Evolution of the IRF Family in Salmonids
Abstract
1. Introduction
2. Materials and Methods
2.1. Phylogenetic and Gene Synteny Analysis
2.2. IRF Primer Design
2.3. Animal Work
2.4. RNA Extraction and Reverse Transcription
2.5. Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
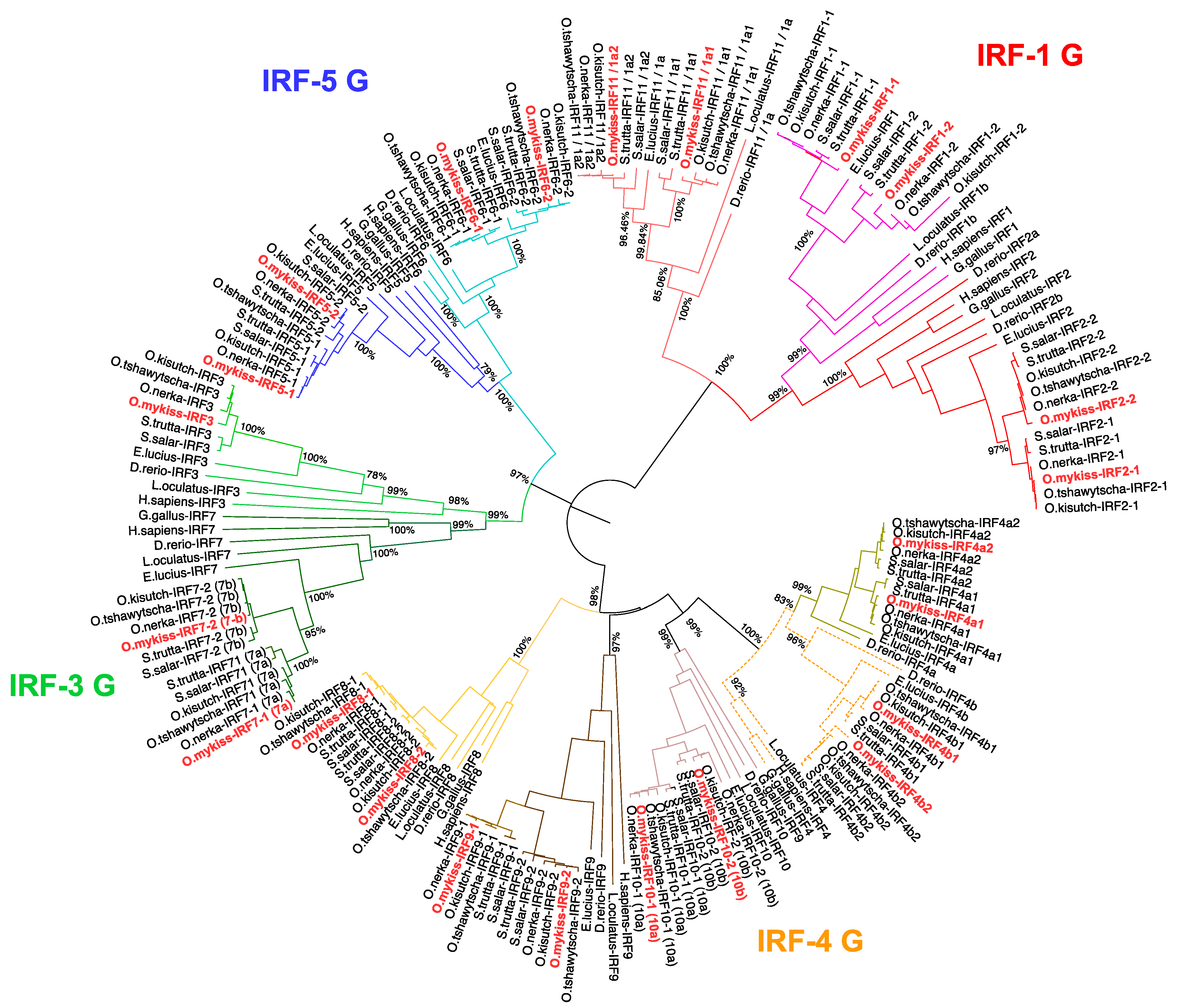
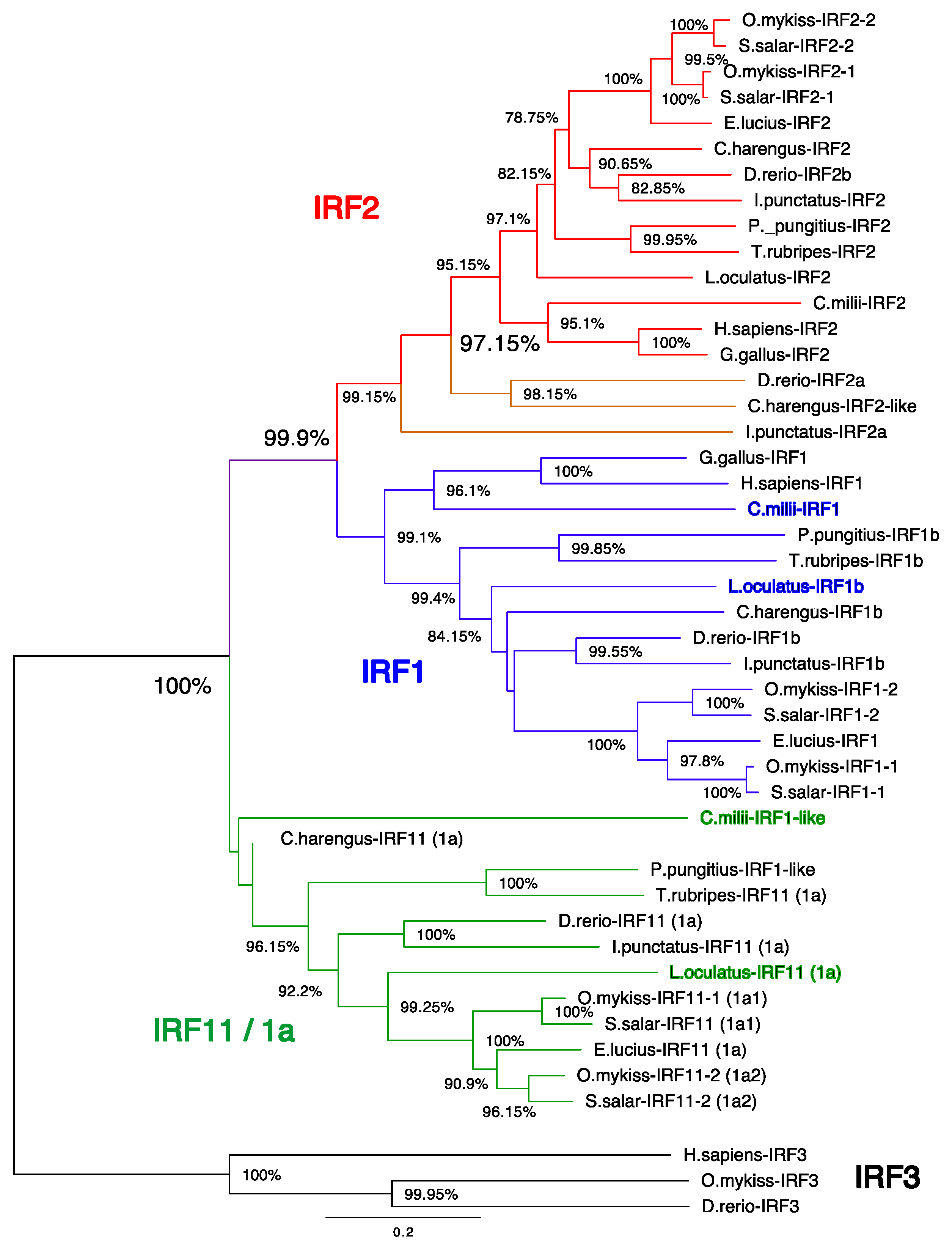
3.1. Phylogenetic Analysis of Salmonid IRF Family
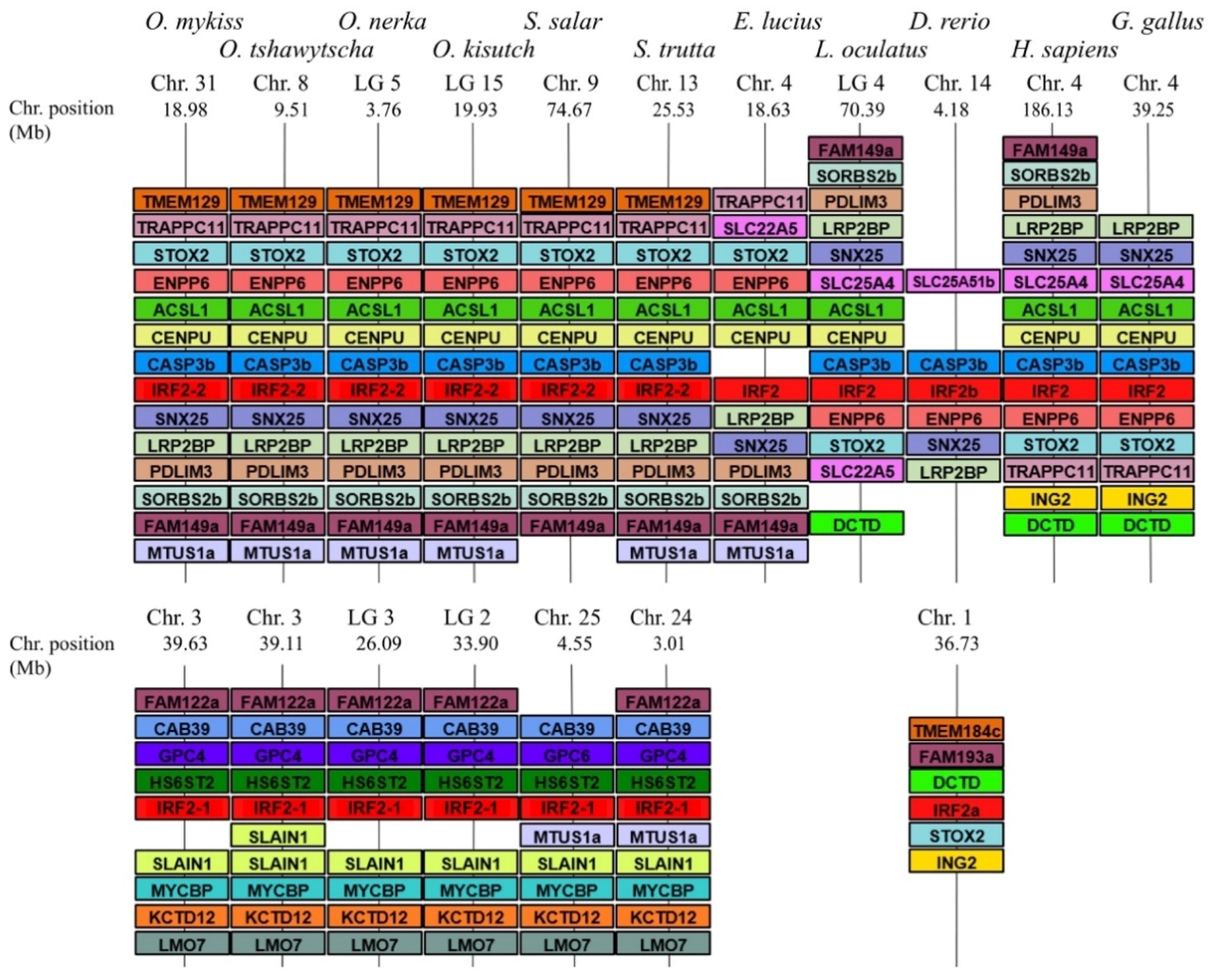
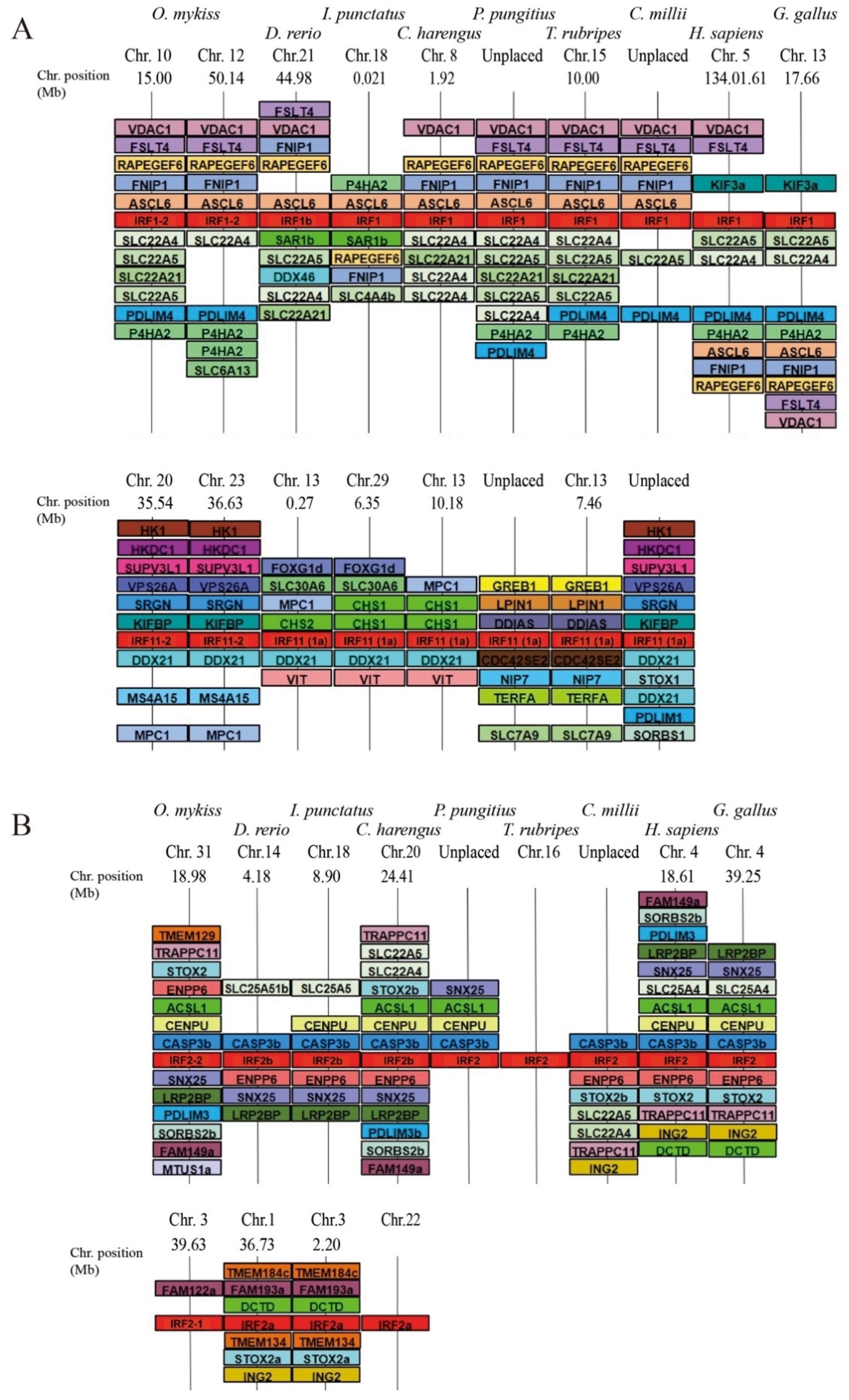
3.2. Comparative Phylogenetic and Synteny Analysis on the Case of Group 1 Salmonid Irfs
3.3. A Consistent Nomenclature for Salmonid IRF Family
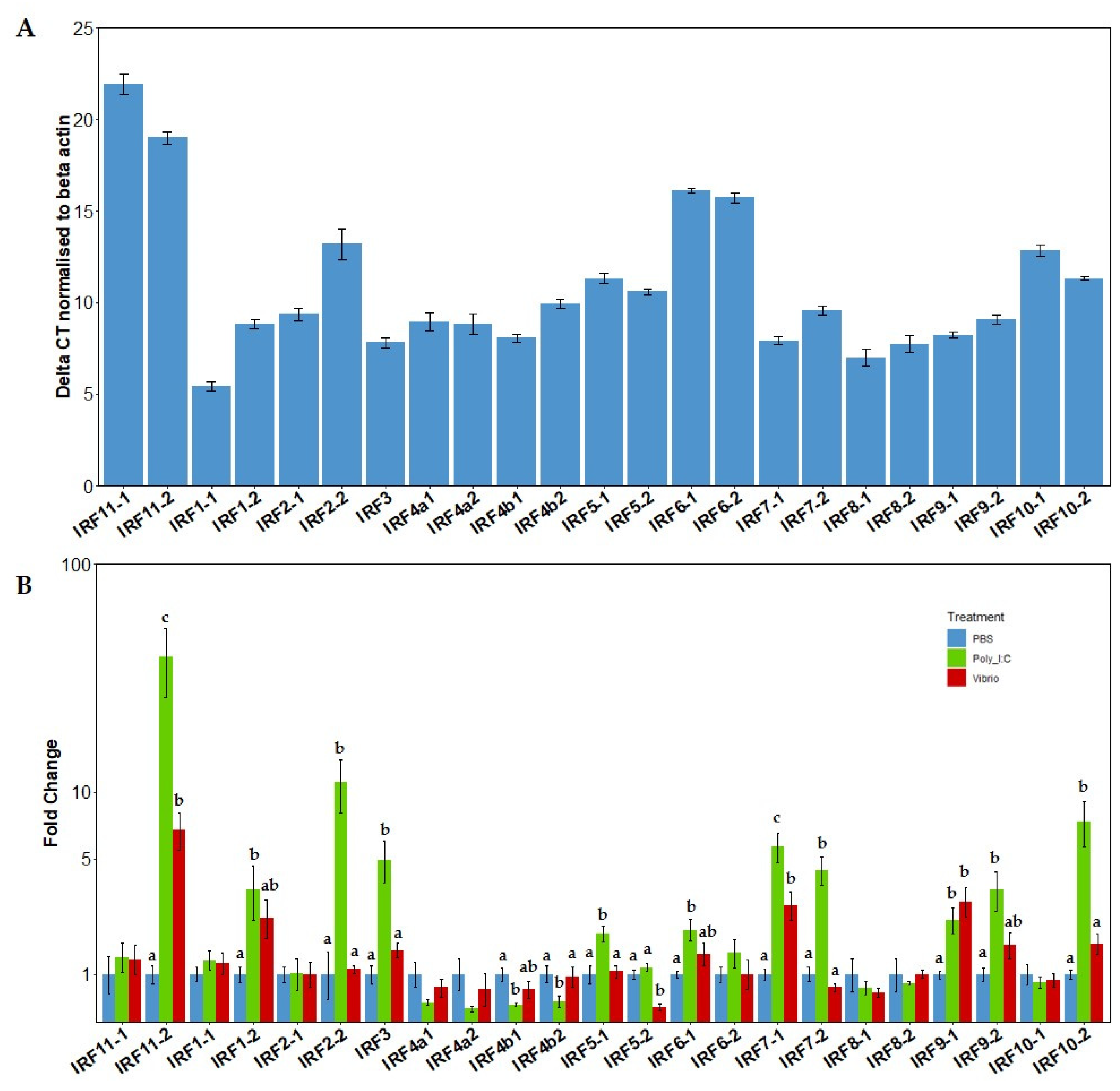
3.4. Constitutive mRNA Expression Levels of the IRF Gene Family
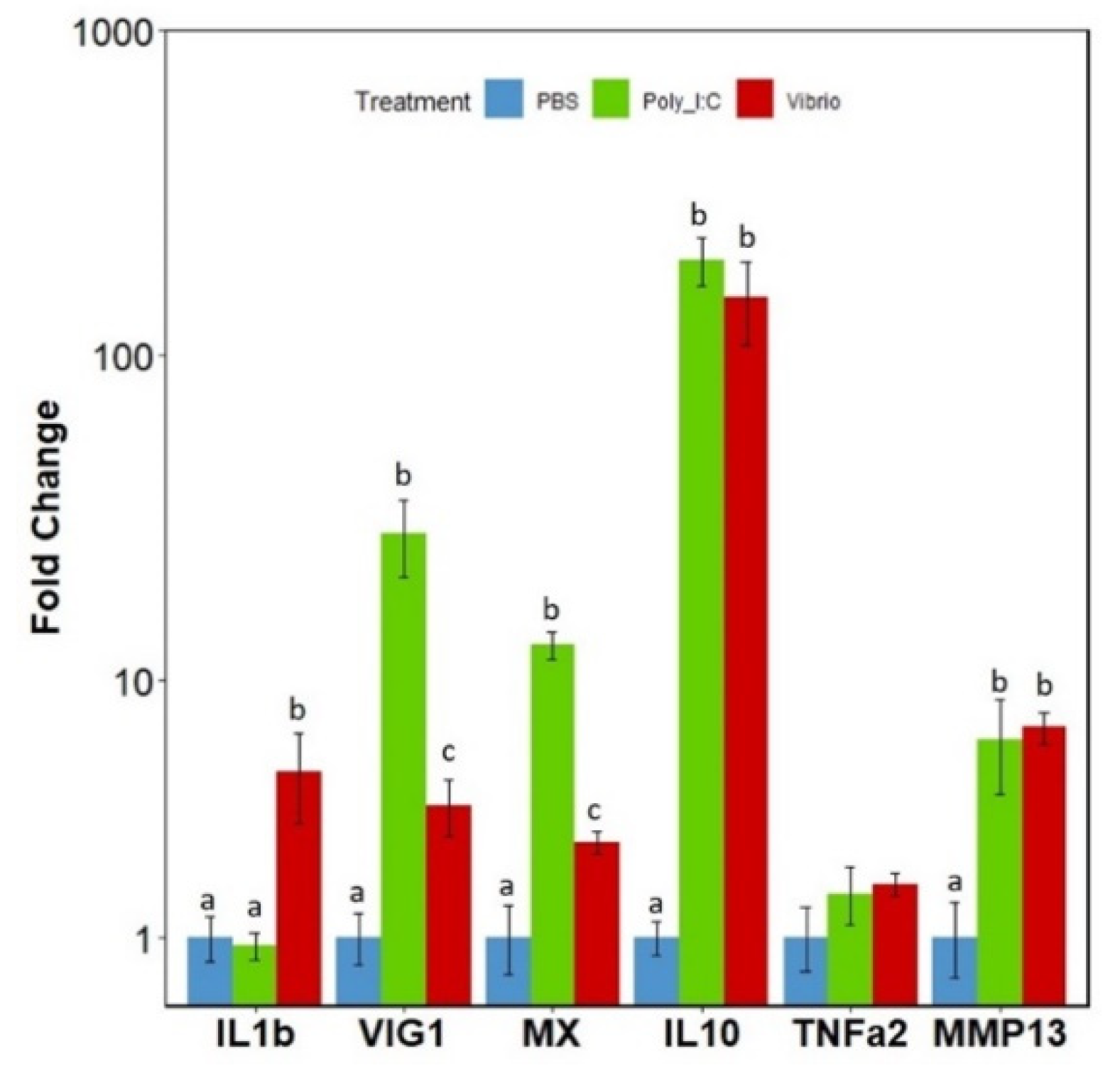
3.5. mRNA Expression Levels of the IRFs in Response to Poly I:C or Vibrio Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nehyba, J.; Hrdličková, R.; Bose, H.R. Dynamic Evolution of Immune System Regulators: The History of the Interferon Regulatory Factor Family. Mol. Biol. Evol. 2009, 26, 2539–2550. [Google Scholar] [CrossRef]
- Yanai, H.; Negishi, H.; Taniguchi, T. The IRF Family of Transcription Factors Inception, Impact and Implications in Oncogenesis. OncoImmunology 2012, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Royer William, E.J.E. Structural Insights into Interferon Regulatory Factor Activation. Cell. Signal. 2010, 22, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Negishi, H.; Taniguchi, T. The IRF Family Transcription Factors at the Interface of Innate and Adaptive Immune Responses. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, C.A. Regulating IRFs in IFN Driven Disease. In Frontiers in Immunology; Frontiers Media: Lausanne, Switzerland, 2019; p. 325. [Google Scholar] [CrossRef]
- Antonczyk, A.; Krist, B.; Sajek, M.; Michalska, A.; Piaszyk-Borychowska, A.; Plens-Galaska, M.; Wesoly, J.; Bluyssen, H.A.R. Direct Inhibition of IRF-Dependent Transcriptional Regulatory Mechanisms Associated with Disease. Front. Immunol. 2019, 10, 1176. [Google Scholar] [CrossRef]
- Honda, K.; Takaoka, A.; Taniguchi, T. Type I Inteferon Gene Induction by the Interferon Regulatory Factor Family of Transcription Factors. Immunity 2006, 25, 349–360. [Google Scholar] [CrossRef]
- Paun, A.; Pitha, P.M. The IRF Family, Revisited. Biochimie 2007, 89, 744–753. [Google Scholar] [CrossRef]
- Eguchi, J.; Yan, Q.W.; Schones, D.E.; Kamal, M.; Hsu, C.H.; Zhang, M.Q.; Crawford, G.E.; Rosen, E.D. Interferon Regulatory Factors Are Transcriptional Regulators of Adipogenesis. Cell Metab. 2008, 7, 86–94. [Google Scholar] [CrossRef]
- Gabriele, L.; Ozato, K. The Role of the Interferon Regulatory Factor (IRF) Family in Dendritic Cell Development and Function. Cytokine Growth Factor Rev. 2007, 18, 503–510. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, K.; Peng, L.; Xiong, H. Regulation of T Helper Cell Differentiation by Interferon Regulatory Factor Family Members. Immunol. Res. 2012, 54, 169–176. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The Impact of Interferon-Regulatory Factors to Macrophage Differentiation and Polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C. The Toll Receptor Family and Microbial Recognition. Trends Microbiol. 2000, 8, 452–456. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA Helicase RIG-I Has an Essential Function in Double-Stranded RNA-Induced Innate Antiviral Responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.A.; Cutrone, E.C.; Kotenko, S. The Class II Cytokine Receptor (CRF2) Family: Overview and Patterns of Receptor-Ligand Interactions. Cytokine Growth Factor Rev. 2004, 15, 33–48. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, Interferon-like Cytokines, and Their Receptors. Immunol. Rev. Immunol. Rev. Dec. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- Levraud, J.-P.; Boudinot, P.; Colin, I.; Benmansour, A.; Peyrieras, N.; Herbomel, P.; Lutfalla, G. Identification of the Zebrafish IFN Receptor: Implications for the Origin of the Vertebrate IFN System. J. Immunol. 2007, 178, 4385–4394. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Innate Immunity to Virus Infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. IRF and STAT Transcription Factors—From Basic Biology to Roles in Infection, Protective Immunity, and Primary Immunodeficiencies. Front. Immunol. 2019, 9, 3047. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E.; Kerr, I.M.; Stark, G.R. Jak-STAT Pathways and Transcriptional Activation in Response to IFNs and Other Extracellular Signaling Proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Bluyssen, H.A.R.; Durbin, J.E.; Levy, D.E. ISGF3γ P48, a Specificity Switch for Interferon Activated Transcription Factors. Cytokine Growth Factor Rev. 1996, 7, 11–17. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ogasawara, K.; Takaoka, A.; Tanaka, N. IRF Family of Transcription Factors as Regulators of Host Defense. Annu. Rev. Immunol. 2001, 19, 623–655. [Google Scholar] [CrossRef] [PubMed]
- Collet, B. Innate Immune Responses of Salmonid Fish to Viral Infections. Dev. Comp. Immunol. 2014, 43, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Qi, Z.T.; Xu, Z.; Nie, P. Global Characterization of Interferon Regulatory Factor (IRF) Genes in Vertebrates: Glimpse of the Diversification in Evolution. BMC Immunol. 2010, 5, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.; Koop, B.F.; Sandve, S.R.; Miller, J.R.; Kent, M.P.; Nome, T.; Hvidsten, T.R.; Leong, J.S.; Minkley, D.R.; Zimin, A.; et al. The Atlantic Salmon Genome Provides Insights into Rediploidization. Nature 2016, 533, 200–205. [Google Scholar] [CrossRef]
- Bergan, V.; Kileng, Ø.; Sun, B.; Robertsen, B. Regulation and Function of Interferon Regulatory Factors of Atlantic Salmon. Mol. Immunol. 2010, 47, 2005–2014. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, Y.; Wangkahart, E.; Zou, J.; Chang, M.; Yang, D.; Secombes, C.J.; Nie, P.; Wang, T. Sequence and Expression Analysis of Interferon Regulatory Factor 10 (IRF10) in Three Diverse Teleost Fish Reveals Its Role in Antiviral Defense. PLoS ONE 2016, 11, e0147181. [Google Scholar] [CrossRef]
- Macqueen, D.J.; Johnston, I.A. A Well-Constrained Estimate for the Timing of the Salmonid Whole Genome Duplication Reveals Major Decoupling from Species Diversification. Proc. R. Soc. B Biol. Sci. 2014, 281, 1778. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hernández, P.P.; Strzelecka, P.M.; Athanasiadis, E.I.; Hall, D.; Robalo, A.F.; Collins, C.M.; Boudinot, P.; Levraud, J.P.; Cvejic, A. Single-Cell Transcriptional Analysis Reveals ILC-like Cells in Zebrafish. Sci. Immunol. 2018, 3, eaau5265. [Google Scholar] [CrossRef]
- Yano, A.; Guyomard, R.; Nicol, B.; Jouanno, E.; Quillet, E.; Klopp, C.; Cabau, C.; Bouchez, O.; Fostier, A.; Guiguen, Y. An Immune-Related Gene Evolved into the Master Sex-Determining Gene in Rainbow Trout, Oncorhynchus Mykiss. Curr. Biol. 2012, 22, 1423–1428. [Google Scholar] [CrossRef]
- Inkpen, S.M.; Solbakken, M.H.; Jentoft, S.; Eslamloo, K.; Rise, M.L. Full Characterization and Transcript Expression Profiling of the Interferon Regulatory Factor (IRF) Gene Family in Atlantic Cod (Gadus Morhua). Dev. Comp. Immunol. 2019, 98, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qi, C.; Shan, S.; Zhang, F.; Li, H.; An, L.; Yang, G. Characterization of Common Carp (Cyprinus Carpio L.) Interferon Regulatory Factor 5 (IRF5) and Its Expression in Response to Viral and Bacterial Challenges. BMC Vet. Res 2016, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Takizawa, F.; Chaara, W.; Lunazzi, A.; Dang, T.H.; Koellner, B.; Quillet, E.; Six, A.; Fischer, U.; Boudinot, P. Contrasted TCRβ Diversity of CD8+ and CD8− T Cells in Rainbow Trout. PLoS ONE 2013, 8, e60175. [Google Scholar] [CrossRef]
- Langevin, C.; Blanco, M.; Martin, S.A.M.; Jouneau, L.; Bernardet, J.-F.; Houel, A.; Lunazzi, A.; Duchaud, E.; Michel, C.; Quillet, E.; et al. Transcriptional Responses of Resistant and Susceptible Fish Clones to the Bacterial Pathogen Flavobacterium Psychrophilum. PLoS ONE 2012, 7, e39126. [Google Scholar] [CrossRef]
- Castro, R.; Jouneau, L.; Tacchi, L.; Macqueen, D.J.; Alzaid, A.; Secombes, C.J.; Martin, S.A.M.; Boudinot, P. Disparate Developmental Patterns of Immune Responses to Bacterial and Viral Infections in Fish. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Boudinot, P.; Bird, S.; Du Pasquier, L.; Collet, B. The Repertoire of Vertebrate STAT Transcription Factors: Origin and Variations in Fish. Dev. Comp. Immunol. 2021, 116, 103929. [Google Scholar] [CrossRef]
- Froschauer, A.; Braasch, I.; Volff, J. Fish Genomes, Comparative Genomics and Vertebrate Evolution. Curr. Genom. 2006, 7, 43–57. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, X.; Liu, S.; Zhang, Y.; Liu, H.; Sun, F.; Bao, L.; Waldbieser, G.; Liu, Z. Whole Genome Comparative Analysis of Channel Catfish (Ictalurus Punctatus) with Four Model Fish Species. BMC Genom. 2013, 14, 780. [Google Scholar] [CrossRef] [PubMed]
- Briolat, V.; Jouneau, L.; Carvalho, R.; Palha, N.; Langevin, C.; Herbomel, P.; Schwartz, O.; Spaink, H.P.; Levraud, J.-P.; Boudinot, P. Contrasted Innate Responses to Two Viruses in Zebrafish: Insights into the Ancestral Repertoire of Vertebrate IFN-Stimulated Genes. J. Immunol. 2014, 192, 4328–4341. [Google Scholar] [CrossRef]
- Venkatesh, B.; Lee, A.P.; Ravi, V.; Maurya, A.K.; Lian, M.M.; Swann, J.B.; Ohta, Y.; Flajnik, M.F.; Sutoh, Y.; Kasahara, M.; et al. Elephant Shark Genome Provides Unique Insights into Gnathostome Evolution. Nature 2014, 505, 174–179. [Google Scholar] [CrossRef]
- Langham, R.J.; Walsh, J.; Dunn, M.; Ko, C.; Goff, S.A.; Freeling, M. Genomic Duplication, Fractionation and the Origin of Regulatory Novelty. Genetics 2004, 166, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, C.; Brunet, F.; Chalopin, D.; Juanchich, A.; Bernard, M.; Noël, B.; Bento, P.; Da Silva, C.; Labadie, K.; Alberti, A.; et al. The Rainbow Trout Genome Provides Novel Insights into Evolution after Whole-Genome Duplication in Vertebrates. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Pasquier, J.; Cabau, C.; Nguyen, T.; Jouanno, E.; Severac, D.; Braasch, I.; Journot, L.; Pontarotti, P.; Klopp, C.; Postlethwait, J.H.; et al. Gene Evolution and Gene Expression after Whole Genome Duplication in Fish: The PhyloFish Database. BMC Genom. 2016, 17, 368. [Google Scholar] [CrossRef]
- Bowie, A.G.; Unterholzner, L. Viral Evasion and Subversion of Pattern-Recognition Receptor Signalling. Nat. Rev. Immunol. 2008, 8, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Puri, M.; Horvath, C.M.; Sen, G.C. Select Paramyxoviral V Proteins Inhibit IRF3 Activation by Acting as Alternative Substrates for Inhibitor of ΚB Kinase ε (IKKe)/TBK1. J. Biol. Chem. 2008, 283, 14269–14276. [Google Scholar] [CrossRef] [PubMed]
- Dehler, C.E.; Lester, K.; Della Pelle, G.; Jouneau, L.; Houel, A.; Collins, C.; Dovgan, T.; Machat, R.; Zou, J.; Boudinot, P.; et al. Viral Resistance and IFN Signaling in STAT2 Knockout Fish Cells. J. Immunol. 2019, 203, 465–475. [Google Scholar] [CrossRef]
- Tamura, T.; Yanai, H.; Savitsky, D.; Taniguchi, T. The IRF Family Transcription Factors in Immunity and Oncogenesis. Annu. Rev. Immunol. 2008, 26, 535–584. [Google Scholar] [CrossRef]
- Negishi, H.; Taniguchi, T.; Yanai, H. The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb. Perspect. Biol. 2018, 10, a028423. [Google Scholar] [CrossRef]
- Taniguchi, T.; Takaoka, A. The Interferon-α/β System in Antiviral Responses: A Multimodal Machinery of Gene Regulation by the IRF Family of Transcription Factors. Curr. Opin. Immunol. 2002, 14, 111–116. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.M.; Maniatis, T. IKKE and TBKI Are Essential Components of the IRF3 Signalling Pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Chen, Z.J. Antiviral Innate Immunity Pathways. Cell Res. 2006, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Forero, A.; Ozarkar, S.; Li, H.; Lee, C.H.; Hemann, E.A.; Nadjsombati, M.S.; Hendricks, M.R.; So, L.; Green, R.; Roy, C.N.; et al. Differential Activation of the Transcription Factor IRF1 Underlies the Distinct Immune Responses Elicited by Type I and Type III Interferons. Immunity 2019, 51, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zhong, Z.; Fang, C.; Dai, W.; Shen, Y.; Gan, X.; He, S. Ancient Duplications and Functional Divergence in the Interferon Regulatory Factors of Vertebrates Provide Insights into the Evolution of Vertebrate Immune Systems. Dev. Comp. Immunol. 2018, 81, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Hida, S.; Ogasawara, K.; Sato, K.; Abe, M.; Takayanagi, H.; Yokochi, T.; Sato, T.; Hirose, S.; Shirai, T.; Taki, S.; et al. CD8+ T Cell-Mediated Skin Disease in Mice Lacking IRF-2, the Transcriptional Attenuator of Interferon-α/β Signaling. Immunity 2000, 13, 643–655. [Google Scholar] [CrossRef]
- Negishi, H.; Ohba, Y.; Yanai, H.; Takaoka, A.; Honma, K.; Yui, K.; Matsuyama, T.; Taniguchi, T.; Honda, K. Negative Regulation of Toll-like-Receptor Signaling by IRF-4. Proc. Natl. Acad. Sci. USA 2005, 102, 15989–15994. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.; Petit, J.; Forlenza, M.; Chen, X.; Chen, L.; Zou, J.; Secombes, C.J. Evolution of IFN Subgroups in Bony Fish—2. Analysis of Subgroup Appearance and Expansion in Teleost Fish with a Focus on Salmonids. Fish Shellfish Immunol. 2020, 98, 564–573. [Google Scholar] [CrossRef]
- Aggad, D.; Mazel, M.; Boudinot, P.; Mogensen, K.E.; Hamming, O.J.; Hartmann, R.; Kotenko, S.; Herbomel, P.; Lutfalla, G.; Levraud, J.-P. The Two Groups of Zebrafish Virus-Induced Interferons Signal via Distinct Receptors with Specific and Shared Chains. J. Immunol. 2009, 183, 3924–3931. [Google Scholar] [CrossRef]
- De Weerd, N.A.; Nguyen, T. The Interferons and Their Receptors-Distribution and Regulation. Immunol. Cell Biol. 2012, 90, 483–491. [Google Scholar] [CrossRef]
- Sun, B.; Greiner-Tollersrud, L.; Koop, B.F.; Robertsen, B. Atlantic Salmon Possesses Two Clusters of Type I Interferon Receptor Genes on Different Chromosomes, Which Allows for a Larger Repertoire of Interferon Receptors than in Zebrafish and Mammals. Dev. Comp. Immunol. 2014, 47, 275–286. [Google Scholar] [CrossRef]
- Laghari, Z.A.; Li, L.; Chen, S.N.; Huo, H.J.; Huang, B.; Zhou, Y.; Nie, P. Composition and Transcription of All Interferon Regulatory Factors (IRFs), IRF1‒11 in a Perciform Fish, the Mandarin Fish, Siniperca Chuatsi. Dev. Comp. Immunol. 2018, 81, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gong, X.Y.; Li, Y.L.; Dan, C.; Gui, J.F.; Zhang, Y.B. Characterization of DNA Binding and Nuclear Retention Identifies Zebrafish IRF11 as a Positive Regulator of IFN Antiviral Response. J. Immunol. 2020, 205, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Sun, Y.; Xu, T. Molecular Characterization of Three IRF1 Subfamily Members Reveals Evolutionary Significance of IRF11 in Miiuy Croaker. Dev. Comp. Immunol. 2015, 53, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, L.-F.; Feng, H.; Wu, N.; Chen, D.-D.; Zhang, Y.-B.; Gui, J.-F.; Nie, P.; Zhang, Y.-A. IFN Regulatory Factor 10 Is a Negative Regulator of the IFN Responses in Fish. J. Immunol. 2014, 193, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shan, S.; Zhao, H.; Liu, R.; Wang, H.; Chen, X.; Yang, G.; Li, H. Identification of an IRF10 Gene in Common Carp (Cyprinus Carpio L.) and Analysis of Its Function in the Antiviral and Antibacterial Immune Response. BMC Vet. Res. 2020, 16, 450. [Google Scholar] [CrossRef] [PubMed]
| Gene ID | Gene Name | Direction | Sequence | Annealing | Product Size (bps) | Accession |
|---|---|---|---|---|---|---|
| 100135845 | β actin 1 | Forward | GGTGGTAGGCCAGAGGC | 60 | 101 | NM_001124235.1 |
| Reverse | GGGAGAAGATGACCCAGATCATG | |||||
| 100136024 | IL1b 2 | Forward | GGAGAGGTTAAAGGGTGGCGA | 60 | 121 | XM_036979104 |
| Reverse | TGCCGACTCCAACTCCAACA | |||||
| 110494493 | MX3 | Forward | CCTCCTGAAATCAGCGAAGAC | 60 | 364 | XM_021569609.2 |
| Reverse | GAGTCTGAAGCATCTCCCTCCTG | |||||
| 100136835 | IL10 2 | Forward | CGACTTTAAATCTCCCATCGAC | 60 | 70 | NM_001245099.1 |
| Reverse | GCATTGGACGATCTCTTTCTTC | |||||
| 100135876 | VIG1 3 | Forward | GGCAACTCCAAGCAGTGTCAA | 60 | 187 | XM_021582972.2 |
| Reverse | GTCGTGTATGAAAGGCTCTCCG | |||||
| 100136064 | TNFa2 2 | Forward | GGAGGCTGTGTGGCGTTCT | 60 | 73 | NM_001124374.1 |
| Reverse | TGCTGACACCAGGCAAAGAG | |||||
| 100136017 | MMP13 2 | Forward | GCACCTTCTCTCTGCCCCGC | 60 | 235 | XM_021618131.2 |
| Reverse | AGGCTCTGTTGTGGTTTGCTGC | |||||
| 110499402 | IRF11-1 (a1) | Forward | TTGATGAGACAGCTCAAGTTTTC | 55 | 146 | XM_021576528.1 |
| Reverse | CTTAGGATCAGGTTCGTCTTTC | |||||
| 110502724 | IRF11-2 (a2) | Forward | CCAGGGGTCACCTGGCG | 62 | 169 | XM_021580977.1 |
| Reverse | TCCATGTCTTCGGATCAGGC | |||||
| 110533376 | IRF1-1 | Forward | TTACAAAATGCTGAGCGTCAG | 55 | 237 | XM_021617512.1 |
| Reverse | GTCTCCCCTACGTTGTCTGA | |||||
| 100135950 | IRF1-2 | Forward | GATGAAGAACGTCCACTCC | 55 | 231 | NM_001124293.1 |
| Reverse | AAATCATCTAGGCTGTCTGT | |||||
| 110519953 | IRF2-1 | Forward | ATGCGAATGCGACCATGGC | 55 | 195 | XM_021596860.1 |
| Reverse | GTATGAATGGCCCAGTTCTTG | |||||
| 100136151 | IRF2-2 | Forward | TGGAACAGATAAACTCTTC | 62 | 130 | NM_001124438.1 |
| Reverse | ATAAATAAAGGAGCGTCTTTC | |||||
| 100750229 | IRF3 | Forward | AGCAATGGTAGGGTTCAAGG | 60 | 179 | NM_001257262.1 |
| Reverse | CATCTGGCCACTGGAACAG | |||||
| 100499175 | IRF4-a1 | Forward | CCCACATGAGCTCAGTCAATAG | 60 | 139 | XM_021613502.1 |
| Reverse | GGGTCGGCTGAGTGGCTG | |||||
| 100499174 | IRF4-a2 | Forward | CGATCAGATTAACAGCAGTAG | 60 | 130 | NM_001310139.1 |
| Reverse | CATCCTCCTCTCGATTGTAG | |||||
| 110521762 | IRF4-b1 | Forward | GCTCGTGCAGCGAAGTCAG | 60 | 181 | XM_021599601.1 |
| Reverse | AGGCATCTGTGTCTGCAGG | |||||
| 110524663 | IRF4-b2 | Forward | TCCGGATTCGGACTACGGC | 60 | 110 | XM_021604510.1 |
| Reverse | TCTCCCACACGAGGCCTGC | |||||
| 110500261 | IRF5-1 | Forward | AGCATTACCATGGCAGCGC | 60 | 130 | XM_021577521.1 |
| Reverse | TGTTGGAGGGTCCTACCG | |||||
| 110500261 | IRF5-2 | Forward | AGCATTACCATGGCAGCGC | 60 | 130 | XM_021577521.1 |
| Reverse | TGTTGGAGGGTCCTACCG | |||||
| 110528098 | IRF6-1 | Forward | GGATGAAGATGAATCAGATGGC | 60 | 209 | XM_021609915.1 |
| Reverse | GGGACGAAGGCTGCATCTC | |||||
| 110494340 | IRF6-2 | Forward | AGACAACAAGCGCTTCAGGG | 60 | 111 | XM_021569306.1 |
| Reverse | TGGAACTTTCCTGTCTCCAC | |||||
| 100750228 | IRF7-1 (a) | Forward | AGCAATACACTGGTTTGTTC | 60 | 145 | XM_021600499 |
| Reverse | GTGGGATGCTCATTGATTTTC | |||||
| 110497044 | IRF7-2 (b) | Forward | GCCGGGTTGTGTTTTGTG | 60 | 144 | XM_021573049.1 |
| Reverse | CTTGTCATTGGGATGCGTG | |||||
| 110526480 | IRF8-1 | Forward | TGGGAGGACGACAGTCGCAC | 60 | 95 | XM_021607480.1 |
| Reverse | GCCTTGAAGATAGAGGCGTCG | |||||
| 110506608 | IRF8-2 | Forward | GTCTGGGAGGACGACAGC | 60 | 98 | XM_021586344.1 |
| Reverse | GCCTTGAAGATAGAAGCGTCT | |||||
| 110535315 | IRF9-1 | Forward | TCCGATGGGGGTCGTGTG | 60 | 160 | XM_021620234.1 |
| Reverse | CCAACACTTGTTCATTCATC | |||||
| 110489699 | IRF9-2 | Forward | TGTCTGAGGGGTGTCATGC | 60 | 146 | XM_021562471 |
| Reverse | GATGGGTACGAGGCGGTAG | |||||
| 110492403 | IRF10-1 (a) | Forward | CTTACCTGGGAGAACGAAG | 60 | 151 | XM_021566691.1 |
| Reverse | GACGAGTCTTCCAGGTG | |||||
| 110532004 | IRF10-2 (b) | Forward | ATCTGAATGAAGATGCAGCC | 60 | 172 | XM_021615574.1 |
| Reverse | CGCTCTGGGACCTCCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, T.C.; Boudinot, P.; Collet, B. Evolution of the IRF Family in Salmonids. Genes 2021, 12, 238. https://doi.org/10.3390/genes12020238
Clark TC, Boudinot P, Collet B. Evolution of the IRF Family in Salmonids. Genes. 2021; 12(2):238. https://doi.org/10.3390/genes12020238
Chicago/Turabian StyleClark, Thomas C., Pierre Boudinot, and Bertrand Collet. 2021. "Evolution of the IRF Family in Salmonids" Genes 12, no. 2: 238. https://doi.org/10.3390/genes12020238
APA StyleClark, T. C., Boudinot, P., & Collet, B. (2021). Evolution of the IRF Family in Salmonids. Genes, 12(2), 238. https://doi.org/10.3390/genes12020238






