Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken
Abstract
:1. Introduction
2. Overview of Lipid Metabolism in Chicken
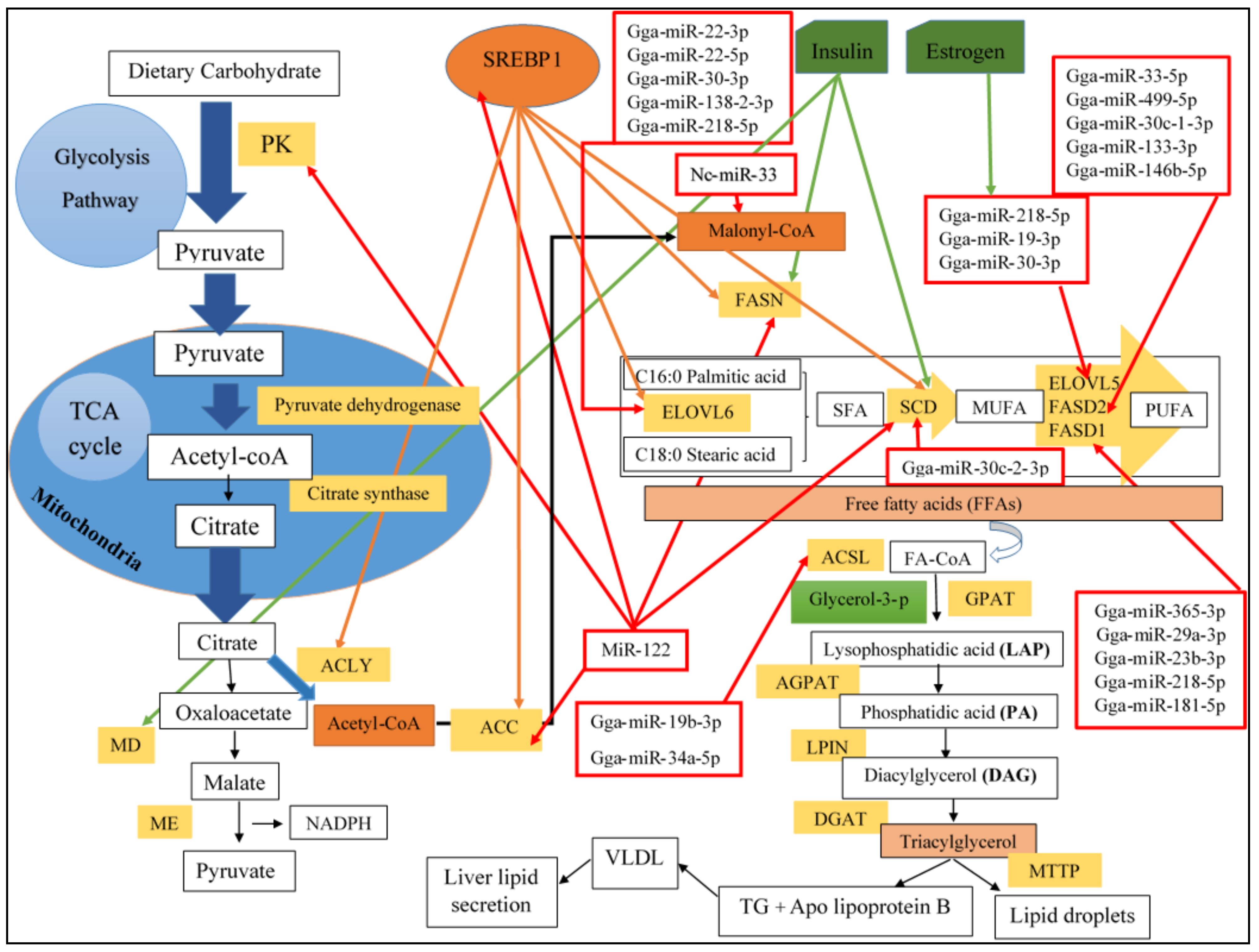
2.1. De Novo Lipogenesis in Chicken Liver
2.1.1. Triglycerides Synthesis in Chicken Liver
2.1.2. Lipoprotein Assembly and Secretion
2.1.3. Lipoprotein Degradation and Fat Deposition
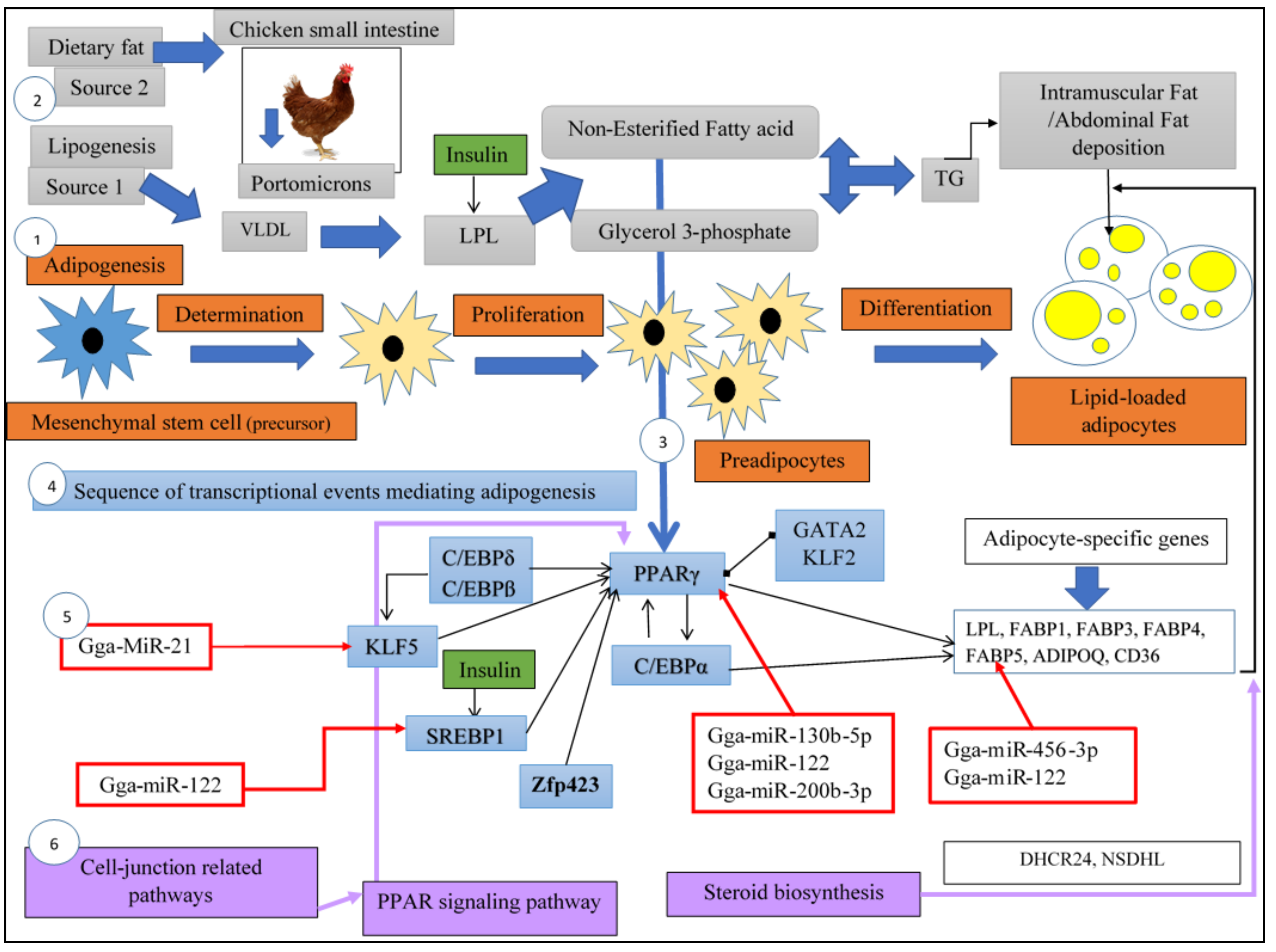
2.2. Adipogenesis in Chicken
3. Important Genes Involved in Chicken Fat Metabolism
3.1. FABP
3.2. GPAM
3.3. ELOVL
3.4. LPL
3.5. ACLY
3.6. ACSL
3.7. ACAA
3.8. SCD
3.9. APOV1
3.10. ACACA
3.11. FAS or FASN
3.12. FADS
4. Main Transcription Factors Involved in Chicken Fat Metabolism
4.1. SREBPs (SREBFs)
4.2. C/EBPβ
4.3. C/EBPα
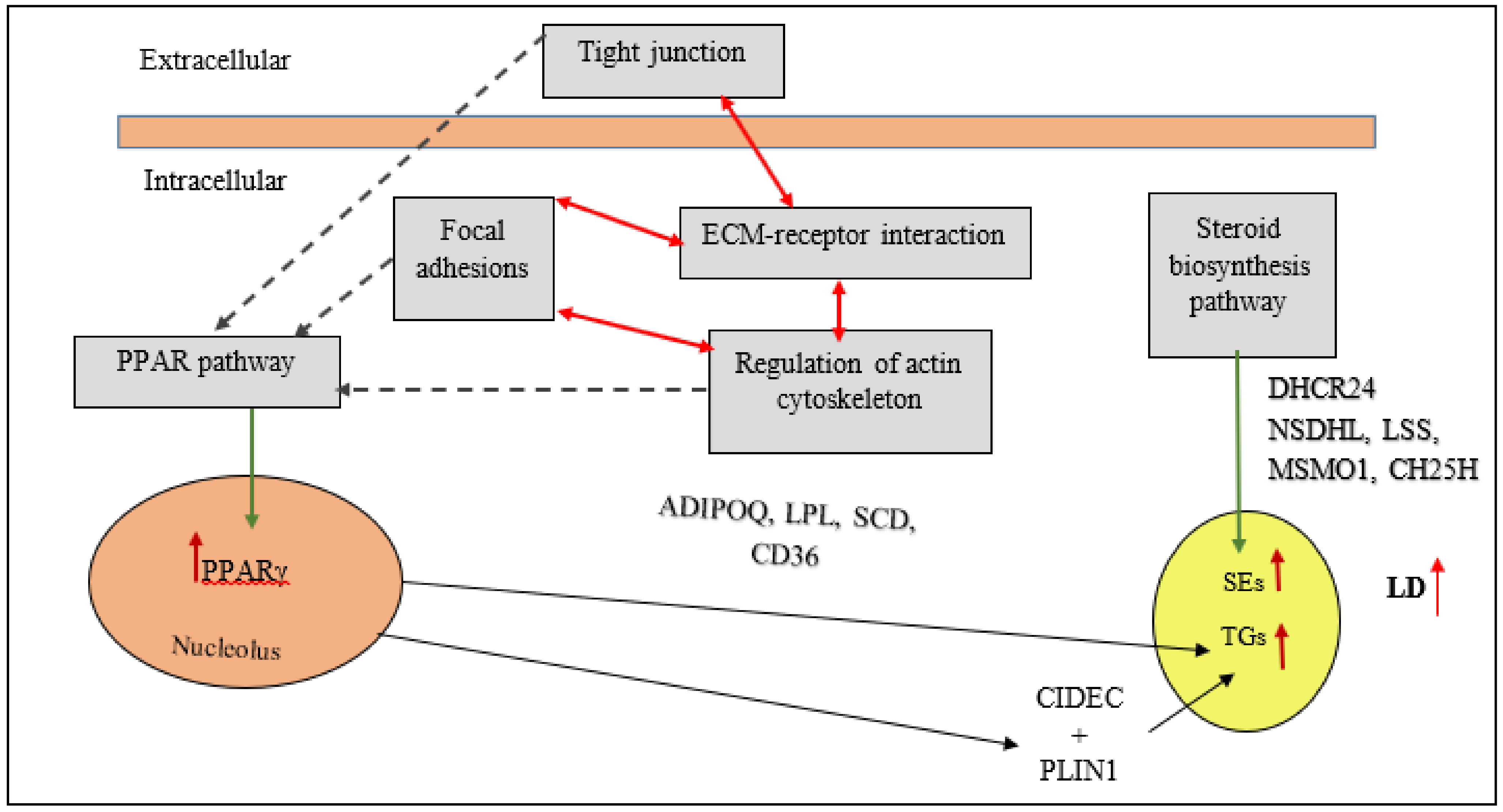
4.4. PPARγ
4.5. PPARα
4.6. PPARβ/δ
4.7. ZFP 423
4.8. KLFs
4.9. GATA2
5. Chicken miRNAs
5.1. MiRNAs in Regulation of Chicken Fat Metabolism
5.1.1. MiRNA-Mediated Regulation of Lipogenesis (Hepatic Lipid Metabolism)
Gga-miR-33
Gga-miR-122
Gga-miR-5
Gga-miR-22-3p
Gga-miR-146b-5p
Gga-miR-24-3b
Gga-miR-34a
Gga-miR-218-5p
Gga-miR-101-2-5p
5.1.2. MiRNAs Involved in Adipogenesis and Fat Deposition
MiRNA and Abdominal Fat Adipogenesis in Chicken
MiRNA and Intramuscular Adipogenesis in Chicken
Gga-miR-223
Gga-miR-140-5p
Gga-miR-18b-3p
Gga-miR-let-7
6. Main Signaling Pathways Involved in Chicken Fat Metabolism
7. Hormonal Regulation of Fat Metabolism in Chicken
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resnyk, C.W.; Carré, W.; Wang, X.; Porter, T.E.; Simon, J.; Le Bihan-Duval, E.; Duclos, M.J.; Aggrey, S.E.; Cogburn, L.A. Transcriptional Analysis of Abdominal Fat in Genetically Fat and Lean Chickens Reveals Adipokines, Lipogenic Genes and a Link between Hemostasis and Leanness. BMC Genom. 2013, 14, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, B.A.; Chen, J.; Nie, Q.; Zhang, X. Genomic Insights into the Multiple Factors Controlling Abdominal Fat Deposition in a Chicken Model. Front. Genet. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Wang, X.; Yu, J.; Shen, K.; Qi, C.; Gu, Z. Expression of MiR-33 from an SREBP2 Intron Inhibits the Expression of the Fatty Acid Oxidation- Regulatory Genes CROT and HADHB in Chicken Liver. Br. Poult. Sci. 2019, 1668. [Google Scholar] [CrossRef] [PubMed]
- Mm, G.; Li, M. Expression of Adipocyte Fatty Acid-Binding Protein Gene in Abdominal Adipose Tissue and Its Association with Growth and Fatness Traits in Commercial Meat Type Chickens. IMedPub J. 2018, 1, 1–10. [Google Scholar]
- Ma, Z.; Li, H.; Zheng, H.; Jiang, K.; Yan, F.; Kang, X.; Wang, Y.; Liu, X.; Tian, Y. Hepatic ELOVL6 MRNA Is Regulated by the Gga-MiR-22-3p in Egg-Laying Hen. Gene 2017, 623, 72–79. [Google Scholar] [CrossRef]
- Cui, H.; Zheng, M.; Zhao, G.; Liu, R.; Wen, J. Identification of Differentially Expressed Genes and Pathways for Intramuscular Fat Metabolism between Breast and Thigh Tissues of Chickens. BMC Genom. 2018, 1–9. [Google Scholar] [CrossRef]
- Zhang, M.; Li, D.H.; Li, F.; Sun, J.W.; Jiang, R.R.; Li, Z.J.; Han, R.L.; Li, G.X.; Liu, X.J.; Kang, X.T.; et al. Integrated Analysis of MiRNA and Genes Associated with Meat Quality Reveals That Gga-MiR-140-5p Affects Intramuscular Fat Deposition in Chickens. Cell. Physiol. Biochem. 2018, 46, 2421–2433. [Google Scholar] [CrossRef]
- Wu, G.Q.; Deng, X.M.; Li, J.Y.; Li, N.; Yang, N. A Potential Molecular Marker for Selection against Abdominal Fatness in Chickens. Poult. Sci. 2006, 85, 1896–1899. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Moreira, M.; Boschiero, C.; Silva, A.; Cesar, M.; Reecy, J.M.; Godoy, T.F.; Pértille, F.; Ledur, M.C.; Silvia, A.; et al. Integration of Genome Wide Association Studies and Whole Genome Sequencing Provides Novel Insights into Fat Deposition in Chicken. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ghedira, K. Introductory Chapter: A Brief Overview of Transcriptional and Post-Transcriptional Regulation. Transcr. Post Transcr. Regul. 2018, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Viscarra, J.; Kim, S.J.; Sul, H.S. Transcriptional Regulation of Hepatic Lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Z.; Liu, X. An Overall View of the Regulation of Hepatic Lipid Metabolism in Chicken Revealed by New-Generation Sequencing; Intech: London, UK, 2017; pp. 133–147. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, L.; Wang, H.; Shao, F.; Yu, J.; Jiang, H.; Han, Y.; Gong, D.; Gu, Z. Growth Hormone-Regulated MRNAs and MiRNAs in Chicken Hepatocytes. PLoS ONE 2014, 9, e112896. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Liu, R.R.; Zhao, G.P.; Li, Q.H.; Zheng, M.Q.; Zhang, J.J.; Li, S.F.; Liang, Z.; Wen, J. Integrated Analysis of MicroRNA and MRNA Expression Profiles in Abdominal Adipose Tissues in Chickens. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Fu, S.; Zhao, Y.; Li, Y.; Li, G.; Chen, Y.; Li, Z.; Sun, G.; Li, H.; Kang, X.; Yan, F. Characterization of MiRNA Transcriptome Profiles Related to Breast Muscle Development and Intramuscular Fat Deposition in Chickens. J. Cell. Biochem. 2018, 119, 7063–7079. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.; Norman, B.P.; Lai, K.; Mohd, N.; Nik, A.; Rahman, A.; Banu, N.; Alitheen, M.; Osman, M.A. The Regulatory Role of MicroRNAs in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef] [Green Version]
- Payne, V.A.; Wo-Shing, A.; Lowe, C.E.; Rahman, S.M.; Jacob, E.F.; O’Rahilly, S.; Rochford, J.J. C/EBP Transcription Factors Regulate SREBP1c Gene Expression during Adipogenesis. Biochem. J. 2010, 425, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Ma, Z.; Jia, L.; Li, Y.; Xu, C.; Wang, T.; Han, R. Systematic Analysis of the Regulatory Functions of MicroRNAs in Chicken Hepatic Lipid Metabolism. Nat. Publ. Gr. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Moreira, G.C.M.; Boschiero, C.; Cesar, A.S.M.; Reecy, J.M.; Godoy, T.F.; Trevisoli, P.A.; Cantão, M.E.; Ledur, M.C.; Ibelli, A.M.G.; Peixoto, J.d.O.; et al. A Genome-Wide Association Study Reveals Novel Genomic Regions and Positional Candidate Genes for Fat Deposition in Broiler Chickens. BMC Genom. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Alvarenga, R.R.; Zangeronimo, M.G.; Pereira, L.J.; Rodrigues, P.B.; Gomide, E.M. Lipoprotein Metabolism in Poultry. Worlds. Poult. Sci. J. 2011, 67, 431–440. [Google Scholar] [CrossRef]
- Hermier, D. Lipoprotein Metabolism and Fattening in Poultry. J. Nutr. 1997, 127, 805S–808S. [Google Scholar] [CrossRef]
- Olaia, U.; Alfonso, L.; Mendizabal, J.A. Cellularity Description of Adipose Depots in Domesticated Animals; Intech: London, UK, 2018; pp. 73–90. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, X.; Cui, H.; Liu, R.; Zhao, G.; Wen, J. Transcriptional Insights into Key Genes and Pathways Controlling Muscle Lipid Metabolism in Broiler Chickens. BMC Genom. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Desert, C.; Baéza, E.; Aite, M.; Boutin, M.; Le Cam, A.; Montfort, J.; Houee-Bigot, M.; Blum, Y.; Roux, P.F.; Hennequet-Antier, C.; et al. Multi-Tissue Transcriptomic Study Reveals the Main Role of Liver in the Chicken Adaptive Response to a Switch in Dietary Energy Source through the Transcriptional Regulation of Lipogenesis. BMC Genom. 2018, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian Liver: The Forgotten Organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laliotis, G.P.; Bizelis, I.; Rogdakis, E. Comparative Approach of the de Novo Fatty Acid Synthesis (Lipogenesis) between Ruminant and Non Ruminant Mammalian Species: From Biochemical Level to the Main Regulatory Lipogenic Genes. Curr. Genom. 2010, 11, 168–183. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhao, Y.; Jin, W.; Li, Y.; Zhang, Y.; Ma, X.; Sun, G.; Han, R. MicroRNAs and Their Regulatory Networks in Chinese Gushi Chicken Abdominal Adipose Tissue during Postnatal Late Development. BMC Genom. 2019, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Y.; Wang, L.; Shi, Z.; Ou, X.; Wu, D.; Zhang, X.; Hu, H.; Yuan, J.; Wang, W.; et al. RNA-Seq Reveals Differentially Expressed Genes Affecting Polyunsaturated Fatty Acids Percentage in the Huangshan Black Chicken Population. PLoS ONE 2018, 13, e0195132. [Google Scholar] [CrossRef] [Green Version]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [Green Version]
- Hicks, J.A.; Porter, T.E.; Liu, H.C. Identification of MicroRNAs Controlling Hepatic MRNA Levels for Metabolic Genes during the Metabolic Transition from Embryonic to Posthatch Development in the Chicken. BMC Genom. 2017, 18, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Kim, W.K.; Cline, M.A.; Gilbert, E.R. Factors Affecting Adipose Tissue Development in Chickens: A Review. Poult. Sci. 2017, 96, 3687–3699. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Ou, K.; Wang, T.; Li, Z.; Tian, Y.; Wang, Y.; Kang, X.; Li, H.; Liu, X. Evolution, Dynamic Expression Changes and Regulatory Characteristics of Gene Families Involved in the Glycerophosphate Pathway of Triglyceride Synthesis in Chicken (Gallus Gallus). Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gunawan, A.; Listyarini, K.; Furqon, A.; Sumantri, C.; Akter, S.H.; Uddin, M.J. Gene Reports RNA Deep Sequencing Reveals Novel Transcripts and Pathways Involved in the Unsaturated Fatty Acid Metabolism in Chicken. Gene Rep. 2019, 15, 100370. [Google Scholar] [CrossRef]
- Na, W.; Wu, Y.Y.; Gong, P.F.; Wu, C.Y.; Cheng, B.H.; Wang, Y.X.; Wang, N.; Du, Z.Q.; Li, H. Embryonic Transcriptome and Proteome Analyses on Hepatic Lipid Metabolism in Chickens Divergently Selected for Abdominal Fat Content. BMC Genom. 2018, 19, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Yonemura, T.; Ishii, H.; Toyomizu, M.; Kamada, T.; Akiba, Y. Role of Peroxisome Proliferator-Activated Receptor β/δ in Chicken Adipogenesis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Resnyk, C.W.; Carré, W.; Wang, X.; Porter, T.E.; Simon, J.; Le Bihan-Duval, E.; Duclos, M.J.; Aggrey, S.E.; Cogburn, L.A. Transcriptional Analysis of Abdominal Fat in Chickens Divergently Selected on Bodyweight at Two Ages Reveals Novel Mechanisms Controlling Adiposity: Validating Visceral Adipose Tissue as a Dynamic Endocrine and Metabolic Organ. BMC Genom. 2017, 18, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, J.; Zhu, S.; Du, Z.; Li, D.; Li, W.; Li, Z.; Tian, Y.; Kang, X.; Sun, G. MiRNAs and MRNAs Analysis during Abdominal Preadipocyte Differentiation in Chickens. Animals 2020, 10, 468. [Google Scholar] [CrossRef] [Green Version]
- Wang, W. Research Advances of Adipocyte Differentiation in Poultry. World’s Poult. Sci. Assoc. 2019, 75, 535–546. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wang, L.; Wang, N.; Li, Y.; Li, H. Transdifferentiation of Fibroblasts into Adipocyte-like Cells by Chicken Adipogenic Transcription Factors. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 502–508. [Google Scholar] [CrossRef]
- Bai, S.; Pan, S.; Zhang, K.; Ding, X.; Wang, J.; Zeng, Q.; Xuan, Y.; Su, Z. Dietary Overload Lithium Decreases the Adipogenesis in Abdominal Adipose Tissue of Broiler Chickens. Environ. Toxicol. Pharmacol. 2017, 49, 163–171. [Google Scholar] [CrossRef]
- Chen, X.; Niu, J.; Geng, Z. Gene Expression and Plasma Lipid Content in Relation to Intramuscular Fat in Chinese Indigenous Wuhua Chicken. J. Appl. Poult. Res. 2017, 26, 391–400. [Google Scholar] [CrossRef]
- Claire D’Andre, H.; Paul, W.; Shen, X.; Jia, X.; Zhang, R.; Sun, L.; Zhang, X. Identification and Characterization of Genes That Control Fat Deposition in Chickens. J. Anim. Sci. Biotechnol. 2013, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Zhang, L.; Xu, S.; Shen, A. ATP-Citrate Lyase (ACLY) in Lipid Metabolism and Atherosclerosis: An Updated Review. Prog. Lipid Res. 2020, 77, 101006. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.C.; Li, F.; Li, H.; Liu, X.J.; Loor, J.J.; Kang, X.T.; Sun, G.R. Estrogen Promotes Hepatic Synthesis of Long-Chain Polyunsaturated Fatty Acids by Regulating ELOVL5 at Post-Transcriptional Level in Laying Hens. Int. J. Mol. Sci. 2017, 18, 1405. [Google Scholar] [CrossRef] [Green Version]
- Burke, A.C.; Huff, M.W. ATP-Citrate Lyase: Genetics, Molecular Biology and Therapeutic Target for Dyslipidemia. Curr. Opin. Lipidol. 2017, 28, 193–200. [Google Scholar] [CrossRef]
- Tian, W.H.; Wang, Z.; Yue, Y.X.; Li, H.; Li, Z.J.; Han, R.L.; Tian, Y.D.; Kang, X.T.; Liu, X.J. MiR-34a-5p Increases Hepatic Triglycerides and Total Cholesterol Levels by Regulating ACSL1 Protein Expression in Laying Hens. Int. J. Mol. Sci. 2019, 20, 4420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, J.A.; Trakooljul, N.; Liu, H.C. Discovery of Chicken MicroRNAs Associated with Lipogenesis and Cell Proliferation. Physiol. Genom. 2010, 41, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.Q.; Liu, R.R.; Zhao, G.P.; Zheng, M.Q.; Chen, J.L.; Wen, J. Expression Profiles of Key Transcription Factors Involved in Lipid Metabolism in Beijing-You Chickens. Gene 2014, 537, 120–125. [Google Scholar] [CrossRef]
- Saneyasu, T.; Kimura, S.; Kitashiro, A.; Tsuchii, N.; Tsuchihashi, T.; Inui, M.; Honda, K.; Kamisoyama, H. Differential Regulation of the Expression of Lipid Metabolism-Related Genes with Skeletal Muscle Type in Growing Chickens. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 189, 1–5. [Google Scholar] [CrossRef]
- Ding, N.; Gao, Y.; Wang, N.; Li, H. Functional Analysis of the Chicken PPARΓ Gene 5′-Flanking Region and C/EBPα-Mediated Gene Regulation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 158, 297–303. [Google Scholar] [CrossRef]
- Hu, G.; Wang, S.Z.; Wang, Z.P.; Li, Y.M.; Li, H. Genetic Epistasis Analysis of 10 Peroxisome Proliferator-Activated Receptor γ-Correlated Genes in Broiler Lines Divergently Selected for Abdominal Fat Content. Poult. Sci. 2010, 89, 2341–2350. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Zhang, M.; Sun, J.; Li, W.; Jiang, R.; Han, R.; Wang, Y.; Tian, Y.; Kang, X.; et al. MiRNA-223 Targets the GPAM Gene and Regulates the differentiation of Intramuscular Adipocytes. Gene 2019, 685, 106–113. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, M.; Qiao, S.; Wang, Y.; Li, H.; Wang, N. Gga-MiR-21 Inhibits Chicken Pre-Adipocyte Proliferation in Part by down-Regulating Kruppel-like Factor 5. Poult. Sci. 2017, 96, 200–210. [Google Scholar] [CrossRef]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, Mechanism, Function, and Recent Clinical Trials. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
- Shao, P.; Zhou, H.; Xiao, Z.D.; He, J.H.; Huang, M.B.; Chen, Y.Q.; Qu, L.H. Identification of Novel Chicken MicroRNAs and Analysis of Their Genomic Organization. Gene 2008, 418, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Du, Z.; Li, N. Identification of MicroRNAs from Different Tissues of Chicken Embryo and Adult Chicken. FEBS Lett. 2006, 580, 3610–3616. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Li, F.; Ma, X.; Sun, J.; Jiang, R.; Tian, Y.; Han, R.; Li, G.; Wang, Y.; Li, Z.; et al. Gga-MiRNA-18b-3p Inhibits Intramuscular Adipocytes Di Erentiation in Chicken by Targeting the ACOT13 Gene. Cells 2019, 8, 556. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Deng, L.; Liang, M.; Xu, L.; Zhang, L.; Ma, Y.; Li, Y. MicroRNAs Expression Profiles in Adipose Tissues and Liver from Sex-Linked Dwarf and Normal Chickens. Acta Biochim. Biophys. Sin. 2014, 46, 723–726. [Google Scholar] [CrossRef] [Green Version]
- Najafi-Shoushtari, S.H.; Kristo, F.; Li, Y.; Shioda, T.; Cohen, E.D.; Gerszten, R.E.; Naar, A.M. MicroRNA-33 and the SREBP Host Genes Cooperate to Control Cholesterol Homeostasis. Science 2013, 20, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Shao, F.; Wang, X.; Yu, J.; Jiang, H.; Zhu, B.; Gu, Z. Expression of MiR-33 from an SREBF2 Intron Targets the FTO Gene in the Chicken. PLoS ONE 2014, 9, e91236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shao, F.; Yu, J.; Jiang, H.; Gong, D.; Gu, Z. MicroRNA-122 Targets Genes Related to Liver Metabolism in Chickens. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 184, 29–35. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yu, J.; Shao, F.; Zhang, Y.; Lu, X. MiR-122 Targets the Vanin 1 Gene to Regulate Its Expression in Chickens. Poult. Sci. 2016, 95, 1145–1150. [Google Scholar] [CrossRef]
- Khatri, B.; Seo, D.; Shouse, S.; Pan, J.H.; Hudson, N.J.; Kim, J.K.; Bottje, W.; Kong, B.C. MicroRNA Profiling Associated with Muscle Growth in Modern Broilers Compared to an Unselected Chicken Breed. BMC Genom. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Ma, Z.; Li, H.; Zheng, H.; Jiang, K.; Jia, L.; Yan, F.; Tian, Y.; Kang, X.; Wang, Y.; Liu, X. MicroRNA-101-2-5p Targets the ApoB Gene in Liver of Chicken (Gallus Gallus). Genome 2017, 60, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Du, Z.Q.; Cheng, B.; Wang, Y.; Yao, J.; Li, Y.; Cao, Z.; Luan, P.; Wang, N.; Li, H. Expression Profiling of Preadipocyte MicroRNAs by Deep Sequencing on Chicken Lines Divergently Selected for Abdominal Fatness. PLoS ONE 2015, 10, e0117843. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Y.; Wang, W.; Wang, N.; Li, H. Solexa Sequencing Analysis of Chicken Pre- Adipocyte MicroRNAs Solexa Sequencing Analysis of Chicken Pre-Adipocyte MicroRNAs. Biosci. Biotechnol. Biochem. 2014, 8451, 54–61. [Google Scholar] [CrossRef]
- Wang, X.G.; Yu, J.F.; Zhang, Y.; Gong, D.Q.; Gu, Z.L. Identification and Characterization of MicroRNA from Chicken Adipose Tissue and Skeletal Muscle. Poult. Sci. 2012, 91, 139–149. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Sun, G.; Liu, X.; Jiang, R.; Han, R.; Li, G.; Kang, X. Association Study of a Common Genetic Variant in Pre-MiR-1596 with Chicken Performance Traits. Mol. Biol. Rep. 2014, 41, 7175–7181. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, S.Z.; Leng, L.; Na, W.; Wang, Q.G.; Li, H. Differentially Expressed Genes in the Liver of Lean and Fat Chickens. Genet. Mol. Res. 2014, 13, 10823–10828. [Google Scholar] [CrossRef]
- Buzala, M.; Janicki, B. Review: Effects of Different Growth Rates in Broiler Breeder and Layer Hens on Some Productive Traits. Poult. Sci. 2016, 95, 2151–2159. [Google Scholar] [CrossRef]

| miRNA | Function | References |
|---|---|---|
| Gga-miR-122 |
| [18,47,61] |
| nc-miR-5 (novel) |
| [47] |
| nc-miR-33 (novel) |
| |
| Gga-miR-33 |
| [3,60] |
| Gga-miR-22-3p |
| [18] |
| Gga-miR-33a |
| |
| Gga-miR-33b | ||
| Gga-miR-146b-5p |
| |
| Gga-miR-24-3b |
| |
| Gga-miR-148a-5p |
| |
| Gga-miR-21-5p | ||
| Gga-let-7F-5p | ||
| Gga-miR-26a-5p | ||
| MiR-126-5p | ||
| Gga-miR-30d | ||
| Gga-miR-10a-5p | ||
| Gga-miR-128-3p |
| |
| Gga-miR-15b |
| [13] |
| Gga-miR-218-5p |
| [44] |
| Gga-miR-19-3p | ||
| Gga-miR-30-5p | ||
| Gga-miR-101-2-5p |
| [64] |
| Gga-34a-5p |
| [46] |
| miRNA | Function | References |
|---|---|---|
| Gga-let-7a |
| [41,65] |
| Gga-let-7j | ||
| Gga-let-7b | ||
| Gga-let-7f | ||
| Gga-let-7c | ||
| Gga-let-7k | ||
| Gga-miR-148a-3p |
| [27,65] |
| Gga-miR-146c |
| [65] |
| Gga-miR-10a | ||
| Gga-miR-33 | ||
| Gga-miR-222 | ||
| Gga-miR-15a | ||
| Gga-miR-22 | ||
| Gga-miR-206 | ||
| Gga-miR-1a | ||
| Gga-miR-29b | ||
| Gga-miR-9 | ||
| Gga-miR-32 | ||
| Gga-miR-429 |
| |
| Gga-miR-200b | ||
| Gga-miR-451 | ||
| Gga-miR-142-5p | ||
| Gga-miR-200a | ||
| Gga-miR-218 | ||
| Gga-miR-454 Gga-miR-30a-5p [27] | ||
| Gga-miR-122-5p |
| [67] |
| Gga-miR-19b-3p |
| [14] |
| Gga-miR-19a-3p |
| |
| Gga-miR-17-5p | ||
| Gga-miR-26a | ||
| Gga-miR-103-3p | ||
| Gga-miR-92-3p | ||
| Gga-miR-34 | Have key roles in:
| [27] |
| Gga-miR-199 | ||
| Gga-miR-8 | ||
| Gga-miR-146b-5p | ||
| Gga-miR-17-3p |
| |
| Gga-miR-215-5p |
| |
| Gga-miR-499-5p | ||
| Gga-miR-135a |
| |
| Gga-miR-21 |
| [9,27,53,65] |
| Gga-miR-101-3p |
| [27,65] |
| Gga-miR-31 |
| [27,65] |
| Gga-miR-30d Gga-miR-27-3p |
| [14,27] |
| miRNA | Function | References |
|---|---|---|
| Gga-Let-7b |
| [15,68] |
| Gga-miR-30a |
| [15] |
| Gga-miR-30d | ||
| Gga-miR-30C |
| |
| Gga-miR-17-5p |
| |
| Gga-miR-20a-5p Gga-miR-10b-5p | ||
| Gga-miR-148a-3p |
| |
| Gga-miR-122-5p |
| |
| Gga-miR-31-5p |
| |
| Gga-miR-27b-3P Gga-miR-24-3p | ||
| Gga-miR-103-3p Gga-miR-146b-5p |
| |
| Gga-miR-22-5p |
| |
| Gga-miR-140-5p |
| [7] |
| Gga-miR-19b-3p |
| |
| Gga-miR-425-5p | ||
| Gga-miR-27a | ||
| Gga-miR-146-5p | ||
| Gga-miR-223 |
| [7,52] |
| Gga-miR-18b-3p |
| [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nematbakhsh, S.; Pei Pei, C.; Selamat, J.; Nordin, N.; Idris, L.H.; Abdull Razis, A.F. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes 2021, 12, 414. https://doi.org/10.3390/genes12030414
Nematbakhsh S, Pei Pei C, Selamat J, Nordin N, Idris LH, Abdull Razis AF. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes. 2021; 12(3):414. https://doi.org/10.3390/genes12030414
Chicago/Turabian StyleNematbakhsh, Sara, Chong Pei Pei, Jinap Selamat, Noordiana Nordin, Lokman Hakim Idris, and Ahmad Faizal Abdull Razis. 2021. "Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken" Genes 12, no. 3: 414. https://doi.org/10.3390/genes12030414
APA StyleNematbakhsh, S., Pei Pei, C., Selamat, J., Nordin, N., Idris, L. H., & Abdull Razis, A. F. (2021). Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes, 12(3), 414. https://doi.org/10.3390/genes12030414








