63. [Google Scholar]
- Baert, Y.; Dvorakova-Hortova, K.; Margaryan, H.; Goossens, E. Mouse in Vitro Spermatogenesis on Alginate-Based 3D Bioprinted Scaffolds. Biofabrication 2019, 11, 035011. [Google Scholar] [CrossRef] [PubMed]
- Alves-Lopes, J.P.; Stukenborg, J.B. Testicular organoids: A new model to study the testicular microenvironment in vitro? Hum. Reprod. Update 2018, 24, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Pendergraft, S.S.; Sadri-Ardekani, H.; Atala, A.; Bishop, C.E. Three-dimensional testicular organoid: A novel tool for the study of human spermatogenesis and gonadotoxicity in vitro dagger. Biol. Reprod. 2017, 96, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; De Kock, J.; Alves-Lopes, J.P.; Söder, O.; Stukenborg, J.-B.; Goossens, E. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Rep. 2017, 1, 30–38. [Google Scholar] [CrossRef]
- Alves-Lopes, J.P.; Soder, O.; Stukenborg, J.B. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 2017, 130, 76–89. [Google Scholar] [CrossRef]
- Topraggaleh, T.R.; Valojerdi, M.R.; Montazeri, L.; Baharvand, H. A testis-derived macroporous 3D scaffold as a platform for the generation of mouse testicular organoids. Biomater. Sci. 2019, 7, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- De Michele, F.; Poels, J.; Weerens, L.; Petit, C.; Evrard, Z.; Ambroise, J.; Gruson, D.; Wyns, C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 2016, 32, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Komeya, M.; Kimura, H.; Nakamura, H.; Yokonishi, T.; Sato, T.; Kojima, K.; Hayashi, K.; Katagiri, K.; Yamanaka, H.; Sanjo, H.; et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016, 6, 21472. [Google Scholar] [CrossRef] [PubMed]
- Komeya, M.; Hayashi, K.; Nakamura, H.; Yamanaka, H.; Sanjo, H.; Kojima, K.; Sato, T.; Yao, M.; Kimura, H.; Fujii, T.; et al. Pumpless Microfluidic System Driven by Hydrostatic Pressure Induces and Maintains Mouse Spermatogenesis in Vitro. Sci. Rep. 2017, 7, 15459. [Google Scholar] [CrossRef]
- Yamanaka, H.; Komeya, M.; Nakamura, H.; Sanjo, H.; Sato, T.; Yao, M.; Kimura, H.; Fujii, T.; Ogawa, T. A monolayer microfluidic device supporting mouse spermatogenesis with improved visibility. Biochem. Biophys. Res. Commun. 2018, 500, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Hou, M.; Petersen, C.; Setchell, B.; Söder, O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001, 61, 706–710. [Google Scholar]
- Sadri-Ardekani, H.; Homburg, C.H.; van Capel, T.M.; Berg, H.V.D.; van der Veen, F.; van der Schoot, C.E.; van Pelt, A.M.; Repping, S. Eliminating acute lymphoblastic leukemia cells from human testicular cell cultures: A pilot study. Fertil. Steril. 2014, 101, 1072–1078.e1. [Google Scholar] [CrossRef]
- Hou, J.; Yang, S.; Yang, H.; Liu, Y.; Hai, Y.; Chen, Z.; Guo, Y.; Gong, Y.; Gao, W.-Q.; Li, Z.; et al. Generation of male differentiated germ cells from various types of stem cells. Reproduction 2014, 147, R179–R188. [Google Scholar] [CrossRef]
- Gauthier-Fisher, A.; Kauffman, A.; Librach, C.L. Potential use of stem cells for fertility preservation. Andrology 2020, 8, 862–878. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kacin, E.; Stamatiadis, P.; Franck, S.; Van der Jeught, M.; Mertes, H.; Pennings, G.; De Sutter, P.; Sermon, K.; Heindryckx, B.; et al. The role of the reprogramming method and pluripotency state in gamete differentiation from patient-specific human pluripotent stem cells. Mol. Hum. Reprod. 2018, 24, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Kee, K.; Angeles, V.T.; Flores, M.; Nguyen, H.N.; Pera, R.A.R. Human dazl, daz and boule genes modulate primordial germ-cell and haploid gamete formation. Nature 2009, 462, 222–225. [Google Scholar] [CrossRef]
- Verma, R.S. Oncogenetics. A new emergieng field of Cancer. Mol. Gen. Genet. 1986, 205, 385–389. [Google Scholar] [CrossRef]
- Zorrilla, M.; Yatsenko, A.N. The Genetics of Infertility: Current Status of the Field. Curr. Genet. Med. Rep. 2013, 1, 247–260. [Google Scholar] [CrossRef]
- Upadhyay, R.D.; Balasinor, N.H.; Kumar, A.V.; Sachdeva, G.; Parte, P.; Dumasia, K. Proteomics in reproductive biology: Beacon for unraveling the molecular complexities. Biochim. Biophys. Acta 2013, 1834, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gilany, K.; Minai-Tehrani, A.; Savadi-Shiraz, E.; Rezadoost, H.; Lakpour, N. Exploring the human seminal plasma proteome: An unexplored gold mine of biomarker for male infertility and male reproduction disorder. J. Reprod. Infertil. 2015, 16, 61–71. [Google Scholar]
- Kolialexi, A.; Mavrou, A.; Spyrou, G.; Tsangaris, G.T. Mass spectrometry-based proteomics in reproductive medicine. Mass Spectrom. Rev. 2008, 27, 624–634. [Google Scholar] [CrossRef]
- Fernandez-Encinas, A.; García-Peiró, A.; Ribas-Maynou, J.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Characterization of Nuclease Activity in Human Seminal Plasma and its Relationship to Semen Parameters, Sperm DNA Fragmentation and Male Infertility. J. Urol. 2016, 195, 213–219. [Google Scholar] [CrossRef]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Abramowitz, L.K.; Kubota, H.; Wu, X.; Niu, Z.; Avarbock, M.R.; Tobias, J.W.; Bartolomei, M.S.; Brinster, R.L. In Vivo and In Vitro Aging Is Detrimental to Mouse Spermatogonial Stem Cell Function. Biol. Reprod. 2010, 84, 698–706. [Google Scholar] [CrossRef][Green Version]
- Chapman, K.M.; Medrano, G.A.; Jaichander, P.; Chaudhary, J.; Waits, A.E.; Nobrega, M.A.; Hotaling, J.M.; Ober, C.; Hamra, F.K. Targeted Germline Modifications in Rats Using CRISPR/Cas9 and Spermatogonial Stem Cells. Cell Rep. 2015, 10, 1828–1835. [Google Scholar] [CrossRef]
- Sato, T.; Sakuma, T.; Yokonishi, T.; Katagiri, K.; Kamimura, S.; Ogonuki, N.; Ogura, A.; Yamamoto, T.; Ogawa, T. Genome Editing in Mouse Spermatogonial Stem Cell Lines Using TALEN and Double-Nicking CRISPR/Cas9. Stem Cell Rep. 2015, 5, 75–82. [Google Scholar] [CrossRef]
- Turocy, J.; Adashi, E.Y.; Egli, D. Herritable human genome editing: Research progress, ethical considerations, and hurdles to clinical practice. Cell 2021, 184, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Zhang, H.; Tang, W.W.C.; Irie, N.; Withey, S.; Klisch, D.; Sybirna, A.; Dietmann, S.; Contreras, D.A.; Webb, R.; et al. Principles of early human development and germ cell program from conserved model systems. Nat. Cell Biol. 2017, 546, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.; et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell 2016, 18, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M.; et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nat. Cell Biol. 2016, 539, 299–303. [Google Scholar] [CrossRef]
- Sugawa, F.; Araúzo-Bravo, M.J.; Yoon, J.; Kim, K.; Aramaki, S.; Wu, G.; Stehling, M.; E Psathaki, O.; Hübner, K.; Schöler, H.R. Human primordial germ cell commitment in vitro associates with a unique PRDM14 expression profile. EMBO J. 2015, 34, 1009–1024. [Google Scholar] [CrossRef]
- Medrano, J.V.; Andres, M.D.M.; Garcia, S.; Herraiz, S.; Vilanova-Perez, T.; Goossens, E.; Pellicer, A. Basic and Clinical Approaches for Fertility Preservation and Restauration in Cancer Patients. Trends Biotehnol. 2018, 36, 199–215. [Google Scholar] [CrossRef]
- Quinn, G.P.; Stearsman, D.K.; Campo-Engelstein, L.; Murphy, D. Preserving the Right to Future Children: An Ethical Case Analysis. Am. J. Bioeth. 2012, 12, 38–43. [Google Scholar] [CrossRef]
- Patrizio, P.; Butts, S.; Caplan, A. Ovarian tissue preservation and future fertility: Emerging technologies and ethical considerations. J. Natl. Cancer Inst. Monogr. 2005, 2005, 107–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dolmans, M.-M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jayasinghe, Y.; Kemertzis, M.A.; Moore, P.; Peate, M. Fertility Preservation in Pediatric and Adolescent Oncology Patients: The Decision-Making Process of Parents. J. Adolesc. Young Adult Oncol. 2017, 6, 213–222. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bîcă, O.; Sârbu, I.; Ciongradi, C.I. Pediatric and Adolescent Oncofertility in Male Patients—From Alpha to Omega. Genes 2021, 12, 701. https://doi.org/10.3390/genes12050701
Bîcă O, Sârbu I, Ciongradi CI. Pediatric and Adolescent Oncofertility in Male Patients—From Alpha to Omega. Genes. 2021; 12(5):701. https://doi.org/10.3390/genes12050701
Chicago/Turabian StyleBîcă, Ovidiu, Ioan Sârbu, and Carmen Iulia Ciongradi. 2021. "Pediatric and Adolescent Oncofertility in Male Patients—From Alpha to Omega" Genes 12, no. 5: 701. https://doi.org/10.3390/genes12050701
APA StyleBîcă, O., Sârbu, I., & Ciongradi, C. I. (2021). Pediatric and Adolescent Oncofertility in Male Patients—From Alpha to Omega. Genes, 12(5), 701. https://doi.org/10.3390/genes12050701