cROStalk for Life: Uncovering ROS Signaling in Plants and Animal Systems, from Gametogenesis to Early Embryonic Development
Abstract
1. Introduction
2. ROS and Physiological and Cellular Responses in Plants
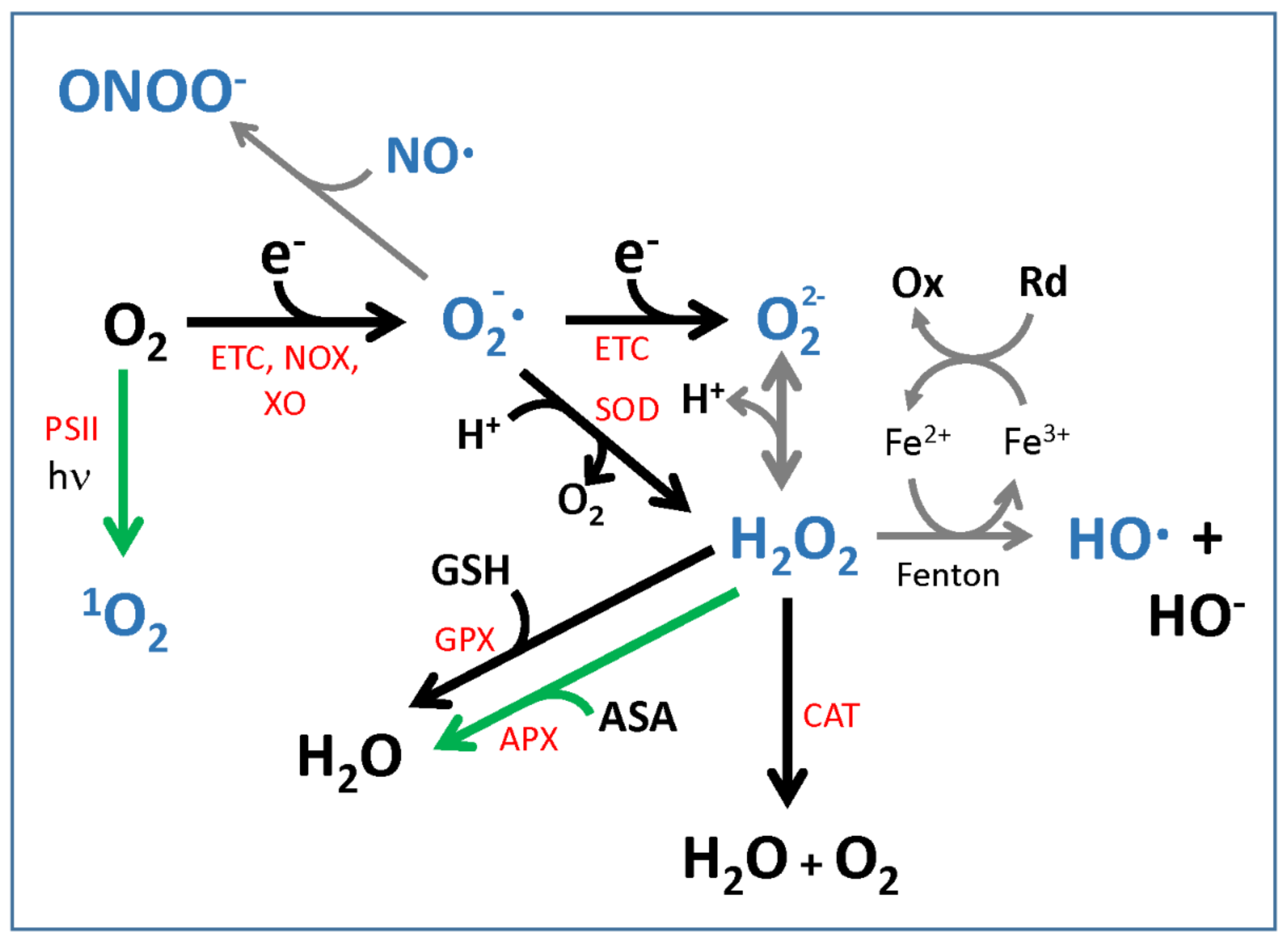
2.1. Reactive Oxygen Species: Production and Detoxification
2.2. ROS Chemistry and Implications for Signaling
2.3. Redox State Communication among Compartments
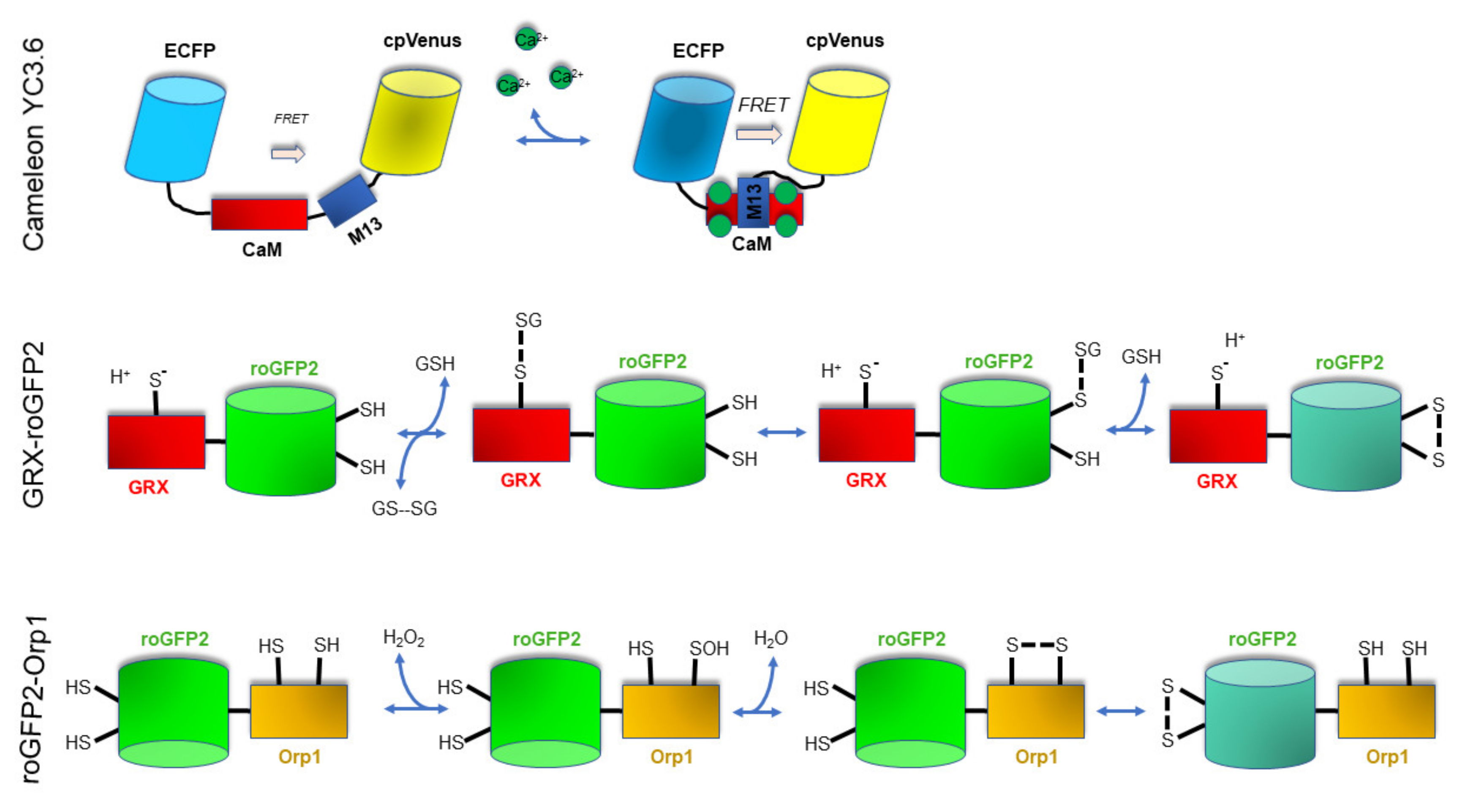
3. Analytical Techniques to Monitor In Vivo Ca2+ and Redox Signaling in Plants: Past and Present Research
3.1. Imaging Techniques to Monitor In Vivo Ca2+ Levels and Dynamics
3.2. Imaging Techniques to Monitor In Vivo the 2GSH/GSSG Redox Status
3.3. Imaging Techniques to Monitor In Vivo H2O2 Levels and Dynamics
4. ROS/Ca2+ and the Impact in Differentiation and Developmental Processes in Plants
4.1. Seeds, ROS, and Germination
4.2. ROS Fine Tune Control on Stem and Root Differentiation in Plants
4.3. ROS Are Crucial in Different Steps during Sexual Plant Reproduction
5. ROS/Ca2+ Crosstalk in Mammalian Embryonic Development
5.1. Overview of Gametogenesis and Early Embryonic Development in Mammals
5.2. Overview of the Ca2+ and ROS Signaling Interplay in Animal Cells
5.3. ROS/Ca2+ Signaling in Mammalian Early Embryonic Development
5.3.1. Ca2+ Signaling
5.3.2. ROS Signaling
5.3.3. Ca2+/ROS Signaling Interplay in Gametes and Early Embryos
6. Conclusions and Future Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podolyan, A.; Maksimov, N.; Breygina, M. Redox-regulation of ion homeostasis in growing lily pollen tubes. J. Plant Physiol. 2019, 243. [Google Scholar] [CrossRef]
- Tola, A.J.; Jaballi, A.; Germain, H.; Missihoun, T.D. Recent development on plant aldehyde dehydrogenase enzymes and their functions in plant development and stress signaling. Genes 2021, 12, 1–18. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A role for Reactive oxygen species produced by NADPH oxidases in the embryo and aleurone cells in barley seed germination. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Cha, J.Y.; Kang, S.; Park, B.; Lee, H.J.; Hong, H.; Chun, H.J.; Kim, D.H.; Kim, M.C.; Lee, S.Y.; et al. The Arabidopsis a zinc finger domain protein ARS1 is essential for seed germination and ROS homeostasis in response to ABA and oxidative stress. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.H.M.; Foyer, C.H.; van Dongen, J.T. Redox regulation in shoot growth, SAM maintenance and flowering. Curr. Opin. Plant Biol. 2016, 29, 121–128. [Google Scholar] [CrossRef]
- Rampon, C.; Volovitch, M.; Joliot, A.; Vriz, S. Hydrogen peroxide and redox regulation of developments. Antioxidants 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Klimenko, E. ROS and ions in cell signaling during sexual plant reproduction. Int. J. Mol. Sci. 2020, 21, 9476. [Google Scholar] [CrossRef]
- Janku, M.; Luhová, L.; Petrivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. Singlet oxygen in plants: Generation, detection, and signaling roles. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Laloi, C.; Stachowiak, M.; Pers-Kamczyc, E.; Warzych, E.; Murgia, I.; Apel, K. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef]
- Foyer, C.H.; Wilson, M.H.; Wright, M.H. Redox regulation of cell proliferation: Bioinformatics and redox proteomics approaches to identify redox-sensitive cell cycle regulators. Free Radic. Biol. Med. 2018, 122, 137–149. [Google Scholar] [CrossRef]
- Del Río, L.A.; López-Huertas, E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Robb, S.J.; Clark, J.B. Nitric oxide and Fenton/Haber-Weiss chemistry: Nitric oxide is a potent antioxidant at physiological concentrations. J. Neurochem. 2003, 87, 386–394. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS Release in Plant and Animal Cells. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0891584917312327 (accessed on 2 February 2021).
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Grulke, N.E.; Heath, R.L. Ozone effects on plants in natural ecosystems. Plant Biol. 2020, 22, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Emberson, L. Effects of ozone on agriculture, forests and grasslands: Improving risk assessment methods for O3. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190327. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.; Kandlbinder, A.; Golldack, D.; Dietz, K.J. Oxidative stress and ozone: Perception, signalling and response. Plantcell Environ. 2005, 28, 1012–1020. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Kangasjärvi, J. Plant signalling in acute ozone exposure. Plantcell Environ. 2015, 38, 240–252. [Google Scholar] [CrossRef]
- Gandin, A.; Dizengremel, P.; Jolivet, Y. Integrative role of plant mitochondria facing oxidative stress: The case of ozone. Plant Physiol. Biochem. 2021, 159, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Hörandl, E.; Hadacek, F. Oxygen, life forms, and the evolution of sexes in multicellular eukaryotes. Heredity 2020, 125. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Signaling in a Higher-CObinf2einf World. Annu. Rev. Plant Biol. 2020, 71, 157–182. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plantcell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Zechmann, B.; Stumpe, M.; Mauch, F. Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta 2011, 233, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, G.; Benedetti, A.; Margittai, É.; Marcolongo, P.; Fulceri, R.; Németh, C.E.; Szarka, A. Subcellular compartmentation of ascorbate and its variation in disease states. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007, 52, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, G.; Ishikawa, T.; Pornsaksit, V.; Smirnoff, N. Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. Elife 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Smirnoff, N.; Cobbett, C.S.; Golz, J.F. Ascorbate-deficient VTC2 mutants in arabidopsis do not exhibit decreased growth. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Aboobucker, S.; Suza, W.; Lorence, A. Characterization of Two Arabidopsis L-Gulono-1,4-lactone Oxidases, AtGulLO3 and AtGulLO5, Involved in Ascorbate Biosynthesis. React. Oxyg. Species 2017. [Google Scholar] [CrossRef]
- Maruta, T.; Ichikawa, Y.; Mieda, T.; Takeda, T.; Tamoi, M.; Yabuta, Y.; Ishikawa, T.; Shigeoka, S. The contribution of Arabidopsis homologs of L-gulono-1,4-lactone oxidase to the biosynthesis of ascorbic acid. Biosci. Biotechnol. Biochem. 2010, 74, 1494–1497. [Google Scholar] [CrossRef]
- Ivanov Kavkova, E.; Blöchl, C.; Tenhaken, R. The Myo-inositol pathway does not contribute to ascorbic acid synthesis. Plant Biol. 2019, 21, 95–102. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants. J. Exp. Bot. 2021, 72, 830–847. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Biological chemistry of superoxide radicals. ChemTexts 2020, 6. [Google Scholar] [CrossRef]
- Tarr, M.; Valenzeno, D.P. Singlet oxygen: The relevance of extracellular production mechanisms to oxidative stress in vivo. Photochem. Photobiol. Sci. 2003, 2, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Indo, H.P.; Yen, H.C.; Nakanishi, I.; Matsumoto, K.I.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002, 3, 1129–1134. [Google Scholar] [CrossRef]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef]
- Medraño-Fernandez, I.; Bestetti, S.; Bertolotti, M.; Bienert, G.P.; Bottino, C.; Laforenza, U.; Rubartelli, A.; Sitia, R. Stress Regulates Aquaporin-8 Permeability to Impact Cell Growth and Survival. Antioxid. Redox Signal. 2016, 24, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef]
- Møller, I.M.; Igamberdiev, A.U.; Bykova, N.V.; Finkemeier, I.; Rasmusson, A.G.; Schwarzländer, M. Matrix redox physiology governs the regulation of plant mitochondrial metabolism through posttranslational protein modifications. Plant Cell 2020, 32, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Willems, P.; Wei, B.; Tian, C.; Ferreira, R.B.; Bodra, N.; Martínez Gache, S.A.; Wahni, K.; Liu, K.; Vertommen, D.; et al. Mining for protein S-sulfenylation in Arabidopsis uncovers redox-sensitive sites. Proc. Natl. Acad. Sci. USA 2019, 116, 20256–20261. [Google Scholar] [CrossRef]
- De Smet, B.; Willems, P.; Fernandez-Fernandez, A.D.; Alseekh, S.; Fernie, A.R.; Messens, J.; Van Breusegem, F. In vivo detection of protein cysteine sulfenylation in plastids. Plant J. 2019, 97, 765–778. [Google Scholar] [CrossRef]
- Pan, J.; Carroll, K.S. Chemical biology approaches to study protein cysteine sulfenylation. Biopolymers 2014, 101, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gurrieri, L.; Distefano, L.; Pirone, C.; Horrer, D.; Seung, D.; Zaffagnini, M.; Rouhier, N.; Trost, P.; Santelia, D.; Sparla, F. The Thioredoxin-Regulated α-Amylase 3 of Arabidopsis thaliana Is a Target of S-Glutathionylation. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.R.; Gaitens, M.; Guan, Q.; Dufresne, C.; Chen, S. S-nitroso-proteome revealed in stomatal guard cell response to flg22. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Sánchez-Vicente, I.; Fernández-Espinosa, M.G.; Lorenzo, O. Nitric oxide molecular targets: Reprogramming plant development upon stress. J. Exp. Bot. 2019, 70, 4441–4460. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Feigl, G.; Bordé, Á.; Molnár, Á.; Erdei, L. Protein tyrosine nitration in plants: Present knowledge, computational prediction and future perspectives. Plant Physiol. Biochem. 2017, 113, 56–63. [Google Scholar] [CrossRef]
- Astier, J.; Lindermayr, C. Nitric oxide-dependent posttranslational modification in plants: An update. Int. J. Mol. Sci. 2012, 13, 15193–15208. [Google Scholar] [CrossRef]
- Pan, L.; De Smet, I. Expanding the Mitogen-Activated Protein Kinase (MAPK) Universe: An Update on MAP4Ks. Front. Plant Sci. 2020, 11, 1220. [Google Scholar] [CrossRef]
- Holzmeister, C.; Gaupels, F.; Geerlof, A.; Sarioglu, H.; Sattler, M.; Durner, J.; Lindermayr, C. Differential inhibition of Arabidopsis superoxide dismutases by peroxynitrite-mediated tyrosine nitration. J. Exp. Bot. 2015, 66, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Feher, J. ATP Production I. In Quantitative Human Physiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 218–226. [Google Scholar]
- Nietzel, T.; Elsässer, M.; Ruberti, C.; Steinbeck, J.; Ugalde, J.M.; Fuchs, P.; Wagner, S.; Ostermann, L.; Moseler, A.; Lemke, P.; et al. The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol. 2019, 221, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, J.; Fuchs, P.; Negroni, Y.L.; Elsässer, M.; Lichtenauer, S.; Stockdreher, Y.; Feitosa-Araujo, E.; Kroll, J.B.; Niemeier, J.O.; Humberg, C.; et al. In vivo nadh/nad1 biosensing reveals the dynamics of cytosolic redox metabolism in plants. Plant Cell 2020, 32, 3324–3345. [Google Scholar] [CrossRef]
- Elsässer, M.; Feitosa-Araujo, E.; Lichtenauer, S.; Wagner, S.; Fuchs, P.; Giese, J.; Kotnik, F.; Hippler, M.; Meyer, A.J.; Maurino, V.G.; et al. Photosynthetic activity triggers pH and NAD redox signatures across different plant cell compartments. bioRxiv 2020. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Zhao, Y.; Xu, Q.; Wu, J.; Ma, H.; Guo, H.; Bai, L.; Zuo, J.; Zhou, J.M.; et al. Regulation of mitochondrial NAD pool via NAD+ transporter 2 is essential for matrix NADH homeostasis and ROS production in Arabidopsis. Sci. China Life Sci. 2019, 62, 991–1002. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, L.; Xu, J.; Xin, P.; Guo, H.; Wu, J.; Bai, L.; Wang, G.; Chu, J.; Zuo, J.; et al. Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana. Cell Res. 2018, 28, 448–461. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, H.; Zhou, J.M.; Smith, S.M.; Li, J. Malate Circulation: Linking Chloroplast Metabolism to Mitochondrial ROS. Trends Plant Sci. 2020, 25, 446–454. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the crossroads of signaling. Plant Cell 2002, 14. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Batistič, O.; Kudla, J. Calcium: Not just another ion. Plant Cell Monogr. 2010, 17, 17–54. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Stael, S.; Wurzinger, B.; Mair, A.; Mehlmer, N.; Vothknecht, U.C.; Teige, M. Plant organellar calcium signalling: An emerging field. J. Exp. Bot. 2012, 63, 1525–1542. [Google Scholar] [CrossRef]
- Costa, A.; Navazio, L.; Szabo, I. The contribution of organelles to plant intracellular calcium signalling. J. Exp. Bot. 2018, 69, 4175–4193. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Allen, G.J.; Hugouvieux, V.; Kwak, J.M.; Waner, D. Guard cell signal transduction. Annu. Rev. Plant Biol. 2001, 52, 627–658. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhard, N.; Angel Torres, M.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Sierla, M.; Waszczak, C.; Vahisalu, T.; Kangasjärvi, J. Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 2016, 171, 1569–1580. [Google Scholar] [CrossRef]
- Pel, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef]
- Mori, I.C.; Murata, Y.; Yang, Y.; Munemasa, S.; Wang, Y.F.; Andreoli, S.; Tiriac, H.; Alonso, J.M.; Harper, J.F.; Ecker, J.R.; et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+- permeable channels and stomatal closure. PLoS Biol. 2006, 4, 1749–1762. [Google Scholar] [CrossRef]
- Vahisalu, T.; Kollist, H.; Wang, Y.F.; Nishimura, N.; Chan, W.Y.; Valerio, G.; Lamminmäki, A.; Brosché, M.; Moldau, H.; Desikan, R.; et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 2008, 452, 487–491. [Google Scholar] [CrossRef]
- Murata, Y.; Pei, Z.-M.; Mori, I.C.; Schroeder, J. Abscisic Acid Activation of Plasma Membrane Ca 2+ Channels in Guard Cells Requires Cytosolic NAD(P)H and Is Differentially Disrupted Upstream and Downstream of Reactive Oxygen Species Production in abi1-1 and abi2-1 Protein Phosphatase 2C Mutants. Plant Cell 2001, 13, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Laanemets, K.; Wang, Y.F.; Lindgren, O.; Wu, J.; Nishimura, N.; Lee, S.; Caddell, D.; Merilo, E.; Brosche, M.; Kilk, K.; et al. Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels. New Phytol. 2013, 197, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Johns, S.; Hagihara, T.; Toyota, M.; Gilroy, S. The Fast and The Furious: Rapid long-range signaling in plants. Plant Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis gp91phox homologues Atrbohd and Atrbohf are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokota, N.; Fujiwara, M.; Shimamoto, K.; Doke, N.; Yoshioka, H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH Oxidase RBOHD during Plant Immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Messerli, M.A.; Gilroy, S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008, 147, 1690–1698. [Google Scholar] [CrossRef]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef]
- Wudick, M.M.; Feijó, J.A. At the intersection: Merging Ca2+ and ROS signaling pathways in pollen. Mol. Plant 2014, 7, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Mangano, S.; Juárez, S.P.D.; Estevez, J.M. ROS regulation of polar growth in plant cells. Plant Physiol. 2016, 171, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Zhang, X.S.; Gao, X.Q. ROS in the Male–Female Interactions During Pollination: Function and Regulation. Front. Plant Sci. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef]
- Evans, M.J.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Gaupels, F.; Durner, J.; Kogel, K.H. Production, amplification and systemic propagation of redox messengers in plants? The phloem can do it all! New Phytol. 2017, 214, 554–560. [Google Scholar] [CrossRef]
- Choi, W.G.; Hilleary, R.; Swanson, S.J.; Kim, S.H.; Gilroy, S. Rapid, Long-Distance Electrical and Calcium Signaling in Plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef]
- Kong, D.; Ju, C.; Parihar, A.; Kim, S.; Cho, D.; Kwak, J.M. Arabidopsis glutamate receptor homolog3.5 modulates cytosolic Ca2+ level to counteract effect of abscisic acid in seed germination. Plant Physiol. 2015, 167, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Hu, H.C.; Okuma, E.; Lee, Y.; Lee, H.S.; Munemasa, S.; Cho, D.; Ju, C.; Pedoeim, L.; Rodriguez, B.; et al. L-Met Activates Arabidopsis GLR Ca2+ Channels Upstream of ROS Production and Regulates Stomatal Movement. Cell Rep. 2016, 17, 2553–2561. [Google Scholar] [CrossRef]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Nietzel, T.; Mostertz, J.; Ruberti, C.; Née, G.; Fuchs, P.; Wagner, S.; Moseler, A.; Müller-Schüssele, S.J.; Benamar, A.; Poschet, G.; et al. Redox-mediated kick-start of mitochondrial energy metabolism drives resource-efficient seed germination. Proc. Natl. Acad. Sci. USA 2020, 117, 741–751. [Google Scholar] [CrossRef]
- De Col, V.; Fuchs, P.; Nietzel, T.; Elsässer, M.; Voon, C.P.; Candeo, A.; Seeliger, I.; Fricker, M.D.; Grefen, C.; Møller, I.M.; et al. ATP sensing in living plant cells reveals tissue gradients and stress dynamics of energy physiology. Elife 2017, 6. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Fermani, S.; Marchand, C.H.; Costa, A.; Sparla, F.; Rouhier, N.; Geigenberger, P.; Lemaire, S.D.; Trost, P. Redox Homeostasis in Photosynthetic Organisms: Novel and Established Thiol-Based Molecular Mechanisms. Antioxid. Redox Signal. 2019, 31, 155–210. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Dick, T.P.; Meyer, A.J.; Morgan, B. Dissecting redox biology using fluorescent protein sensors. Antioxid. Redox Signal. 2016, 24, 680–712. [Google Scholar] [CrossRef]
- Walia, A.; Waadt, R.; Jones, A.M. Genetically Encoded Biosensors in Plants: Pathways to Discovery. Annu. Rev. Plant Biol. 2018, 69, 497–524. [Google Scholar] [CrossRef]
- Nagai, T.; Yamada, S.; Tominaga, T.; Ichikawa, M.; Miyawaki, A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 10554–10559. [Google Scholar] [CrossRef]
- Beneloujaephajri, E.; Costa, A.; L’Haridon, F.; Métraux, J.P.; Binda, M. Production of reactive oxygen species and wound-induced resistance in Arabidopsis thaliana against Botrytis cinerea are preceded and depend on a burst of calcium. BMC Plant Biol. 2013, 13, 160. [Google Scholar] [CrossRef]
- Benikhlef, L.; L’Haridon, F.; Abou-Mansour, E.; Serrano, M.; Binda, M.; Costa, A.; Lehmann, S.; Métraux, J.P. Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol. 2013, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Doccula, F.G.; Luoni, L.; Behera, S.; Bonza, M.C.; Costa, A. In vivo analysis of calcium levels and glutathione redox status in Arabidopsis epidermal leaf cells infected with the hypersensitive response-inducing bacteria Pseudomonas syringae pv. tomato AvrB (PstAvrB). In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1743, pp. 125–141. [Google Scholar]
- Storti, M.; Costa, A.; Golin, S.; Zottini, M.; Morosinotto, T.; Alboresi, A. Systemic calcium wave propagation in physcomitrella patens. Plant Cell Physiol. 2018, 59, 1377–1384. [Google Scholar] [CrossRef]
- Wagner, S.; Steinbeck, J.; Fuchs, P.; Lichtenauer, S.; Elsässer, M.; Schippers, J.H.M.; Nietzel, T.; Ruberti, C.; Van Aken, O.; Meyer, A.J.; et al. Multiparametric real-time sensing of cytosolic physiology links hypoxia responses to mitochondrial electron transport. New Phytol. 2019, 224, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- Hilleary, R.; Paez-Valencia, J.; Vens, C.; Toyota, M.; Palmgren, M.; Gilroy, S. Tonoplast-localized Ca2+ pumps regulate Ca2+ signals during pattern-triggered immunity in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2020, 117, 18849–18857. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Kudla, J. Live Cell Imaging of Cytoplasmic Ca2+ Dynamics in Arabidopsis Guard Cells. Cold Spring Harb. Protoc. 2013, 8, 665–669. [Google Scholar] [CrossRef]
- Miyawaki, A.; Llopis, J.; Heim, R.; Michael McCaffery, J.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Candeo, A.; Fieramonti, L.; Valentini, G.; Bassi, A. Calcium Dynamics in Root Cells of Arabidopsis thaliana Visualized with Selective Plane Illumination Microscopy. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Krebs, M.; Held, K.; Binder, A.; Hashimoto, K.; Den Herder, G.; Parniske, M.; Kudla, J.; Schumacher, K. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 2012, 69, 181–192. [Google Scholar] [CrossRef]
- Vigani, G.; Costa, A. Harnessing the new emerging imaging technologies to uncover the role of Ca2+ signalling in plant nutrient homeostasis. Plant Cell Environ. 2019, 42, 2885–2901. [Google Scholar] [CrossRef]
- Bischof, H.; Burgstaller, S.; Waldeck-Weiermair, M.; Rauter, T.; Schinagl, M.; Ramadani-Muja, J.; Graier, W.F.; Malli, R. Live-Cell Imaging of Physiologically Relevant Metal Ions Using Genetically Encoded FRET-Based Probes. Cells 2019, 8, 492. [Google Scholar] [CrossRef]
- Dooley, C.T.; Dore, T.M.; Hanson, G.T.; Jackson, W.C.; Remington, S.J.; Tsien, R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004, 279, 22284–22293. [Google Scholar] [CrossRef]
- Meyer, A.J.; Dick, T.P. Fluorescent protein-based redox probes. Antioxid. Redox Signal. 2010, 13, 621–650. [Google Scholar] [CrossRef]
- Meyer, A.J.; Brach, T.; Marty, L.; Kreye, S.; Rouhier, N.; Jacquot, J.P.; Hell, R. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 2007, 52, 973–986. [Google Scholar] [CrossRef]
- Gutscher, M.; Pauleau, A.L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Harrison, D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef]
- Wagener, K.C.; Kolbrink, B.; Dietrich, K.; Kizina, K.M.; Terwitte, L.S.; Kempkes, B.; Bao, G.; Müller, M. Redox Indicator Mice Stably Expressing Genetically Encoded Neuronal roGFP: Versatile Tools to Decipher Subcellular Redox Dynamics in Neuropathophysiology. Antioxid. Redox Signal. 2016, 25, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gibhardt, C.; Cappello, S.; Zimmermann, K.; Vultur, A.; Bogeski, I. Measuring Mitochondrial ROS in Mammalian Cells with a Genetically Encoded Protein Sensor. Bio Protoc. 2018, 8. [Google Scholar] [CrossRef]
- Abo, M.; Weerapana, E. Chemical Probes for Redox Signaling and Oxidative Stress. Antioxid. Redox Signal. 2019, 30, 1369–1386. [Google Scholar] [CrossRef]
- Breckwoldt, M.O.; Wittmann, C.; Misgeld, T.; Kerschensteiner, M.; Grabher, C. Redox imaging using genetically encoded redox indicators in zebrafish and mice. Biol. Chem. 2015, 396, 511–522. [Google Scholar] [CrossRef]
- Pak, V.V.; Ezeriņa, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Martínez Gache, S.A.; et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab. 2020, 31, 642–653.e6. [Google Scholar] [CrossRef]
- Kärkönen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef]
- Muller, K.; Linkies, A.; Vreeburg, R.A.M.; Fry, S.C.; Krieger-Liszkay, A.; Leubner-Metzger, G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 2009, 150, 1855–1865. [Google Scholar] [CrossRef]
- Novo-Uzal, E.; Fernández-Pérez, F.; Herrero, J.; Gutiérrez, J.; Gómez-Ros, L.V.; Bernal, M.Á.; Díaz, J.; Cuello, J.; Pomar, F.; Pedreño, M.Á. From Zinnia to Arabidopsis: Approaching the involvement of peroxidases in lignification. J. Exp. Bot. 2013, 64, 3499–3518. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Yang, B.; Schmidt, M.H.W.; Günl, M.; Usadel, B. Starting to gel: How arabidopsis seed coat epidermal cells produce specialized secondary cell walls. Int. J. Mol. Sci. 2015, 16, 3452–3473. [Google Scholar] [CrossRef]
- Prasad, A.; Sedlářová, M.; Balukova, A.; Rác, M.; Pospíšil, P. Reactive Oxygen Species as a Response to Wounding: In Vivo Imaging in Arabidopsis thaliana. Front. Plant Sci. 2020, 10, 1660. [Google Scholar] [CrossRef]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide-a central hub for information flow in plant cells. Aob Plants 2012, 12, pls014. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Suzuki, N. ROS as key players of abiotic stress responses in plants. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 57–82. ISBN 9783319204215. [Google Scholar]
- Ezquer, I.; Li, J.; Ovecka, M.; Baroja-Fernández, E.; Muñoz, F.J.; Montero, M.; Díaz De Cerio, J.; Hidalgo, M.; Sesma, M.T.; Bahaji, A.; et al. Microbial volatile emissions promote accumulation of exceptionally high levels of starch in leaves in Mono- and dicotyledonous plants. Plant Cell Physiol. 2010, 51, 1674–1693. [Google Scholar] [CrossRef]
- Li, J.; Ezquer, I.; Bahaji, A.; Montero, M.; Ovecka, M.; Baroja-Fernández, E.; José Muñoz, F.; Mérida, Á.; Almagro, G.; Hidalgo, M.; et al. Microbial Volatile-Induced Accumulation of Exceptionally High Levels of Starch in Arabidopsis Leaves is a Process Involving NTRC and Starch Synthase Classes III and IV. Available online: https://pubmed.ncbi.nlm.nih.gov/21649509/ (accessed on 2 February 2021).
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7815643/ (accessed on 2 February 2021).
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plantcell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, I.; Salameh, I.; Colombo, L.; Kalaitzis, P. Plant cell walls tackling climate change: Insights into plant cell wall remodeling, its regulation, and biotechnological strategies to improve crop adaptations and photosynthesis in response to global warming. Plants 2020, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Kamada, S.; Takehara, S.; Takeuchi, H.; Nakamura, A.; Satoh, S.; Iwai, H. Rice Putative Methyltransferase Gene OsPMT16 Is Required for Pistil Development Involving Pectin Modification. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Pospíšil, P.; Tada, M. Editorial: Reactive oxygen species (ros) detection methods in biological system. Front. Physiol. 2019, 10, 1316. [Google Scholar] [CrossRef]
- Seifert, G.J. The FLA4-FEI pathway: A unique and mysterious signaling module related to cell wall structure and stress signaling. Genes 2021, 12, 145. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef]
- del Pozo, J.C. Reactive Oxygen Species: From Harmful Molecules to Fine-Tuning Regulators of Stem Cell Niche Maintenance. PLoS Genet. 2016, 12. [Google Scholar] [CrossRef]
- Lee, Y. Redox Control on Stem Cell Fate and Maintenance in the Root. J. Plant Biol. 2019, 62, 320–328. [Google Scholar] [CrossRef]
- Yu, Q.; Tian, H.; Yue, K.; Liu, J.; Zhang, B.; Li, X.; Ding, Z. A P-Loop NTPase Regulates Quiescent Center Cell Division and Distal Stem Cell Identity through the Regulation of ROS Homeostasis in Arabidopsis Root. PLoS Genet. 2016, 12. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, Y.; Li, C.; Yu, G. Modulatory Role of Reactive Oxygen Species in Root Development in Model Plant of Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; He, J.; Qin, Y.; Hua, D.; Duan, Y.; Chen, Z.; Gong, Z. ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling PLETHORA Expression in Arabidopsis. PLoS Genet. 2014, 10, e1004791. [Google Scholar] [CrossRef]
- Kong, X.; Tian, H.; Yu, Q.; Zhang, F.; Wang, R.; Gao, S.; Xu, W.; Liu, J.; Shani, E.; Fu, C.; et al. PHB3 Maintains Root Stem Cell Niche Identity through ROS-Responsive AP2/ERF Transcription Factors in Arabidopsis. Cell Rep. 2018, 22, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ries, A.; Wu, K.; Yang, A.; Crawford, N.M. The arabidopsis prohibitin gene phb3 functions in nitric oxide-mediated responses and in hydrogenperoxide-induced nitric oxide accumulation. Plant Cell 2010, 22, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Han, X.; Benfey, P.N. RGF1 controls root meristem size through ROS signalling. Nature 2020, 577, 85–88. [Google Scholar] [CrossRef]
- Yamada, M.; Hsiao, Y.C. The roles of peptide hormones and their receptors during plant root development. Genes 2021, 12, 1–13. [Google Scholar]
- Martin, M.V.; Distéfano, A.M.; Zabaleta, E.J.; Pagnussat, G.C. New insights into the functional roles of reactive oxygen species during embryo sac development and fertilization in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef]
- Victoria Martin, M.; Fernando Fiol, D.; Sundaresan, V.; Julián Zabaleta, E.; Pagnussat, G.C. Oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 2013, 25, 1573–1591. [Google Scholar] [CrossRef]
- Pratibha, P.; Singh, S.K.; Srinivasan, R.; Bhat, S.R.; Sreenivasulu, Y. Gametophyte development needs mitochondrial coproporphyrinogen III oxidase function. Plant Physiol. 2017, 174, 258–275. [Google Scholar] [CrossRef]
- Takeuchi, H.; Higashiyama, T. A Species-Specific Cluster of Defensin-Like Genes Encodes Diffusible Pollen Tube Attractants in Arabidopsis. PLoS Biol. 2012, 10, e1001449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Nagae, T.T.; Takeuchi, H.; Zhang, H.; Han, Z.; Higashiyama, T.; Chai, J. Structural basis for receptor recognition of pollen tube attraction peptides. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 2016, 531, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Trigo, S.; Blanco-Touriñán, N.; DeFalco, T.A.; Wells, E.S.; Gray, J.E.; Zipfel, C.; Smith, L.M. Cr RLK 1L receptor-like kinases HERK 1 and ANJEA are female determinants of pollen tube reception. EMBO Rep. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Restrepo, J.M.; Huck, N.; Kessler, S.; Gagliardini, V.; Gheyselinck, J.; Yang, W.C.; Grossniklaus, U. The Feronia receptor-like kinase mediates male-female interactions during pollen tube reception. Science 2007, 317, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.A.; Shimosato-Asano, H.; Keinath, N.F.; Wuest, S.E.; Ingram, G.; Panstruga, R.; Grossniklaus, U. Conserved molecular components for pollen tube reception and fungal invasion. Science 2010, 330, 968–971. [Google Scholar] [CrossRef]
- Guo, H.; Ye, H.; Li, L.; Yin, Y. A family of receptor-like kinases are regulated by BES1 and involved in plant growth in Arabidopsis thaliana. Plant Signal. Behav. 2009, 4, 784–786. [Google Scholar] [CrossRef]
- Boisson-Dernier, A.; Lituiev, D.S.; Nestorova, A.; Franck, C.M.; Thirugnanarajah, S.; Grossniklaus, U. ANXUR Receptor-Like Kinases Coordinate Cell Wall Integrity with Growth at the Pollen Tube Tip Via NADPH Oxidases. PLoS Biol. 2013, 11, e1001719. [Google Scholar] [CrossRef]
- Boisson-Dernier, A.; Roy, S.; Kritsas, K.; Grobei, M.A.; Jaciubek, M.; Schroeder, J.I.; Grossniklaus, U. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 2009, 136, 3279–3288. [Google Scholar] [CrossRef]
- Meng, J.G.; Liang, L.; Jia, P.F.; Wang, Y.C.; Li, H.J.; Yang, W.C. Integration of ovular signals and exocytosis of a Ca2+ channel by MLOs in pollen tube guidance. Nat. Plants 2020, 6, 143–153. [Google Scholar] [CrossRef]
- Ju, Y.; Kessler, S.A. Keeping pollen tubes on track. Nat. Plants 2020, 6, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Yuan, J.; Smith, B.E.; Willoughby, A.C.; Kumimoto, E.L.; Kessler, S.A. MILDEW RESISTANCE LOCUS O function in pollen tube reception is linked to its oligomerization and subcellular distribution. Plant Physiol. 2017, 175, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Iwano, M.; Takeda, S.; Kanaoka, M.M.; Kimura, S.; Abe, M.; Kuchitsu, K. Apoplastic ros production upon pollination by rbohH and RbohJ in arabidopsis. Plant Signal. Behav. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Hartung, F.; Wurz-Wildersinn, R.; Fuchs, J.; Schubert, I.; Suer, S.; Puchta, H. The catalytically active tyrosine residues of both SPO11-1 and SPO11-2 are required for meiotic double-strand break induction in Arabidopsis. Plant Cell 2007, 19, 3090–3099. [Google Scholar] [CrossRef]
- Murgia, I.; Morandini, P. Iron deficiency prolongs seed dormancy in Arabidopsis plants. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Veasey, E.A.; Karasawa, M.G.; Santos, P.P.; Rosa, M.S.; Mamani, E.; Oliveira, G.C.X. Variation in the loss of seed dormancy during after-ripening of wild and cultivated rice species. Ann. Bot. 2004, 94, 875–882. [Google Scholar] [CrossRef]
- Willis, C.G.; Baskin, C.C.; Baskin, J.M.; Auld, J.R.; Venable, D.L.; Cavender-Bares, J.; Donohue, K.; de Casas, R.R.; Bradford, K.; Burghardt, L.; et al. The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol. 2014, 203, 300–309. [Google Scholar] [CrossRef]
- Longo, C.; Holness, S.; De Angelis, V.; Lepri, A.; Occhigrossi, S.; Ruta, V.; Vittorioso, P. From the outside to the inside: New insights on the main factors that guide seed dormancy and germination. Genes 2021, 12, 52. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Née, G.; Xiang, Y.; Soppe, W.J. The release of dormancy, a wake-up call for seeds to germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef]
- Ventura, L.; Donà, M.; Macovei, A.; Carbonera, D.; Buttafava, A.; Mondoni, A.; Rossi, G.; Balestrazzi, A. Understanding the molecular pathways associated with seed vigor. Plant Physiol. Biochem. 2012, 60, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Leymarie, J.; Vitkauskaité, G.; Hoang, H.H.; Gendreau, E.; Chazoule, V.; Meimoun, P.; Corbineau, F.; El-Maarouf-Bouteau, H.; Bailly, C. Role of reactive oxygen species in the regulation of arabidopsis seed dormancy. Plant Cell Physiol. 2012, 53, 96–106. [Google Scholar] [CrossRef]
- Murgia, I.; Tarantino, D.; Vannini, C.; Bracale, M.; Carravieri, S.; Soave, C. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to Paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 2004, 38, 940–953. [Google Scholar] [CrossRef]
- Murgia, I.; Giacometti, S.; Balestrazzi, A.; Paparella, S.; Pagliano, C.; Morandini, P. Analysis of the transgenerational iron deficiency stress memory in Arabidopsis thaliana plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishibashi, Y.; Aoki, N.; Kasa, S.; Sakamoto, M.; Kai, K.; Tomokiyo, R.; Watabe, G.; Yuasa, T.; Iwaya-Inoue, M. The interrelationship between abscisic acid and reactive oxygen species plays a key role in barley seed dormancy and germination. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf-Bouteau, H.; Bailly, C. Targeted mRNA oxidation regulates sunflower seed dormancy alleviation during dry after-ripening. Plant Cell 2011, 23, 2196–2208. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Sajjad, Y.; Bazin, J.; Langlade, N.; Cristescu, S.M.; Balzergue, S.; Baudouin, E.; Bailly, C. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, Z.; Wu, H.; Tian, Z.; Zhao, Z. Redox regulation of plant stem cell fate. Embo J. 2017, 36, 2844–2855. [Google Scholar] [CrossRef] [PubMed]
- Negin, B.; Shemer, O.; Sorek, Y.; Williams, L.E. Shoot stem cell specification in roots by the WUSCHEL transcription factor. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Dunand, C.; Crèvecoeur, M.; Penel, C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 2007, 174, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.M.; Wilson, M.H.; Bennett, M.J. Feeling UPBEAT about Growth: Linking ROS Gradients and Cell Proliferation. Dev. Cell 2010, 19, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14. [Google Scholar] [CrossRef]
- Breygina, M.A.; Abramochkin, D.V.; Maksimov, N.M.; Yermakov, I.P. Hydrogen peroxide affects ion channels in lily pollen grain protoplasts. Plant Biol. 2016, 18, 761–767. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Lin, X.J.; Liang, H.M.; Wang, F.F.; Chen, L.Y. The long journey of pollen tube in the pistil. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Ju, Y.; Kessler, S.A. Reactive Oxygen Species as Mediators of Gametophyte Development and Double Fertilization in Flowering Plants. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7419745/ (accessed on 14 December 2020).
- Klatt, S.; Hadacek, F.; Hodač, L.; Brinkmann, G.; Eilerts, M.; Hojsgaard, D.; Hörandl, E. Photoperiod Extension Enhances Sexual Megaspore Formation and Triggers Metabolic Reprogramming in Facultative Apomictic Ranunculus auricomus. Front. Plant Sci. 2016, 7, 278. [Google Scholar] [CrossRef]
- Mateo de Arias, M.; Gao, L.; Sherwood, D.A.; Dwivedi, K.K.; Price, B.J.; Jamison, M.; Kowallis, B.M.; Carman, J.G. Whether Gametophytes Are Reduced or Unreduced in Angiosperms Might Be Determined Metabolically. Genes 2020, 11, 1449. [Google Scholar] [CrossRef]
- Song, Q.; Ando, A.; Jiang, N.; Ikeda, Y.; Chen, Z.J. Single-cell RNA-seq analysis reveals ploidy-dependent and cell-specific transcriptome changes in Arabidopsis female gametophytes. Genome Biol. 2020, 21. [Google Scholar] [CrossRef]
- Sandaklie-Nikolova, L.; Palanivelu, R.; King, E.J.; Copenhaver, G.P.; Drews, G.N. Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol. 2007, 144, 1753–1762. [Google Scholar] [CrossRef]
- Okuda, S.; Tsutsui, H.; Shiina, K.; Sprunck, S.; Takeuchi, H.; Yui, R.; Kasahara, R.D.; Hamamura, Y.; Mizukami, A.; Susaki, D.; et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 2009, 458, 357–361. [Google Scholar] [CrossRef]
- Higashiyama, T.; Takeuchi, H. The mechanism and key molecules involved in pollen tube guidance. Annu. Rev. Plant Biol. 2015, 66, 393–413. [Google Scholar] [CrossRef]
- Kanaoka, M.M.; Higashiyama, T. Peptide signaling in pollen tube guidance. Curr. Opin. Plant Biol. 2015, 28, 127–136. [Google Scholar] [CrossRef]
- Duan, Q.; Kita, D.; Johnson, E.A.; Aggarwal, M.; Gates, L.; Wu, H.M.; Cheung, A.Y. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 2014, 5, 3129. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.A.; Vogler, H.; Lituiev, D.S.; Nestorova, A.; Grossniklaus, U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev. Cell 2014, 29, 491–500. [Google Scholar] [CrossRef]
- Yuan, J.; Ju, Y.; Jones, D.S.; Zhang, W.; Lucca, N.; Staiger, C.J.; Kessler, S.A. Pollen tube-triggered accumulation of NORTIA at the filiform apparatus facilitates fertilization in Arabidopsis thaliana. bioRxiv 2019. [Google Scholar] [CrossRef]
- Leydon, A.R.; Tsukamoto, T.; Dunatunga, D.; Qin, Y.; Johnson, M.A.; Palanivelu, R. Pollen tube discharge completes the process of synergid degeneration that is initiated by pollen tube-synergid interaction in arabidopsis. Plant Physiol. 2015, 169, 485–496. [Google Scholar] [CrossRef]
- Gao, X.Q.; Liu, C.Z.; Li, D.D.; Zhao, T.T.; Li, F.; Jia, X.N.; Zhao, X.Y.; Zhang, X.S. The Arabidopsis KINβγ Subunit of the SnRK1 Complex Regulates Pollen Hydration on the Stigma by Mediating the Level of Reactive Oxygen Species in Pollen. PLoS Genet. 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Guan, H.; Li, F.; Liu, C.Z.; Dong, Y.X.; Zhang, X.S.; Gao, X.Q. Arabidopsis shaker pollen inward K+ channel SPIK functions in SnRK1 complex-regulated pollen hydration on the stigma. J. Integr. Plant Biol. 2017, 59, 604–611. [Google Scholar] [CrossRef]
- Manrique, S.; Friel, J.; Gramazio, P.; Hasing, T.; Ezquer, I.; Bombarely, A. Genetic Insights into the Modification of the Pre-fertilization Mechanisms during Plant Domestication. Available online: https://academic.oup.com/jxb/article-abstract/70/11/3007/5509875 (accessed on 14 January 2021).
- Zafra, A.; Rejón, J.D.; Hiscock, S.J.; de Dios Alché, J. Patterns of ROS accumulation in the stigmas of angiosperms and visions into their multi-functionality in plant reproduction. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Romero-Puertas, M.C.; Sandalio, L.M.; Olmedilla, A. The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J. Exp. Bot. 2015, 66, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, S.J.; Allen, A.M. Diverse cell signalling pathways regulate pollen-stigma interactions: The search for consensus. New Phytol. 2008, 179, 286–317. [Google Scholar] [CrossRef] [PubMed]
- Hyttel, P.; Sinowatz, F.; Vejlsted, M. Essentiels of Domestic Animal Embryology; Saunders Ltd.: Nottingham, UK, 2010; Volume 1, pp. 68–103. [Google Scholar]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- Hidalgo, C.; Donoso, P. Crosstalk between calcium and redox signaling: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1275–1312. [Google Scholar] [CrossRef]
- Feissner, R.F.; Skalska, J.; Gaum, W.E.; Sheu, S.S. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front. Biosci. 2009, 14, 1197–1218. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in Mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef]
- Feno, S.; Butera, G.; Reane, D.V.; Rizzuto, R.; Raffaello, A. Crosstalk between calcium and ROS in pathophysiological conditions. Oxid. Med. Cell. Longev. 2019, 2019, 9324018. [Google Scholar] [CrossRef]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef]
- Jain, R.; Watson, U.; Vasudevan, L.; Saini, D.K. ERK Activation Pathways Downstream of GPCRs. In International Review of Cell and Molecular Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 338, pp. 79–109. ISBN 9780128137727. [Google Scholar]
- Prole, D.L.; Taylor, C.W. Structure and function of ip3 receptors. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Giorgi, C.; Galluzzi, L.; Pinton, P. Ca2+ Fluxes and Cancer. Mol. Cell 2020, 78, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Iizumi, S.; Satoh, K.; Ooka, H.; Kawai, J.; Carninci, P.; Hayashizaki, Y.; Otomo, Y.; Murakami, K.; Matsubara, K.; et al. Comparative analysis of plant and animal calcium signal transduction element using plant full-length cDNA data. Mol. Biol. Evol. 2004, 21, 1855–1870. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria and Ca2+ in cell physiology and pathophysiology. Cell Calcium 2000, 28, 339–348. [Google Scholar] [CrossRef]
- Ramalho-Santos, J.; Varum, S.; Amaral, S.; Mota, P.C.; Sousa, A.P.; Amaral, A. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 2009, 15, 553–572. [Google Scholar] [CrossRef]
- Tsunoda, S.; Kimura, N.; Fujii, J. Oxidative stress and redox regulation of gametogenesis, fertilization, and embryonic development. Reprod. Med. Biol. 2014, 13, 71–79. [Google Scholar] [CrossRef]
- Deluca, H.F.; Engstrom, G.W. Calcium uptake by rat kidney mitochondria. Proc. Natl. Acad. Sci. USA 1961, 47, 1744–1750. [Google Scholar] [CrossRef]
- Vasington, F.D.; Murphy, J.V. Ca++ Uptake by Rat Kidney Mitochondria and Its Dependence on Respiration and Phosphorylation. J. Biol. Chem. 1962, 237, 2670–2677. [Google Scholar] [CrossRef]
- Rizzuto, R.; Brini, M.; Pizzo, P.; Murgia, M.; Pozzan, T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr. Biol. 1995, 5, 635–642. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef]
- Csordás, G.; Thomas, A.P.; Hajnóczky, G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999, 18, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Simpson, A.W.M.; Brini, M.; Pozzan, T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 1992, 358, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Di Lisa, F.; Bernardi, P. A CaPful of mechanisms regulating the mitochondrial permeability transition. J. Mol. Cell. Cardiol. 2009, 46, 775–780. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef]
- Wakai, T.; Mehregan, A.; Fissore, R.A. Ca2+ signaling and homeostasis in mammalian oocytes and eggs. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef]
- Whitaker, M. Calcium at fertilization and in early development. Physiol. Rev. 2006, 86, 25–88. [Google Scholar] [CrossRef]
- Horner, V.L.; Wolfner, M.F. Transitioning from egg to embryo: Triggers and mechanisms of egg activation. Dev. Dyn. 2008, 237, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S. Thirty years of calcium signals at fertilization. Semin. Cell Dev. Biol. 2006, 17, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Swann, K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J. Biol. Chem. 1992, 267, 11196–11201. [Google Scholar] [CrossRef]
- Ajduk, A.; Małagocki, A.; Maleszewski, M. Cytoplasmic maturation of mammalian oocytes: Development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod. Biol. 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Wakai, T.; Fissore, R.A. Constitutive IP3R1-mediated Ca2+ release reduces Ca2+ store content and stimulates mitochondrial metabolism in mouse GV oocytes. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef]
- Tiwari, M.; Prasad, S.; Shrivastav, T.G.; Chaube, S.K. Calcium Signaling During Meiotic Cell Cycle Regulation and Apoptosis in Mammalian Oocytes. J. Cell. Physiol. 2017, 232, 976–981. [Google Scholar] [CrossRef]
- Pandey, A.N.; Chaube, S.K. A Moderate Increase of Hydrogen Peroxide Level Is Beneficial for Spontaneous Resumption of Meiosis from Diplotene Arrest in Rat Oocytes Cultured In Vitro. Biores. Open Access 2014, 3, 183–191. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Pandey, G.K.; Grant, J.J.; Batistic, O.; Li, L.; Kim, B.G.; Lee, S.C.; Kudla, J.; Luan, S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007, 52, 223–239. [Google Scholar] [CrossRef]
- Wakai, T.; Fissore, R.A. Ca2+ homeostasis and regulation of ER Ca2+ in mammalian oocytes/eggs. Cell Calcium 2013, 53, 63–67. [Google Scholar] [CrossRef]
- Wakai, T.; Vanderheyden, V.; Yoon, S.Y.; Cheon, B.; Zhang, N.; Parys, J.B.; Fissore, R.A. Regulation of inositol 1,4,5-trisphosphate receptor function during mouse oocyte maturation. J. Cell. Physiol. 2012, 227, 705–717. [Google Scholar] [CrossRef]
- Wakai, T.; Zhang, N.; Vangheluwe, P.; Fissore, R.A. Regulation of endoplasmic reticulum Ca2+ oscillations in mammalian eggs. J. Cell Sci. 2013, 126, 5714–5724. [Google Scholar] [CrossRef]
- Lee, B.; Vermassen, E.; Yoon, S.Y.; Vanderheyden, V.; Ito, J.; Alfandari, D.; De Smedt, H.; Parys, J.B.; Fissore, R.A. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: A role for the MAP kinase pathway. Development 2006, 133, 4355–4365. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y. Role of Type 1 Inositol 1,4,5-triphosphate Receptors in Mammalian Oocytes. Dev. Reprod. 2019, 23, 1–9. [Google Scholar] [CrossRef]
- Dumollard, R.; Marangos, P.; Fitzharris, G.; Swann, K.; Duchen, M.; Carroll, J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 2004, 131, 3057–3067. [Google Scholar] [CrossRef]
- Dumollard, R.; Campbell, K.; Halet, G.; Carroll, J.; Swann, K. Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes. Dev. Biol. 2008, 316, 431–440. [Google Scholar] [CrossRef]
- Hachem, A.; Godwin, J.; Ruas, M.; Lee, H.C.; Buitrago, M.F.; Ardestani, G.; Bassett, A.; Fox, S.; Navarrete, F.; De Sutter, P.; et al. Plcζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 2017, 144, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Satouh, Y.; Fujimoto, T.; Oji, A.; Ikawa, M. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Escoffier, J.; Lee, H.C.; Yassine, S.; Zouari, R.; Martinez, G.; Karaouzène, T.; Coutton, C.; Kherraf, Z.E.; Halouani, L.; Triki, C.; et al. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum. Mol. Genet. 2016, 25, 878–891. [Google Scholar] [CrossRef]
- Nomikos, M.; Kashir, J.; Lai, F.A. The role and mechanism of action of sperm PLC-zeta in mammalian fertilisation. Biochem. J. 2017, 474, 3659–3673. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Stamatiadis, P.; Sanders, J.R.; Beck, K.; Calver, B.L.; Buntwal, L.; Lofty, M.; Sideratou, Z.; Swann, K.; Lai, F.A. Male infertility-linked point mutation reveals a vital binding role for the C2 domain of sperm PLCζ. Biochem. J. 2017, 474, 1003–1016. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Von Mengden, L.; Klamt, F.; Smitz, J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid. Redox Signal. 2020, 32, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels with inflammatory processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed]
- Nasr-esfahani, M.; Johnson, M.H.; Aitken, R.J. The effect of iron and iron chelators on the in-vitro block to development of the mouse preimplantation embryo: BAT6 a new medium for improved culture of mouse embryos in vitro. Hum. Reprod. 1990, 5, 997–1003. [Google Scholar] [CrossRef]
- Fujii, J.; Imai, H. Redox reactions in mammalian spermatogenesis and the potential targets of reactive oxygen species under oxidative stress. Spermatogenesis 2014, 4, e979108. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2021, 53, e13577. [Google Scholar] [CrossRef]
- Morales, H.; Tilquin, P.; Rees, J.F.; Massip, A.; Dessy, F.; Van Langendonckt, A. Pyruvate prevents peroxide-induced injury of in vitro preimplantation bovine embryos. Mol. Reprod. Dev. 1999, 52, 149–157. [Google Scholar] [CrossRef]
- Dumollard, R.; Ward, Z.; Carroll, J.; Duchen, M.R. Regulation of redox metabolism in the mouse oocyte and embryo. Development 2007, 134, 455–465. [Google Scholar] [CrossRef]
- Lopes, A.S.; Lane, M.; Thompson, J.G. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum. Reprod. 2010, 25, 2762–2773. [Google Scholar] [CrossRef]
- Dumollard, R.; Carroll, J.; Duchen, M.R.; Campbell, K.; Swann, K. Mitochondrial function and redox state in mammalian embryos. Semin. Cell Dev. Biol. 2009, 20, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ishibashi, S.; Iglesias-Gonzalez, J.; Chen, Y.; Love, N.R.; Amaya, E. Ca2+ -Induced Mitochondrial ROS Regulate the Early Embryonic Cell Cycle. Cell Rep. 2018, 22, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Hörandl, E.; Speijer, D. How oxygen gave rise to eukaryotic sex. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172706. [Google Scholar] [CrossRef]
- Wuest, S.E.; Vijverberg, K.; Schmidt, A.; Weiss, M.; Gheyselinck, J.; Lohr, M.; Wellmer, F.; Rahnenführer, J.; von Mering, C.; Grossniklaus, U. Arabidopsis Female Gametophyte Gene Expression Map Reveals Similarities between Plant and Animal Gametes. Curr. Biol. 2010, 20, 506–512. [Google Scholar] [CrossRef]
- Bernasconi, G.; Ashman, T.L.; Birkhead, T.R.; Bishop, J.D.D.; Grossniklaus, U.; Kubli, E.; Marshall, D.L.; Schmid, B.; Skogsmyr, I.; Snook, R.R.; et al. Evolutionary Ecology of the Prezygotic Stage. Science 2004, 303, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Kleene, K.C. Sexual selection, genetic conflict, selfish genes, and the atypical patterns of gene expression in spermatogenic cells. Dev. Biol. 2005, 277, 16–26. [Google Scholar] [CrossRef]
- Márton, M.L.; Dresselhaus, T. A comparison of early molecular fertilization mechanisms in animals and flowering plants. Sex. Plant Reprod. 2008, 21, 37–52. [Google Scholar] [CrossRef]
- Randerson, J.P.; Hurst, L.D. A comparative test of a theory for the evolution of anisogamy. Proc. R. Soc. B Biol. Sci. 2001, 268, 879–884. [Google Scholar] [CrossRef]
- Cox, P.A.; Sethian, J.A. Gamete Motion, Search, and the Evolution of Anisogamy, Oogamy, and Chemotaxis. Am. Nat. 1985, 125, 74–101. [Google Scholar] [CrossRef]
- Blute, M. The Evolution of Anisogamy: More Questions than Answers. Biol. Theory 2013, 7, 3–9. [Google Scholar] [CrossRef]
- Miller, C.W. VII.5 Sexual Selection: Male-Male Competition. Princet. Guide Evol. 2013, 2013, 641–646. [Google Scholar]
- de Lamirande, E.; O’Flaherty, C. Sperm activation: Role of reactive oxygen species and kinases. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.C.L. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update 2004, 10, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.X.; Feng, Q.N.; Xie, H.T.; Li, S.; Zhang, Y. Reactive oxygen species mediate tapetal programmed cell death in tobacco and tomato. BMC Plant Biol. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, X.; Patel, S.; Clapham, D.E. Insights into the early evolution of animal calcium signaling machinery: A unicellular point of view. Cell Calcium 2015, 57, 166–173. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Ge, L.L.; Xie, C.T.; Tian, H.Q.; Russell, S.D. Distribution of calcium in the stigma and style of tobacco during pollen germination and tube elongation. Sex. Plant Reprod. 2009, 22, 87–96. [Google Scholar] [CrossRef]
- Denninger, P.; Bleckmann, A.; Lausser, A.; Vogler, F.; Ott, T.; Ehrhardt, D.W.; Frommer, W.B.; Sprunck, S.; Dresselhaus, T.; Grossmann, G. Male-female communication triggers calcium signatures during fertilization in arabidopsis. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Li, L.G.; Hui, Q.T.; Russell, S.D. Calcium function and distribution during fertilization in angiosperms. Am. J. Bot. 2007, 94, 1046–1060. [Google Scholar] [CrossRef]
- Leng, L.; Sun, J.; Huang, J.; Gong, F.; Yang, L.; Zhang, S.; Yuan, X.; Fang, F.; Xu, X.; Luo, Y.; et al. Single-Cell Transcriptome Analysis of Uniparental Embryos Reveals Parent-of-Origin Effects on Human Preimplantation Development. Cell Stem Cell 2019, 25, 697–712.e6. [Google Scholar] [CrossRef]
- Zhu, P.; Guo, H.; Ren, Y.; Hou, Y.; Dong, J.; Li, R.; Lian, Y.; Fan, X.; Hu, B.; Gao, Y.; et al. Single-cell DNA methylome sequencing of human preimplantation embryos. Nat. Genet. 2018, 50, 12–19. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Williams, B.A.; Trout, D.; Marinov, G.K.; Amrhein, H.; Berghella, L.; Goh, S.T.; Plajzer-Frick, I.; Afzal, V.; Pennacchio, L.A.; et al. The changing mouse embryo transcriptome at whole tissue and single-cell resolution. Nature 2020, 583, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Sladitschek, H.L.; Fiuza, U.M.; Pavlinic, D.; Benes, V.; Hufnagel, L.; Neveu, P.A. MorphoSeq: Full Single-Cell Transcriptome Dynamics Up to Gastrulation in a Chordate. Cell 2020, 181, 922–935.e21. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Schon, M.A.; Nodine, M.D. The embryonic transcriptome of Arabidopsis thaliana. Plant Reprod. 2019, 32, 77–91. [Google Scholar] [CrossRef]
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G. High CO2 primes plant biotic stress defences through redox-linked pathways. Plant Physiol. 2016, 172, 929–942. [Google Scholar] [CrossRef]
- Liu, U.; Cossu, T.A.; Davies, R.M.; Forest, F.; Dickie, J.B.; Breman, E. Conserving orthodox seeds of globally threatened plants ex situ in the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK: The status of seed collections. Biodivers. Conserv. 2020, 29, 2901–2949. [Google Scholar] [CrossRef]
- Bailly, C.; Kranner, I. Analyses of reactive oxygen species and antioxidants in relation to seed longevity and germination. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 773, pp. 343–367. ISBN 9781617792304. [Google Scholar]

| Gene ID | Full Gene Name | Plant Structure Affected | Reference | Impact on ROS and Development |
|---|---|---|---|---|
| At2g47270 | UPBEAT1 (UPB1), a transcription factor with a bHLH domain | Root differentiation | [164,165] | Regulates the expression of a set of peroxidases that modulate the balance of ROS between the zones of cell proliferation and the zone of cell elongation where differentiation begins. Disruption of UPB1 activity alters this ROS balance, leading to a delay in the onset of differentiation. |
| At5g53540 | P-loop NTPase APP1 | Root differentiation | [166,167] | Encodes a P-loop NTPase APP1. The disruption of APP1 is accompanied by a reduction in ROS level, a rise in the rate of cell division in the quiescent center (QC) and the promotion of root distal stem cell (DSC) differentiation. |
| At4g11690 | ABA OVERLY SENSITIVE MUTANT(ABO8), a pentatricopeptide repeat (PPR) protein | Root differentiation | [168,169] | Abo8 mutants accumulate more ROS in root tips than the wild type. |
| At5g40770 | PROHIBITIN 3 (PHB3) | Root differentiation | [168,170,171] | PHB3 coordinates cell division and differentiation in the root apical meristem via ROS-dependent signaling. |
| At2g12646 | RGF1-INDUCIBLE TRANSCRIPTION FACTOR 1 (RITF1) | Root meristem | [172,173] | Controls root meristem size through ROS signaling. |
| At3g10920 | MANGANESE SUPEROXIDE DISMUTASE (MSD1) | Pollen | [174,175] | Female gametophytic mutant impaired in mitochondrial manganese-superoxide dismutase (MSD1) displays high levels of ROS detectable in the central cell and micropylar cells. |
| At5g63290 | ATHEMN1, HEMN1 | Female gametophyte | [175,176] | athemn1 mutant defective in tetrapyrrole biosynthesis which had increased ROS accumulation in developing embryo sacs and defects in female gametophyte development with embryo sacs displaying unfused polar nuclei. |
| At5g43285 | LURE1.1 | Synergid cell at female gametophyte | [177,178,179] | Encodes a cysteine-rich peptide that acts as a pollen tube attractant guiding pollen tubes to the ovular micropyle. |
| At4g08869 | LURE1.7 | Pollen tube | [177,178,179] | Encodes a defensin-like family protein. Pollen tube emergence accelerator that favors conspecific pollen over pollen from other species and thus promotes reproductive isolation. |
| At4g08875 | LURE1.8 | Pollen tube | [177,178,179] | Encodes a defensin-like family protein. Pollen tube emergence accelerator that favors conspecific pollen over pollen from other species and thus promotes reproductive isolation. |
| At3g51550 | FER, FERONIA | Synergid cell at female gametophyte | [180,181,182] | Receptor-like kinase involved in pollen tube reception. |
| At3g46290 | HERCULES RECEPTOR KINASE 1 (HERK1) | Pollen tube | [180,183] | Receptor-like kinase involved in pollen tube reception. |
| At3g04690 | ANXUR1 (ANX1) | Pollen tube growth | [184,185] | Receptor-like kinase involved in pollen tube reception. |
| At2g17430 | NORTIA (NTA, MLO7) | Pollen tube reception | [182] | Studies performed with the Arabidopsis synergid-expressed gene NORTIA have shown how calcium oscillation in synergids is fundamental to proper pollen tube reception. nta mutants affected in Ca2+ display the pollen tube overgrowth phenotype. |
| At2g33670 | MILDEW RESISTANCE LOCUS O 5 (MLO5) | Stigma, anther, and pollen grains | [186] | MLO5 and MLO9 selectively recruit Ca2+ channel CNGC18-containing vesicles to the plasma membrane through the R-SNARE proteins in order to modify Ca2+ gradients in the pollen tube. |
| At1g42560 | MILDEW RESISTANCE LOCUS O 9 (MLO9) | Pollen | [186,187,188] | MLO5 and MLO9 selectively recruit Ca2+ channel CNGC18-containing vesicles to the plasma membrane through the R-SNARE proteins in order to modify Ca2+ gradients in the pollen tube. |
| At2g44110 | MILDEW RESISTANCE LOCUS O 15 (MLO15) | Seedlings, root tips, and flower structure | [186,187,188] | MLOs; together with MLO5 and MLO9, MLO15 is required for proper pollen tube sensitivity to ovule signals in Arabidopsis thaliana. |
| At5g60010 | RESPIRATORY BURST OXIDASE HOMOLOG H (RBOHH) | Pollen | [189] | ROS production by RbohH and RbohJ is essential for proper pollen tube tip growth. Double mutant pollen tubes cease their growth and burst in vitro and fail to reach the female gametophytes in vivo. |
| At3g45810 | RESPIRATORY BURST OXIDASE HOMOLOG J (RBOHJ) | Pollen | [189] | ROS production by RbohH and RbohJ is essential for proper pollen tube tip growth. Double mutant pollen tubes stop growth, burst in vitro and fail to reach the female gametophytes in vivo. |
| AT1G63990 | SPORULATION 11 (SPO11) | Floral organs | [35,190] | SPO11 is a DNA topoisomerase whose activity is exposed to redox regulation. SPO11 is required for meiotic recombination. Plants homozygous for atspo11-2 exhibit a strong sterility phenotype and this is associated with severe defects in synapsis during the first meiotic division and reduced meiotic recombination. |
| Gene ID | Short_Description | Egg Cell | Central Cell | Synergid Cell |
|---|---|---|---|---|
| At1g20630 | Catalase 1 | 52.04 | 4757.34 | 265.66 |
| At4g35090 | Catalase 2 | 0.00 | 495.80 | 0.00 |
| At1g20620 | Catalase 3 | 9.72 | 177.36 | 0.00 |
| At1g08830 | Copper/Zinc Superoxide Dismutase 1 | 6.62 | 911.31 | 25.49 |
| At1g09090 | Respiratory Burst Oxidase Homolog B | 0.00 | 2.85 | 0.00 |
| At1g12520 | Copper Chaperone For SOD1 | 5.02 | 6.81 | 0.00 |
| At1g14920 | GAI /GRAS Family Transcription Factor Family Protein | 3.14 | 1.10 | 0.00 |
| At1g32230 | WWE Protein-Protein Interaction Domain Protein Family | 20.53 | 258.70 | 3.41 |
| At1g66350 | RGA-Like 1 | 0.00 | 1.01 | 0.00 |
| At1g17020 | Senescence-Related Gene 1 | 19.50 | 3.59 | 0.00 |
| At4g12420 | Cupredoxin Superfamily Protein | 10.86 | 7.81 | 0.00 |
| At4g39830 | Plant L-Ascorbate Oxidase | 0.00 | 16.79 | 0.00 |
| At5g21100 | Plant L-Ascorbate Oxidase | 0.00 | 2.26 | 0.00 |
| At5g21105 | Plant L-Ascorbate Oxidase | 3.20 | 7.96 | 8.67 |
| At2g25080 | Glutathione Peroxidase 1 | 13.18 | 272.94 | 0.00 |
| At2g31570 | Glutathione Peroxidase 2 | 1.82 | 5543.76 | 0.00 |
| At2g43350 | Glutathione Peroxidase 3 | 10.49 | 358.55 | 102.44 |
| At2g48150 | Glutathione Peroxidase 4 | 0.00 | 25.73 | 0.00 |
| At3g63080 | Glutathione Peroxidase 5 | 5.18 | 83.62 | 66.53 |
| At4g11600 | Glutathione Peroxidase 6 | 153.48 | 4766.73 | 206.43 |
| At4g31870 | Glutathione Peroxidase 7 | 0.00 | 12.64 | 0.00 |
| At1g63460 | Glutathione Peroxidase 8 | 13.60 | 259.42 | 12.65 |
| At2g30860 | Glutathione S-Transferase PHI 9 | 0.00 | 0.00 | 0.00 |
| At5g27380 | Glutathione Synthetase 2 GSH2 | 1.82 | 402.09 | 0.00 |
| At4g23100 | Glutamate-Cysteine Ligase GSH1 | 65.25 | 1411.02 | 0.00 |
| At4g33670 | Gal-DH NAD(P)-Linked Oxidoreductase Superfamily Protein | 0.00 | 273.91 | 0.00 |
| At3g55590 | Glucose-1-Phosphate Adenylyltransferase Family Protein | 3.42 | 131.82 | 53.29 |
| At2g39770 | GDP-Mannose VTC1 | 2.18 | 326.29 | 0.00 |
| At3g02870 | Inositol Monophosphatase Family Protein VTC4 | 124.00 | 616.27 | 0.00 |
| At5g55120 | Galactose-1-Phosphate Guanylyltransferase VTC5 | 2.88 | 139.24 | 16.42 |
| At4g26850 | Mannose-1-Phosphate Guanylyltransferase VTC2 | 6.67 | 118.31 | 0.00 |
| At3g47930 | GalDH L-galactono-1,4-lactone dehydrogenase | 0.00 | 204.95 | 5.26 |
| At5g56490 | D-arabinono-1,4-lactone oxidase GulLO | 1.26 | 182.31 | 3.41 |
| At5g28840 | GDP-D-mannose 3’,5’-epimerase GME | 86.90 | 395.34 | 0.00 |
| At1g32300 | D-arabinono-1,4-lactone oxidase family protein | 0.00 | 0.00 | 0.00 |
| At4g39120 | IMPL2 myo-inositol monophosphatase like 2 | 4.97 | 163.22 | 0.00 |
| At1g31190 | IMPL1 myo-inositol monophosphatase like 1 | 2.17 | 212.95 | 0.00 |
| At2g46740 | D-arabinono-1,4-lactone oxidase family protein | 0.00 | 13.04 | 0.00 |
| At2g46750 | D-arabinono-1,4-lactone oxidase family protein | 0.00 | 0.00 | 16.42 |
| At2g46760 | D-arabinono-1,4-lactone oxidase family protein | 0.00 | 0.00 | 0.00 |
| At5g11540 | D-arabinono-1,4-lactone oxidase family protein | 0.00 | 784.19 | 0.00 |
| At5g56470 | FAD-dependent oxidoreductase family protein | 2.93 | 4.91 | 0.00 |
| At5g56490 | D-arabinono-1,4-lactone oxidase family protein | 1.26 | 182.31 | 3.41 |
| At4g32770 | Tocopherol cyclase / vitamin E deficient 1 (VTE1) | 0.00 | 175.02 | 0.00 |
| At3g63410 | S-adenosyl-L-methionine-dependent methyltransferase | 0.00 | 414.27 | 0.00 |
| At1g64970 | Gamma-tocopherol methyltransferase | 0.00 | 0.00 | 0.00 |
| At2g18950 | Homogentisate phytyltransferase 1 | 0.00 | 0.00 | 0.00 |
| At3g11945 | Homogentisate prenyltransferase | 0.00 | 180.24 | 5.89 |
| At1g06570 | Phytoene desaturation 1 | 8.55 | 50.43 | 5.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodde, V.; Morandini, P.; Costa, A.; Murgia, I.; Ezquer, I. cROStalk for Life: Uncovering ROS Signaling in Plants and Animal Systems, from Gametogenesis to Early Embryonic Development. Genes 2021, 12, 525. https://doi.org/10.3390/genes12040525
Lodde V, Morandini P, Costa A, Murgia I, Ezquer I. cROStalk for Life: Uncovering ROS Signaling in Plants and Animal Systems, from Gametogenesis to Early Embryonic Development. Genes. 2021; 12(4):525. https://doi.org/10.3390/genes12040525
Chicago/Turabian StyleLodde, Valentina, Piero Morandini, Alex Costa, Irene Murgia, and Ignacio Ezquer. 2021. "cROStalk for Life: Uncovering ROS Signaling in Plants and Animal Systems, from Gametogenesis to Early Embryonic Development" Genes 12, no. 4: 525. https://doi.org/10.3390/genes12040525
APA StyleLodde, V., Morandini, P., Costa, A., Murgia, I., & Ezquer, I. (2021). cROStalk for Life: Uncovering ROS Signaling in Plants and Animal Systems, from Gametogenesis to Early Embryonic Development. Genes, 12(4), 525. https://doi.org/10.3390/genes12040525






