Aphids and Ants, Mutualistic Species, Share a Mariner Element with an Unusual Location on Aphid Chromosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material, DNA Extraction, PCR Amplification and Cloning
2.2. Sequence Analyses and Molecular Evolutionary Analyses
2.3. Chromosome Preparation, Ag-Stain Technique and In Situ Hybridization Procedures
3. Results
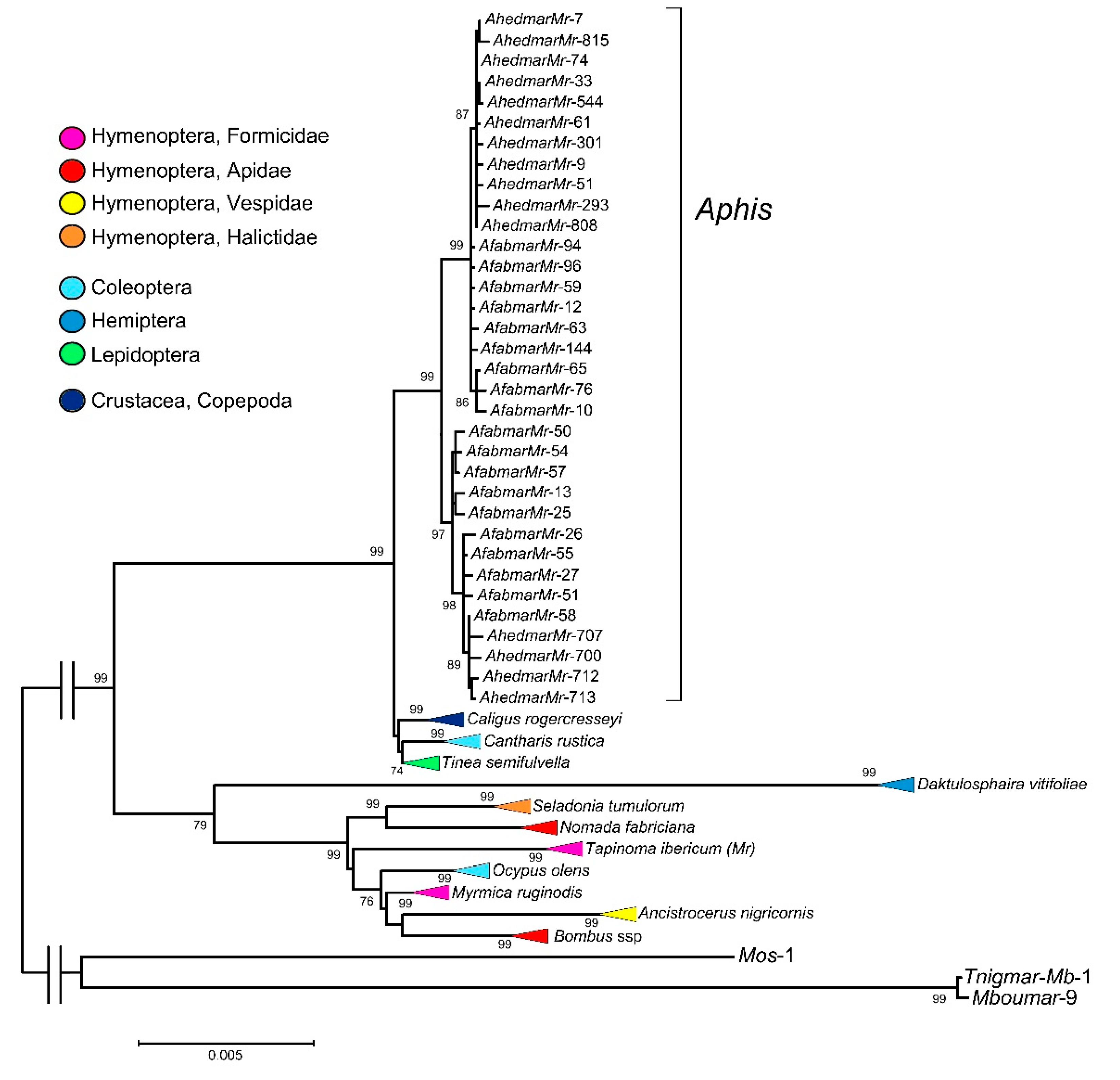
3.1. Isolation of Mariner Elements from A. fabae and A. hederae Genomes, Sequence and Phylogenetic Analyses
3.2. Localization of Transposable Elements by FISH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vantaux, A.; Billen, J.; Wenseleers, T. Levels of clonal mixing in the black bean aphid Aphis fabae, a facultative ant mutualist. Mol. Ecol. 2011, 20, 4772–4785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidolin, A.S.; Cônsoli, F.L. Diversity of the most commonly reported facultative symbionts in two closely-related aphids with different host ranges. Neotrop. Entomol. 2018, 47, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Chen, Z.; Li, Q.; Deng, J.; Lin, X.; Huang, X. DNA barcoding of aphid-associated ants (Hymenoptera, Formicidae) in a subtropical area of southern China. ZooKeys 2019, 879, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Wamonje, F.O.; Michuki, G.N.; Braidwood, L.A.; Njuguna, J.N.; Musembi Mutuku, J.; Djikeng, A.; Harvey, J.; Carr, J.P. Viral metagenomics of aphids present in bean and maize plots on mixed-use farms in Kenya reveals the presence of three dicistroviruses including a novel Big Sioux River virus-like dicistrovirus. Virol. J. 2017, 14, 188. [Google Scholar] [CrossRef] [Green Version]
- Wamonje, F.O.; Tungadi, T.D.; Murphy, A.M.; Pate, A.E.; Woodcock, C.; Caulfield, J.C.; Mutuku, J.M.; Cunniffe, N.J.; Bruce, T.; Gilligan, C.A.; et al. Three aphid-transmitted viruses encourage vector migration from infected common bean (Phaseolus vulgaris) plants through a combination of volatile and surface cues. Front. Plant Sci. 2020, 11, 613772. [Google Scholar] [CrossRef] [PubMed]
- Novgorodova, T.A. Quarantining behaviour in ants: Are Myrmica aphid milkers able to detect and get rid of fungus-infected aphids? Entomol. Exp. Appl. 2020, 168, 869–877. [Google Scholar] [CrossRef]
- Mandrioli, M.; Manicardi, G.C. Holocentric chromosomes. PLoS Genet. 2020, 16, e1008918. [Google Scholar] [CrossRef] [PubMed]
- Monti, V.; Manicardi, G.C.; Mandrioli, M. Cytogenetic and molecular analysis of the holocentric chromosomes of the potato aphid Macrosiphum euphorbiae (Thomas, 1878). Comp. Cytogenet. 2011, 5, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monti, V.; Mandrioli, M.; Rivi, M.; Manicardi, G.C. The vanishing clone: Karyotypic evidence for extensive intraclonal genetic variation in the peach potato aphid, Myzus persicae (Hemiptera: Aphididae). Biol. J. Linnean Soc. 2012, 105, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Monti, V.; Serafini, C.; Manicardi, G.C.; Mandrioli, M. Characterization of non-LTR retrotransposable TRAS elements in the aphids Acyrthosiphon pisum and Myzus persicae (Aphididae, Hemiptera). J. Hered. 2013, 104, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaquiéry, J.; Rispe, C.; Roze, D.; Legeai, F.; Le Trionnaire, G.; Stoeckel, S.; Mieuzet, L.; Da Silva, C.; Poulain, J.; Prunier-Leterme, N.; et al. Masculinization of the X chromosome in the pea aphid. PLoS Genet. 2013, 9, e1003690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manicardi, G.C.; Mandrioli, M.; Blackman, R.L. The cytogenetic architecture of the aphid genome. Biol. Rev. Camb. Philos. Soc. 2015, 90, 112–125. [Google Scholar] [CrossRef]
- Gavrilov-Zimin, I.A.; Stekolshchikov, A.V.; Gautam, D.C. General trends of chromosomal evolution in Aphidococca (Insecta, Homoptera, Aphidinea + Coccinea). Comp. Cytogenet. 2015, 9, 335–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caillaud, M.C.; Boutin, M.; Braendle, C.; Simon, J.C. A sex-linked locus controls wing polymorphism in males of the pea aphid, Acyrthosiphon pisum (Harris). Heredity 2002, 89, 346–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.W. Inbreeding, male viability, and the remarkable evolutionary stability of the aphid X chromosome. Heredity 2021, 127, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Barberà, M.; Escrivá, L.; Collantes-Alegre, J.M.; Meca, G.; Rosato, E.; Martínez-Torres, D. Melatonin in the seasonal response of the aphid Acyrthosiphon pisum. Insect Sci. 2020, 27, 224–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaquiéry, J.; Peccoud, J.; Ouisse, T.; Legeai, F.; Prunier-Leterme, N.; Gouin, A.; Nouhaud, P.; Brisson, J.A.; Bickel, R.; Purandare, S.; et al. Disentangling the causes for faster-X evolution in aphids. Genome Biol. Evol. 2018, 10, 507–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, H.M.; Robertson, E.; Walden, K.; Enders, L.S.; Miller, N.J. The chemoreceptors and odorant binding proteins of the soybean and pea aphids. Insect Biochem. Mol. Biol. 2019, 105, 69–78. [Google Scholar] [CrossRef]
- Mathers, T.C.; Wouters, R.; Mugford, S.T.; Swarbreck, D.; van Oosterhout, C.; Hogenhout, S.A. Chromosome-scale genome assemblies of aphids reveal extensively rearranged autosomes and long-term conservation of the X chromosome. Mol. Biol. Evol. 2021, 38, 856–875. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Moran, N.A. The aphid X Chromosome is a dangerous place for functionally important genes: Diverse evolution of hemipteran genomes based on chromosome-level assemblies. Mol. Biol. Evol. 2020, 37, 2357–2368. [Google Scholar] [CrossRef] [Green Version]
- Kapitonov, V.V.; Jurka, J. A universal classification of eukaryotic transposable elements implemented in Repbase. Nat. Rev. Genet. 2008, 9, 411–414. [Google Scholar] [CrossRef]
- Jacobson, J.W.; Medhora, M.M.; Hartl, D.L. Molecular structure of a somatically unstable transposable element in Drosophila. Proc. Natl. Acad. Sci. USA 1986, 83, 8684–8688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, H.M. The Tc1-mariner superfamily of transposons in animals. J. Insect Physiol. 1995, 41, 99–105. [Google Scholar] [CrossRef]
- Bradic, M.; Warring, S.D.; Low, V.; Carlton, J.M. The Tc1/mariner transposable element family shapes genetic variation and gene expression in the protist Trichomonas vaginalis. Mob. DNA 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotto, B.R.; Carvalho, E.L.; da Silva, A.F.; Dezordi, F.Z.; Pinto, P.M.; Campos, T.L.; Rezende, A.M.; Wallau, G. HTT-DB: New features and updates. Database J. Biol. Databases Curation 2018, 2018, bax102. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, I.R.; Yushenova, I.A. Giant transposons in eukaryotes: Is bigger better? Genome Biol. Evol. 2019, 11, 906–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, K.K. Structural and sequence diversity of eukaryotic transposable elements. Genes Genet. Syst. 2020, 94, 233–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, C.; Peccoud, J.; Cordaux, R. Transposable elements and the evolution of insects. Annu. Rev. Entomol. 2021, 66, 355–372. [Google Scholar] [CrossRef]
- Lorite, P.; Maside, X.; Sanllorente, O.; Torres, M.I.; Periquet, G.; Palomeque, T. The ant genomes have been invaded by several types of mariner transposable elements. Naturwissenschaften 2012, 99, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Palomeque, T.; Sanllorente, O.; Maside, X.; Vela, J.; Mora, P.; Torres, M.I.; Periquet, G.; Lorite, P. Evolutionary history of the Azteca-like mariner transposons and their host ants. Naturwissenschaften 2015, 102, 44. [Google Scholar] [CrossRef] [PubMed]
- Sanllorente, O.; Vela, J.; Mora, P.; Ruiz-Mena, A.; Torres, M.I.; Lorite, P.; Palomeque, T. Complex evolutionary history of Mboumar, a mariner element widely represented in ant genomes. Sci. Rep. 2020, 10, 2610. [Google Scholar] [CrossRef]
- Filée, J.; Rouault, J.D.; Harry, M.; Hua-Van, A. Mariner transposons are sailing in the genome of the blood-sucking bug Rhodnius prolixus. BMC Genom. 2015, 16, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, C.; Cordaux, R. Viruses as vectors of horizontal transfer of genetic material in eukaryotes. Curr. Opin. Virol. 2017, 25, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Feschotte, C. Horizontal acquisition of transposable elements and viral sequences: Patterns and consequences. Curr. Opin. Genet. Dev. 2018, 49, 15–24. [Google Scholar] [CrossRef]
- Krieger, M.J.; Ross, K.G. Molecular evolutionary analyses of mariners and other transposable elements in fire ants (Hymenoptera: Formicidae). Insect Mol. Biol. 2003, 12, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bigot, Y.; Hamelin, M.H.; Capy, P.; Periquet, G. Mariner-like elements in hymenopteran species: Insertion site and distribution. Proc. Natl. Acad. Sci. USA 1994, 91, 3408–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouleux-Bonnin, F.; Petit, A.; Demattei, M.V.; Bigot, Y. Evolution of full-length and deleted forms of the mariner-like element, Botmar1, in the Genome of the bumble bee, Bombus terrestris (Hymenoptera: Apidae). J. Mol. Evol. 2005, 60, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Palomeque, T.; Antonio Carrillo, J.; Muñoz-López, M.; Lorite, P. Detection of a mariner-like element and a miniature inverted-repeat transposable element (MITE) associated with the heterochromatin from ants of the genus Messor and their possible involvement for satellite DNA evolution. Gene 2006, 371, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Combet, C.; Blanchet, C.; Geourjon, C.; Deléage, G. NPS@: Network protein sequence analysis. Trends Biochem. Sci. 2000, 25, 147–150. [Google Scholar] [CrossRef]
- Seifert, B.; D’Eustacchio, D.; Kaufmann, B.; Centorame, M.; Lorite, P.; Modica, M.V. Four species within the supercolonial ants of the Tapinoma nigerrimum complex revealed by integrative taxonomy (Hymenoptera: Formicidae). Myrmecol. News 2017, 24, 107–122. [Google Scholar] [CrossRef]
- Manicardi, G.C.; Bizzaro, D.; Galli, E.; Bianchi, U. Heterochromatin heterogeneity in the holocentric X chromatin of Megoura viciae (Homoptera, Aphididae). Genome 1996, 39, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Rufas, J.S.; Iturra, P.; Desouza, W.; Esponda, P. Simple silver staining procedures for the location of nucleolus and nucleolar organizer under light and electron microscopy. Arch. Biol. 1982, 93, 267–274. [Google Scholar]
- Endow, S.A. Polytenization of the ribosomal genes on the X and Y chromosomes of Drosophila melanogaster. Genetics 1982, 100, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Palomeque, T.; Muñoz-López, M.; Carrillo, J.A.; Lorite, P. Characterization and evolutionary dynamics of a complex family of satellite DNA in the leaf beetle Chrysolina carnifex (Coleoptera, Chrysomelidae). Chromosome Res. 2005, 13, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.M.; Asplund, M.L. Bmmar1: A basal lineage of the mariner family of transposable elements in the silkworm moth, Bombyx mori. Insect. Biochem. Mol. Biol. 1996, 26, 945–954. [Google Scholar] [CrossRef]
- Muñoz-López, M.; Siddique, A.; Bischerour, J.; Lorite, P.; Chalmers, R.; Palomeque, T. Transposition of Mboumar-9: Identification of a new naturally active mariner-family transposon. J. Mol. Biol. 2008, 382, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dornan, J.; Grey, H.; Richardson, J.M. Structural role of the flanking DNA in mariner transposon excision. Nucleic Acids Res. 2015, 43, 2424–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rispe, C.; Legeai, F.; Nabity, P.D.; Fernández, R.; Arora, A.K.; Baa-Puyoulet, P.; Banfill, C.R.; Bao, L.; Barberà, M.; Bouallegue, M.; et al. The genome sequence of the grape phylloxera provides insights into the evolution, adaptation, and invasion routes of an iconic pest. BMC Biol. 2000, 18, 90. [Google Scholar] [CrossRef]
- Blackman, R.L. Chromosome numbers in the Aphididae and their taxonomic significance. Syst. Entomol. 1980, 5, 7–25. [Google Scholar] [CrossRef]
- Bakhtadze, N.; Kintsurashvili, N.; Bakhtadze, G.; Barjadze, S.; Zhukovskaya, N.; Chakvetadze, N. Karyological study of three species of the genus Aphis (Hemiptera: Aphididae) from Georgia. Bull. Georg. Natl. Acad. Sci. 2010, 4, 130–132. [Google Scholar]
- Marco, R.; Cassanelli, S.; Mazzoni, E.; Bizzaro, D.; Manicardi, G.C. Heterochromatin and rDNA localization on the holocentric chromosomes of black bean aphid, Aphis fabae Scop. (Hemiptera: Aphididae). Caryologia 2009, 62, 341–346. [Google Scholar] [CrossRef]
- Rivi, M.; Monti, V.; Mazzoni, E.; Cassanelli, S.; Panini, M.; Bizzaro, D.; Mandrioli, M.; Manicardi, G.C. Karyotype variations in Italian populations of the peach-potato aphid Myzus persicae (Hemiptera: Aphididae). Bull. Entomol. Res. 2012, 102, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, O.; Rivera-Vega, L.; Bhandary, B.; Bautista, M.A.; Mamidala, P.; Michel, A.P.; Shukle, R.H.; Mian, M.A. Cloning and characterization of mariner-like elements in the soybean aphid, Aphis glycines Matsumura. Bull. Entomol. Res. 2011, 101, 697–704. [Google Scholar] [CrossRef]
- Kharrat, I.; Mezghani, M.; Casse, N.; Denis, F.; Caruso, A.; Makni, H.; Capy, P.; Rouault, J.D.; Chénais, B.; Makni, M. Characterization of mariner-like transposons of the mauritiana subfamily in seven tree aphid species. Genetica 2015, 143, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bouallègue, M.; Filée, J.; Kharrat, I.; Mezghani-Khemakhem, M.; Rouault, J.D.; Makni, M.; Capy, P. Diversity and evolution of mariner-like elements in aphid genomes. BMC Genom. 2017, 18, 494. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S. A molecular phylogeny of the tribe Aphidini (Insecta: Hemiptera: Aphididae) based on the mitochondrial tRNA/COII, 12S/16S and the nuclear EF1a genes. Syst. Entomol. 2008, 33, 711–721. [Google Scholar] [CrossRef]
- Lagos, D.M.; Voegtlin, D.J.; Coeur d’acier, A.; Giordano, R. Aphis (Hemiptera: Aphididae) species groups found in the Midwestern United States and their contribution to the phylogenetic knowledge of the genus. Insect Sci. 2014, 21, 374–391. [Google Scholar] [CrossRef]
- Pedersen, V. European bumblebees (Hymenoptera: Bombini)—Phylogenetic relationships inferred from DNA sequences. Insect Syst. Evol. 2002, 33, 361–386. [Google Scholar] [CrossRef]
- Dupeyron, M.; Leclercq, S.; Cerveau, N.; Bouchon, D.; Gilbert, C. Horizontal transfer of transposons between and within crustaceans and insects. Mob. DNA 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, E.S.; Wallau, G.L. Mosquito genomes are frequently invaded by transposable elements through horizontal transfer. PLoS Genet. 2020, 16, e1008946. [Google Scholar] [CrossRef] [PubMed]
- Peccoud, J.; Loiseau, V.; Cordaux, R.; Gilbert, C. Massive horizontal transfer of transposable elements in insects. Proc. Natl. Acad. Sci. USA 2017, 114, 4721–4726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrioli, M.; Melchiori, G.; Panini, M.; Chiesa, O.; Giordano, R.; Mazzoni, E.; Manicardi, G.C. Analysis of the extent of synteny and conservation in the gene order in aphids: A first glimpse from the Aphis glycines genome. Insect Biochem. Mol. Biol. 2019, 113, 103228. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, J.G.; Wolf, I.R.; Vilas-Boas, L.A.; Heslop-Harrison, J.S.; Schwarzacher, T.; Dias, A.L. Repetitive DNA in the catfish genome: rDNA, microsatellites, and Tc1-mariner transposon sequences in Imparfinis species (Siluriformes, Heptapteridae). J. Hered. 2017, 108, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Montiel, E.E.; Cabrero, J.; Camacho, J.P.; López-León, M.D. Gypsy, RTE and mariner transposable elements populate Eyprepocnemis plorans genome. Genetica 2012, 140, 365–374. [Google Scholar] [CrossRef]
- Amorim, I.C.; Sotero-Caio, C.G.; Costa, R.; Xavier, C.; de Moura, R.C. Comprehensive mapping of transposable elements reveals distinct patterns of element accumulation on chromosomes of wild beetles. Chromosome Res. 2021, 29, 203–218. [Google Scholar] [CrossRef]
- Mandrioli, M.; Manicardi, G.C. Unlocking holocentric chromosomes: New perspectives from comparative and functional genomics? Curr. Genom. 2012, 13, 343–349. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vela, J.; Montiel, E.E.; Mora, P.; Lorite, P.; Palomeque, T. Aphids and Ants, Mutualistic Species, Share a Mariner Element with an Unusual Location on Aphid Chromosomes. Genes 2021, 12, 1966. https://doi.org/10.3390/genes12121966
Vela J, Montiel EE, Mora P, Lorite P, Palomeque T. Aphids and Ants, Mutualistic Species, Share a Mariner Element with an Unusual Location on Aphid Chromosomes. Genes. 2021; 12(12):1966. https://doi.org/10.3390/genes12121966
Chicago/Turabian StyleVela, Jesús, Eugenia E. Montiel, Pablo Mora, Pedro Lorite, and Teresa Palomeque. 2021. "Aphids and Ants, Mutualistic Species, Share a Mariner Element with an Unusual Location on Aphid Chromosomes" Genes 12, no. 12: 1966. https://doi.org/10.3390/genes12121966
APA StyleVela, J., Montiel, E. E., Mora, P., Lorite, P., & Palomeque, T. (2021). Aphids and Ants, Mutualistic Species, Share a Mariner Element with an Unusual Location on Aphid Chromosomes. Genes, 12(12), 1966. https://doi.org/10.3390/genes12121966






