Cdx2 Regulates Intestinal EphrinB1 through the Notch Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Stable Cell Lines
2.2. Western Blot Analysis
2.3. Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-qPCR)
2.4. Animals
2.5. Histology and Immunohistochemistry
2.6. In Situ Hybridization
2.7. Chromatin Immunoprecipitation (ChIP)-PCR
2.8. Promoter Analysis
2.9. DAPT and Valproic Acid Treatment
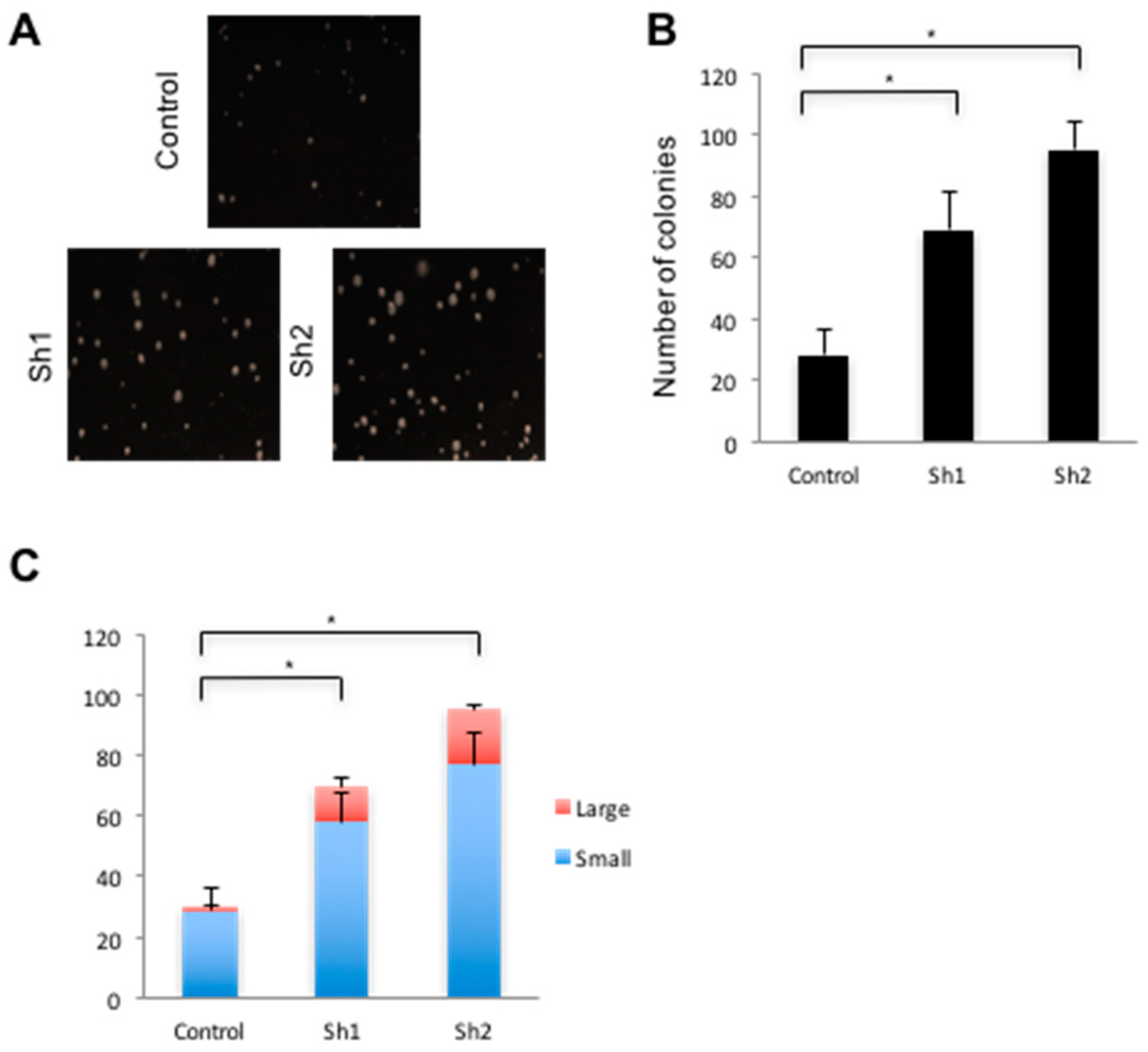
2.10. Anchorage Independent Growth Assays
3. Results
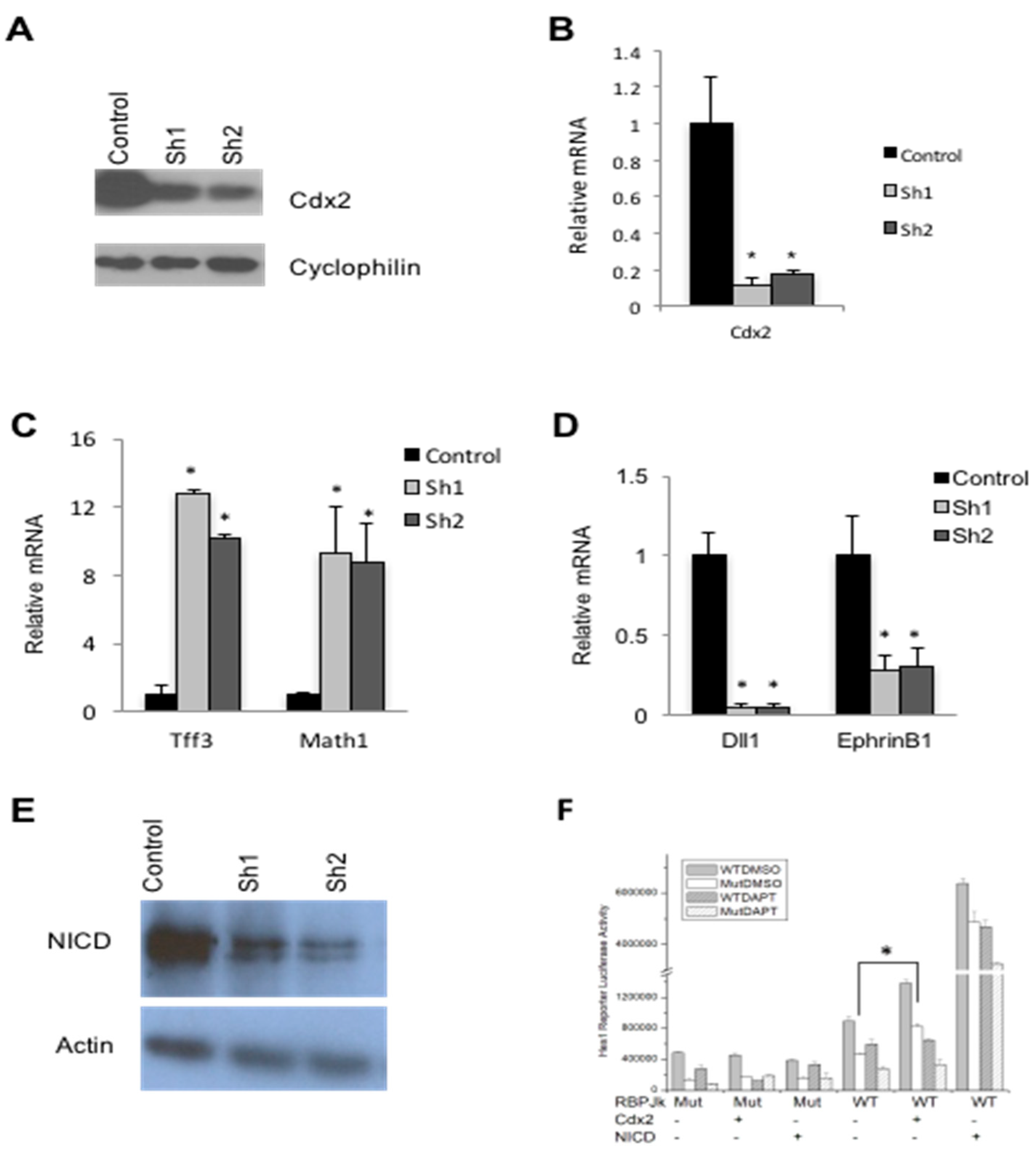
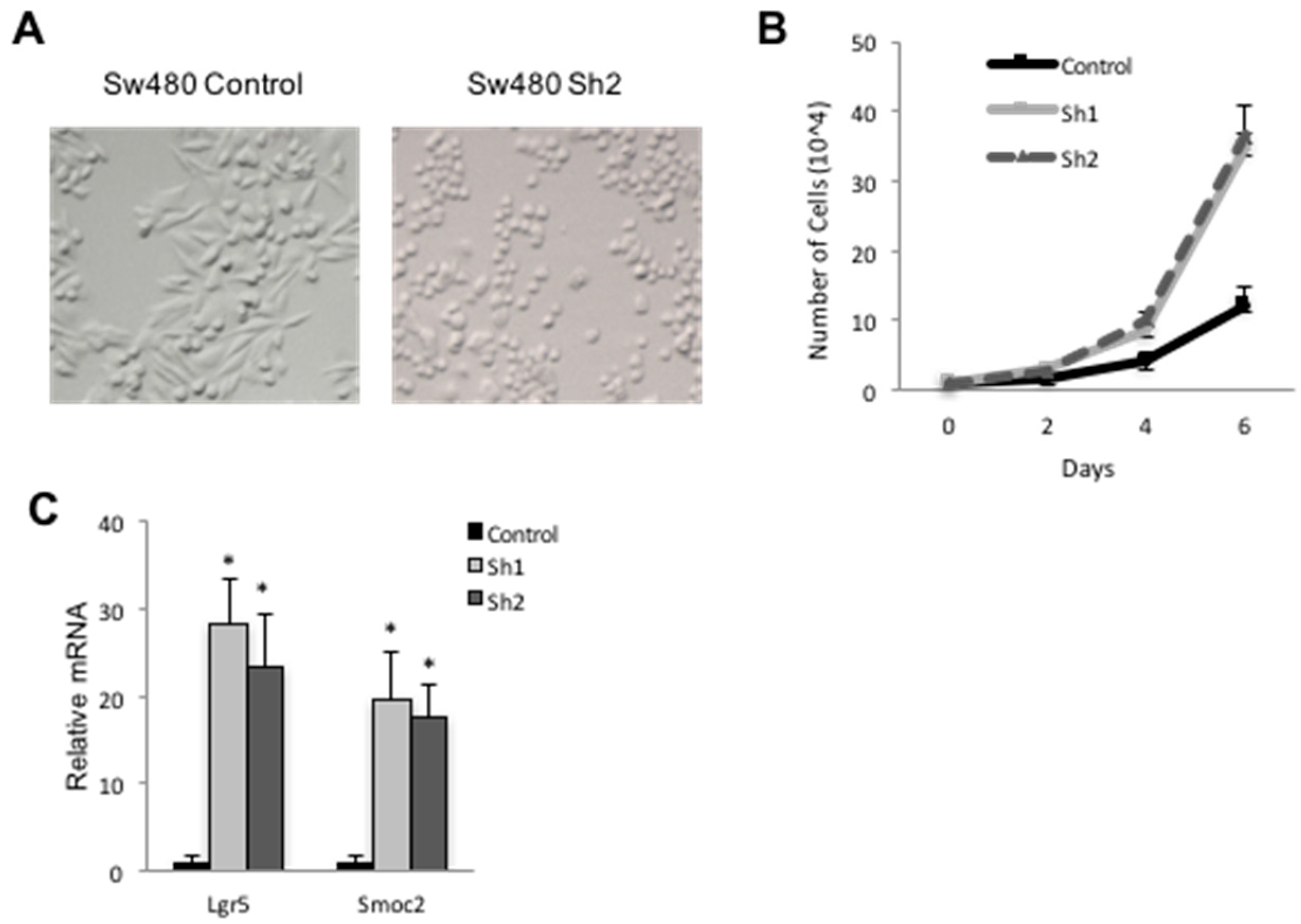
3.1. Derivation of CDX2-Deficient SW480 Cells
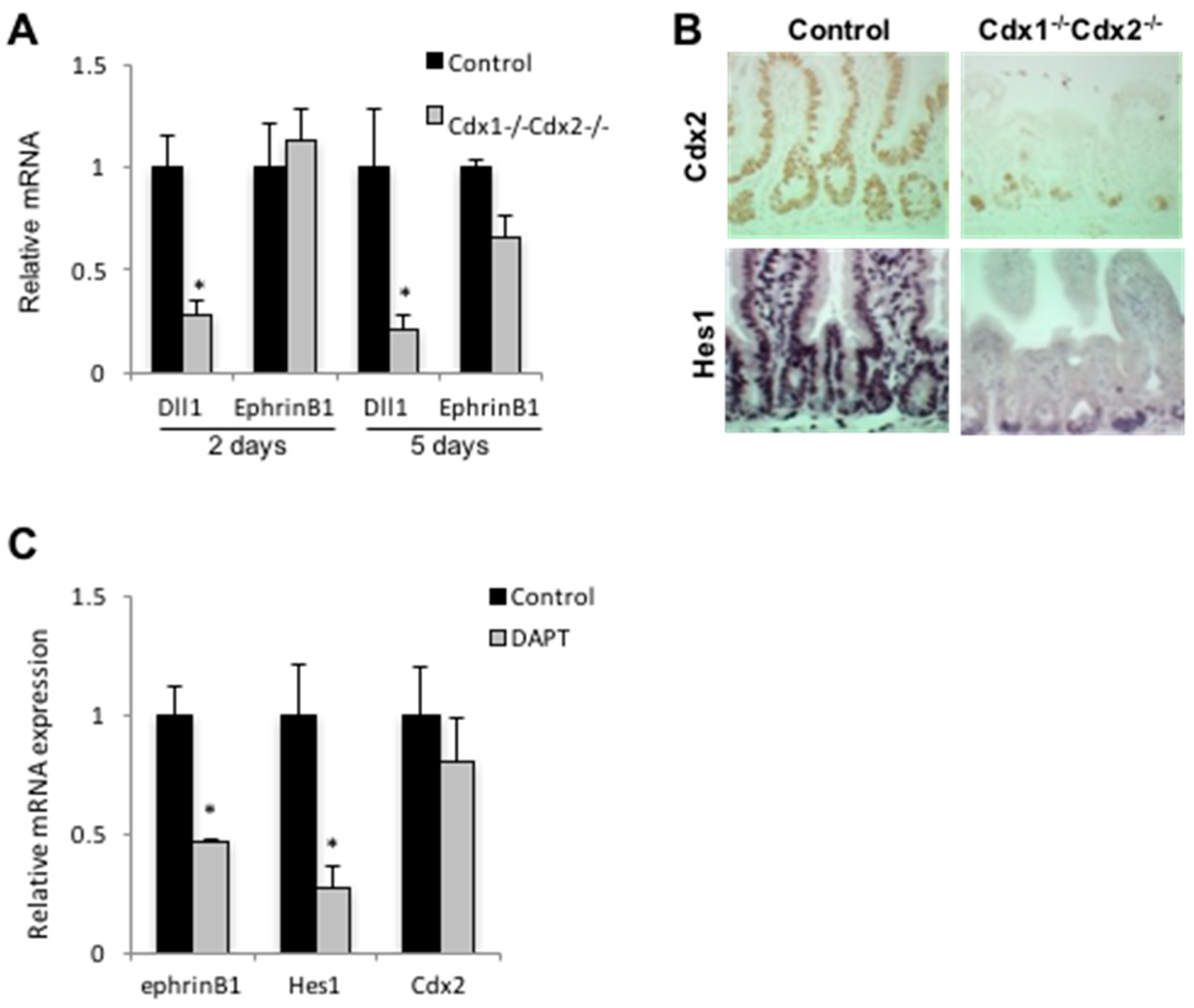
3.2. Cdx2 Impacts Notch Signaling in SW480 Cells
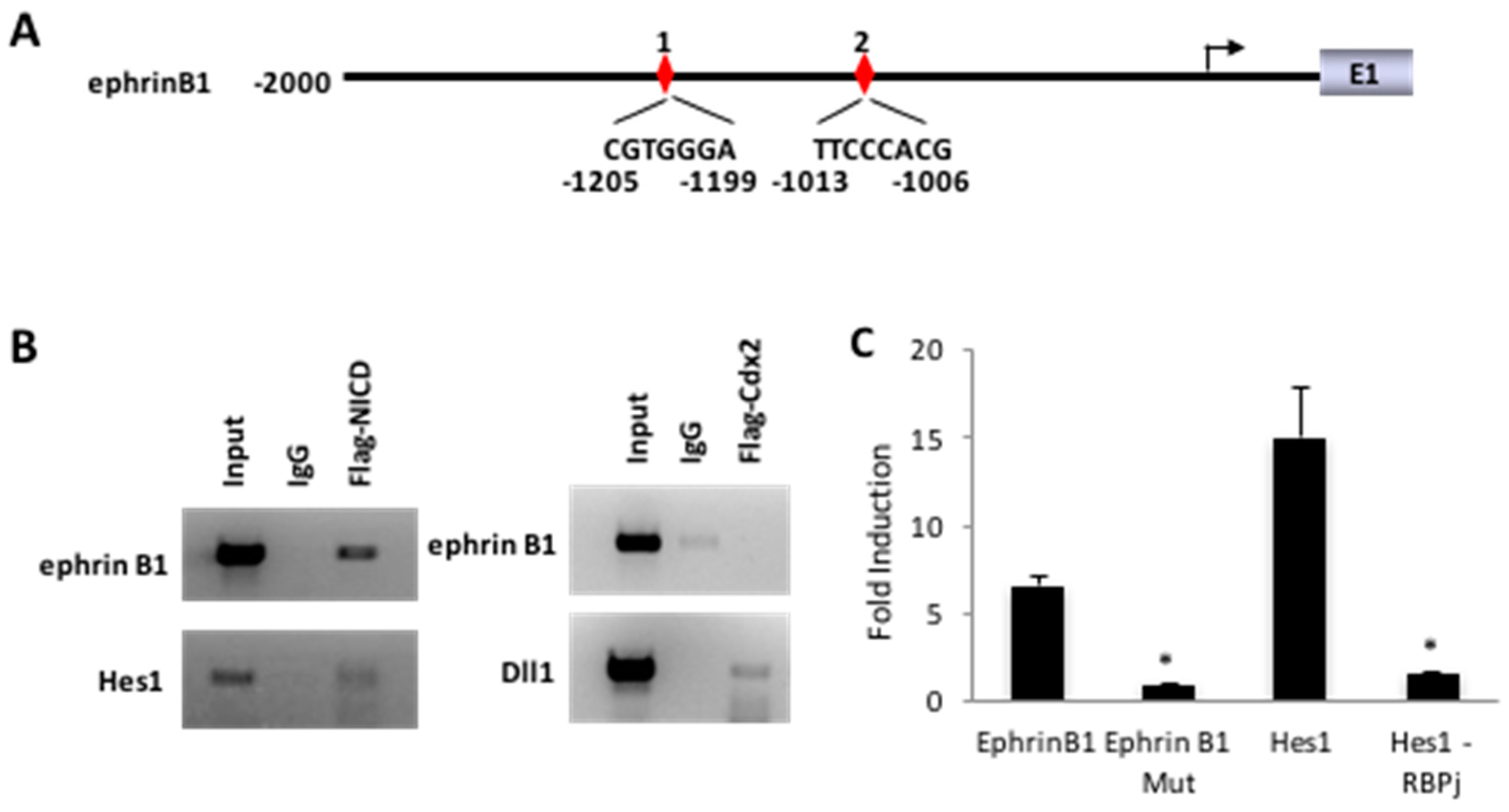
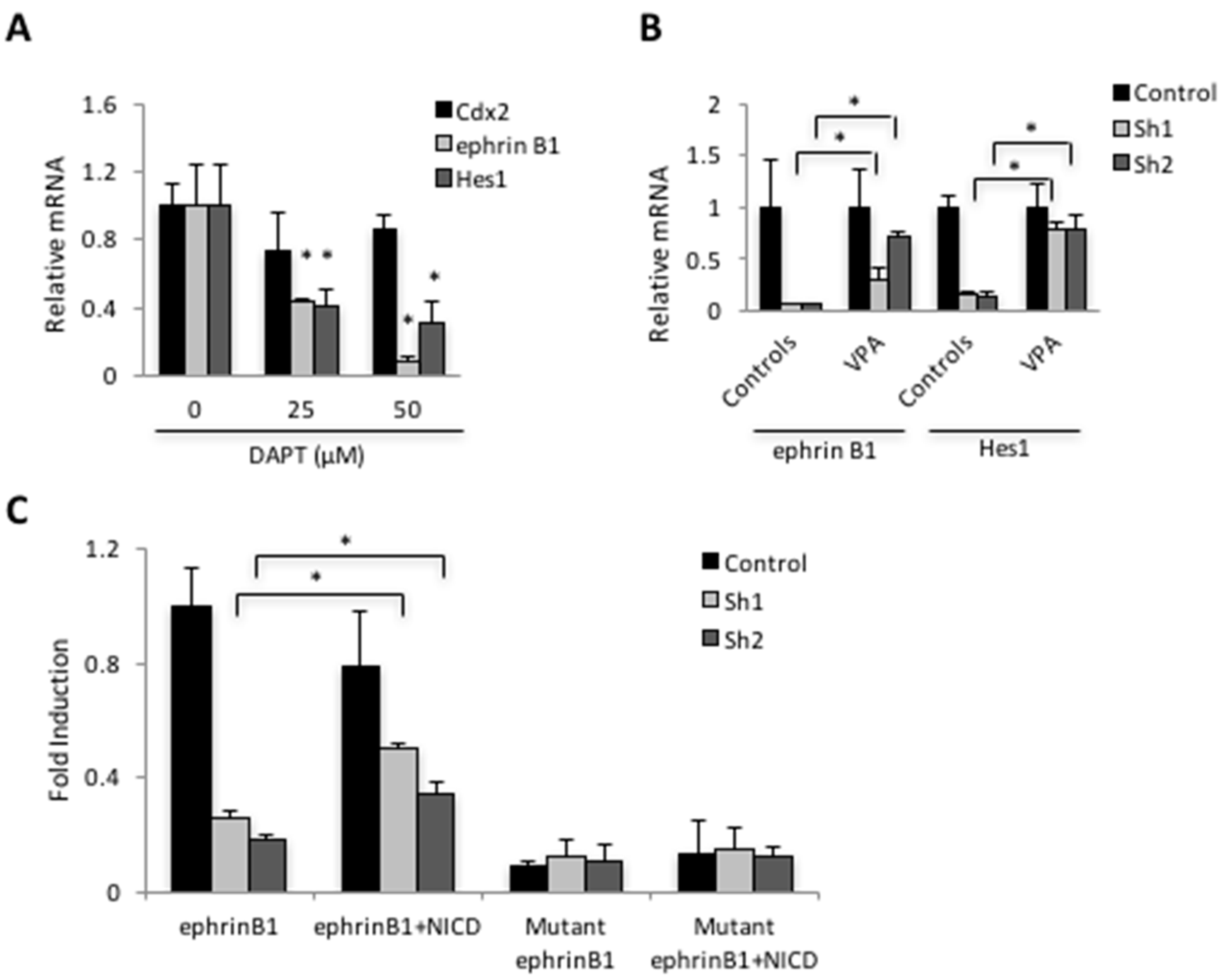
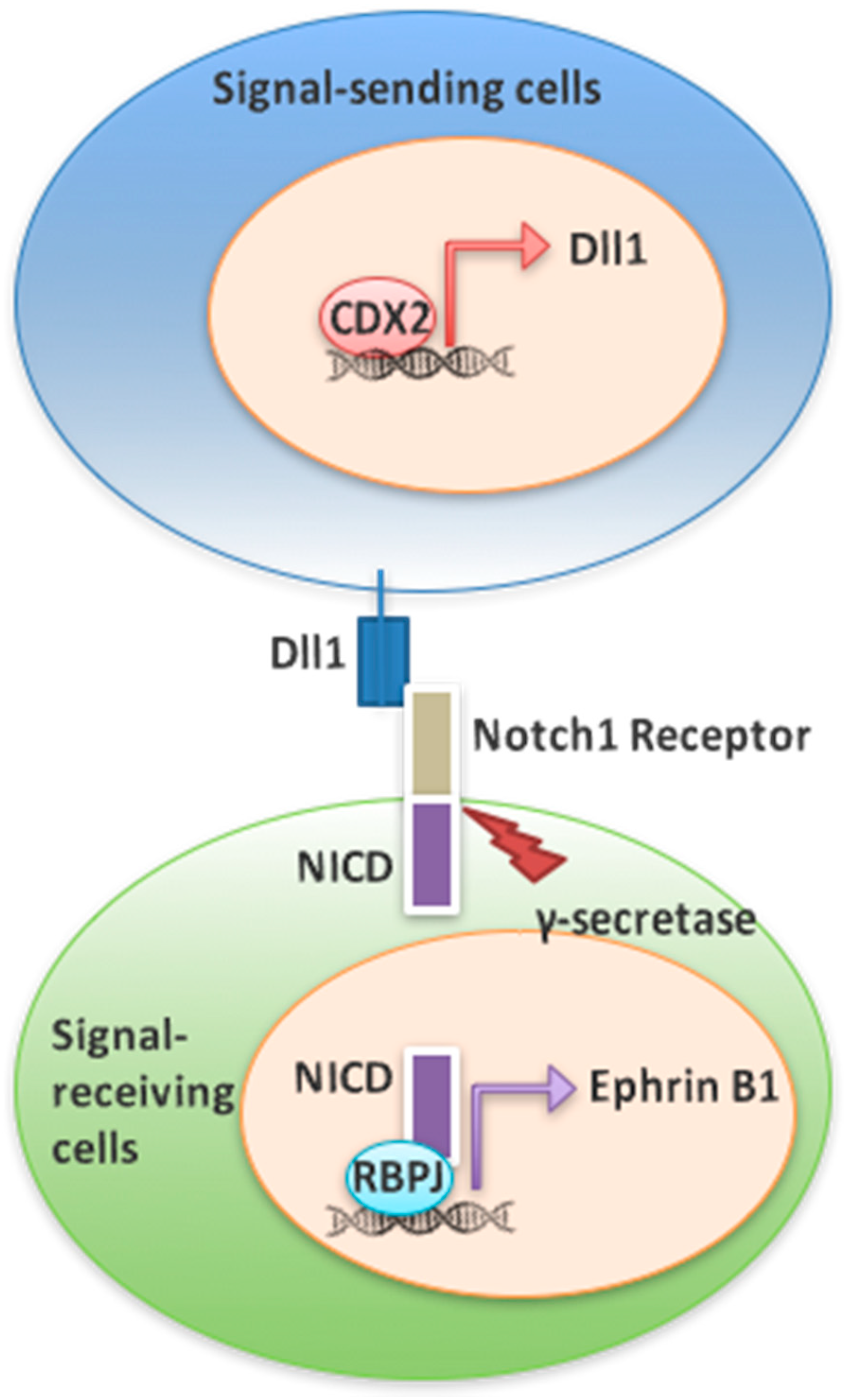
3.3. Cdx2 Regulates EphrinB1 through the Notch Pathway
3.4. Loss of Cdx2 Enhances Stem Cell Character
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangiorgi, E.; Capecchi, M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008, 40, 915–920. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Umar, S. Intestinal Stem Cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Nosho, K.; Shima, K.; Freed, E.; Irahara, N.; Philips, J.; Meyerhardt, J.A.; Hornick, J.L.; Shivdasani, R.A.; Fuchs, C.S.; et al. Relationship of CDX2 loss with molecular features and prognosis in colorectal cancer. Clin. Cancer Res. 2009, 15, 4665–4673. [Google Scholar] [CrossRef]
- Hinoi, T.; Tani, M.; Lucas, P.C.; Caca, K.; Dunn, R.L.; Macri, E.; Loda, M.; Appelman, H.D.; Cho, K.R.; Fearon, E.R. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am. J. Pathol. 2001, 159, 2239–2248. [Google Scholar] [CrossRef]
- Lugli, A.; Tzankov, A.; Zlobec, I.; Terracciano, L.M. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod. Pathol. 2008, 21, 1403–1412. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Hankey, W.; Frankel, W.L.; Groden, J. Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: Implications for therapeutic targeting. Cancer Metastasis Rev. 2018, 37, 159–172. [Google Scholar] [CrossRef]
- Dalerba, P.; Sahoo, D.; Paik, S.; Guo, X.; Yothers, G.; Song, N.; Wilcox-Fogel, N.; Forgó, E.; Rajendran, P.S.; Miranda, S.P.; et al. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. N. Engl. J. Med. 2016, 374, 211–222. [Google Scholar] [CrossRef]
- Grainger, S.; Savory, J.G.; Lohnes, D. Cdx2 regulates patterning of the intestinal epithelium. Dev. Biol. 2010, 339, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, I.; Duluc, I.; Martin, E.; Guenot, D.; Freund, J.N.; Gross, I. Cdx2 Controls Expression of the Protocadherin Mucdhl, an Inhibitor of Growth and β-Catenin Activity in Colon Cancer Cells. Gastroenterology 2012, 142, 875–885.e3. [Google Scholar] [CrossRef] [PubMed]
- Hryniuk, A.; Grainger, S.; Savory, J.G.; Lohnes, D. Cdx function is required for maintenance of intestinal identity in the adult. Dev. Biol. 2012, 363, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Verzi, M.P.; Shin, H.; He, H.H.; Sulahian, R.; Meyer, C.A.; Montgomery, R.K.; Fleet, J.C.; Brown, M.; Liu, X.S.; Shivdasani, R.A. Differentiation-Specific Histone Modifications Reveal Dynamic Chromatin Interactions and Partners for the Intestinal Transcription Factor CDX2. Dev. Cell 2014, 31, 801. [Google Scholar] [CrossRef]
- Verzi, M.P.; Shin, H.; Ho, L.L.; Liu, X.S.; Shivdasani, R.A. Essential and redundant functions of caudal family proteins in activating adult intestinal genes. Mol. Cell Biol. 2011, 31, 2026–2039. [Google Scholar] [CrossRef]
- Aoki, K.; Tamai, Y.; Horiike, S.; Oshima, M.; Taketo, M.M. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Δ716 Cdx2+/− compound mutant mice. Nat. Genet. 2003, 35, 323–330. [Google Scholar] [CrossRef]
- Bonhomme, C.; Duluc, I.; Martin, E.; Chawengsaksophak, K.; Chenard, M.P.; Kedinger, M.; Beck, F.; Freund, J.N.; Domon-Dell, C. The Cdx2 homeobox gene has a tumour suppressor function in the distal colon in addition to a homeotic role during gut development. Gut 2003, 52, 1465–1471. [Google Scholar] [CrossRef]
- Chawengsaksophak, K.; James, R.; Hammond, V.E.; Köntgen, F.; Beck, F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 1997, 386, 84–87. [Google Scholar] [CrossRef]
- Hryniuk, A.; Grainger, S.; Savory, J.G.; Lohnes, D. Cdx1 and Cdx2 function as tumor suppressors. J. Biol. Chem. 2014, 289, 33343–33354. [Google Scholar] [CrossRef]
- Pitulescu, M.E.; Adams, R.H. Eph/ephrin molecules—A hub for signaling and endocytosis. Genes Dev. 2010, 24, 2480–2492. [Google Scholar] [CrossRef]
- Grainger, S.; Lam, J.; Savory, J.G.; Mears, A.J.; Rijli, F.M.; Lohnes, D. Cdx regulates Dll1 in multiple lineages. Dev. Biol. 2012, 361, 1–11. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Savory, J.G.; Brooke-Bisschop, T.; Ringuette, R.; Foley, T.; Hess, B.L.; Mulatz, K.J.; Trinkle-Mulcahy, L.; Lohnes, D. Cdx2 Regulates Gene Expression through Recruitment of Brg1-associated Switch-Sucrose Non-fermentable (SWI-SNF) Chromatin Remodeling Activity. J. Biol. Chem. 2017, 292, 3389–3399. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. Berl. Ger. 2006, 84, 901–910. [Google Scholar] [CrossRef]
- Savory, J.G.A.; Bouchard, N.; Pierre, V.; Rijli, F.M.; De Repentigny, Y.; Kothary, R.; Lohnes, D. Cdx2 regulation of posterior development through non-HOX targets. J. Dev. 2009, 136, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Meyer, B.I.; Gruss, P. Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of HOX genes. Cell 1995, 83, 641–653. [Google Scholar] [CrossRef]
- Gregorieff, A.; Clevers, H. In situ hybridization to identify gut stem cells. Curr. Protoc. Stem Cell Biol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Béland, M.; Pilon, N.; Houle, M.; Oh, K.; Sylvestre, J.-R.; Prinos, P.; Lohnes, D. Cdx 1Autoregulation Is Governed by a Novel Cdx1-LEF1 Transcription Complex. J. Mol. Cell. Biol. 2004, 24, 5028–5038. [Google Scholar] [CrossRef]
- Ilagan, M.X.G.; Lim, S.; Fulbright, M.; Piwnica-Worms, D.; Kopan, R. Real-time imaging of notch activation with a luciferase complementation-based reporter. Sci. Signal. 2011, 4, rs7. [Google Scholar] [CrossRef]
- San Roman, A.K.; Tovaglieri, A.; Breault, D.T.; Shivdasani, R.A. Distinct Processes and Transcriptional Targets Underlie CDX2 Requirements in Intestinal Stem Cells and Differentiated Villus Cells. Stem Cell Rep. 2015, 5, 673–681. [Google Scholar] [CrossRef]
- van Es, J.H.; de Geest, N.; van de Born, M.; Clevers, H.; Hassan, B.A. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat. Commun. 2010, 1, 18. [Google Scholar] [CrossRef]
- van Es, J.H.; van Gijn, M.E.; Riccio, O.; van den Born, M.; Vooijs, M.; Begthel, H.; Cozijnsen, M.; Robine, S.; Winton, D.J.; Radtke, F.; et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005, 435, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev. Cell 2017, 41, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Weerkamp, F.; Luis, T.C.; Naber, B.A.E.; Koster, E.E.L.; Jeannotte, L.; van Dongen, J.J.M.; Staal, F.J.T. Identification of Notch target genes in uncommitted T-cell progenitors: No direct induction of a T-cell specific gene program. Leukemia 2006, 20, 1967–1977. [Google Scholar] [CrossRef][Green Version]
- Dovey, H.F.; John, V.; Anderson, J.P.; Chen, L.Z.; De Saint Andrieu, P.; Fang, L.Y.; Freedman, S.B.; Folmer, B.; Goldbach, E.; Holsztynska, E.J.; et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J. Neurochem. 2001, 76, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Lim, H.S.; Chang, H.J.; Yoon, M.J.; Choi, Y.; Kong, M.P.; Kim, C.H.; Kim, J.M.; Park, J.G.; Kong, Y.Y. Notch signaling promotes the generation of EphrinB1-positive intestinal epithelial cells. Gastroenterology 2009, 137, 145–155.e3. [Google Scholar] [CrossRef] [PubMed]
- Suksaweang, S.; Jiang, T.-X.; Roybal, P.; Chuong, C.-M.; Widelitz, R. Roles of EphB3/ephrin-B1 in feather morphogenesis. Int. J. Dev. Biol. 2012, 56, 719–728. [Google Scholar] [CrossRef]
- Greenblatt, D.Y.; Vaccaro, A.M.; Jaskula-Sztul, R.; Ning, L.; Haymart, M.; Kunnimalaiyaan, M.; Chen, H. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist 2007, 12, 942–951. [Google Scholar] [CrossRef]
- Kim, H.A.; Koo, B.K.; Cho, J.H.; Kim, Y.Y.; Seong, J.; Chang, H.J.; Oh, Y.M.; Stange, D.E.; Park, J.G.; Hwang, D.; et al. Notch1 counteracts WNT/β-catenin signaling through chromatin modification in colorectal cancer. J. Clin. Investig. 2012, 122, 3248–3259. [Google Scholar] [CrossRef]
- Fre, S.; Pallavi, S.K.; Huyghe, M.; Laé, M.; Janssen, K.-P.; Robine, S.; Artavanis-Tsakonas, S.; Louvard, D. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc. Natl. Acad. Sci. USA 2009, 106, 6309–6314. [Google Scholar] [CrossRef]
- Tomita, N.; Jiang, W.; Hibshoosh, H.; Warburton, D.; Kahn, S.M.; Weinstein, I.B. Isolation and characterization of a highly malignant variant of the SW480 human colon cancer cell line. Cancer Res. 1992, 52, 6840–6847. [Google Scholar]
- Jägle, S.; Rönsch, K.; Timme, S.; Andrlová, H.; Bertrand, M.; Jäger, M.; Proske, A.; Schrempp, M.; Yousaf, A.; Michoel, T.; et al. Silencing of the EPHB3 tumor-suppressor gene in human colorectal cancer through decommissioning of a transcriptional enhancer. Proc. Natl. Acad. Sci. USA 2014, 111, 4886–4891. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, S.; Fingerle, A.A.; Cammareri, P.; Nebelsiek, T.; Göktuna, S.I.; Ziegler, P.K.; Canli, O.; Heijmans, J.; Huels, D.J.; Moreaux, G.; et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 2013, 152, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Rispal, J.; Escaffit, F.; Trouche, D. Chromatin Dynamics in Intestinal Epithelial Homeostasis: A Paradigm of Cell Fate Determination versus Cell Plasticity. Stem Cell Rev. Rep. 2020, 16, 1062–1080. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, T.; Kageyama, N.; Watanabe, H. Identifying target genes regulated downstream of Cdx2 by microarray analysis. J. Mol. Biol. 2004, 337, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinet, L.; Rodilla, V.; Liu, Z.; Chen, S.; Koch, U.; Espinosa, L.; Kaestner, K.H.; Kopan, R.; Lewis, J.; Radtke, F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 2011, 140, 1230–1240.e7. [Google Scholar] [CrossRef] [PubMed]
- Rodilla, V.; Villanueva, A.; Obrador-Hevia, A.; Robert-Moreno, A.; Fernández-Majada, V.; Grilli, A.; López-Bigas, N.; Bellora, N.; Albà, M.M.; Torres, F.; et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 6315–6320. [Google Scholar] [CrossRef]
- Guo, R.-J.; Funakoshi, S.; Lee, H.H.; Kong, J.; Lynch, J.P. The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin-TCF protein complex. Carcinogenesis 2010, 31, 159–166. [Google Scholar] [CrossRef]
- Natoli, M.; Christensen, J.; El-Gebali, S.; Felsani, A.; Anderle, P. The role of CDX2 in Caco-2 cell differentiation. Eur. J. Pharm. Biopharm. 2013, 85, 20–25. [Google Scholar] [CrossRef]
- Gross, I.; Duluc, I.; Benameur, T.; Calon, A.; Martin, E.; Brabletz, T.; Kedinger, M.; Domon-Dell, C.; Freund, J.N. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene 2008, 27, 107–115. [Google Scholar] [CrossRef][Green Version]
- Domon-Dell, C.; Schneider, A.; Moucadel, V.; Guerin, E.; Guenot, D.; Aguillon, S.; Duluc, I.; Martin, E.; Iovanna, J.; Launay, J.-F.; et al. Cdx1 homeobox gene during human colon cancer progression. Oncogene 2003, 22, 7913–7921. [Google Scholar] [CrossRef]
- Salari, K.; Spulak, M.E.; Cuff, J.; Forster, A.D.; Giacomini, C.P.; Huang, S.; Ko, M.E.; Lin, A.Y.; van de Rijn, M.; Pollack, J.R. CDX2 is an amplified lineage-survival oncogene in colorectal cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E3196–E3205. [Google Scholar] [CrossRef] [PubMed]
- Subtil, C.; Guérin, E.; Schneider, A.; Chenard, M.P.; Martin, E.; Domon-Dell, C.; Duluc, I.; Brabletz, T.; Kedinger, M.; Duclos, B.; et al. Frequent rearrangements and amplification of the CDX2 homeobox gene in human sporadic colorectal cancers with chromosomal instability. Cancer Lett. 2007, 247, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Witek, M.E.; Nielsen, K.; Walters, R.; Hyslop, T.; Palazzo, J.; Schulz, S.; Waldman, S.A. The putative tumor suppressor Cdx2 is overexpressed by human colorectal adenocarcinomas. Clin. Cancer Res. 2005, 11, 8549–8556. [Google Scholar] [CrossRef] [PubMed]
- Pilozzi, E.; Onelli, M.R.; Ziparo, V.; Mercantini, P.; Ruco, L. CDX1 expression is reduced in colorectal carcinoma and is associated with promoter hypermethylation. J. Pathol. 2004, 204, 289–295. [Google Scholar] [CrossRef]
- Wong, N.A.C.S.; Britton, M.P.; Choi, G.S.; Stanton, T.K.; Bicknell, D.C.; Wilding, J.L.; Bodmer, W.F. Loss of CDX1 expression in colorectal carcinoma: Promoter methylation, mutation, and loss of heterozygosity analyses of 37 cell lines. Proc. Natl. Acad. Sci. USA 2004, 101, 574–579. [Google Scholar] [CrossRef]
- Peignon, G.; Durand, A.; Cacheux, W.; Ayrault, O.; Terris, B.; Laurent-Puig, P.; Shroyer, N.F.; Van Seuningen, I.; Honjo, T.; Perret, C.; et al. Complex interplay between β-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut 2011, 60, 166–176. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Hryniuk, A.; Foley, T.; Hess, B.; Lohnes, D. Cdx2 Regulates Intestinal EphrinB1 through the Notch Pathway. Genes 2021, 12, 188. https://doi.org/10.3390/genes12020188
Zhu Y, Hryniuk A, Foley T, Hess B, Lohnes D. Cdx2 Regulates Intestinal EphrinB1 through the Notch Pathway. Genes. 2021; 12(2):188. https://doi.org/10.3390/genes12020188
Chicago/Turabian StyleZhu, Yalun, Alexa Hryniuk, Tanya Foley, Bradley Hess, and David Lohnes. 2021. "Cdx2 Regulates Intestinal EphrinB1 through the Notch Pathway" Genes 12, no. 2: 188. https://doi.org/10.3390/genes12020188
APA StyleZhu, Y., Hryniuk, A., Foley, T., Hess, B., & Lohnes, D. (2021). Cdx2 Regulates Intestinal EphrinB1 through the Notch Pathway. Genes, 12(2), 188. https://doi.org/10.3390/genes12020188




