Nuclear Sirtuins and the Aging of the Immune System
Abstract
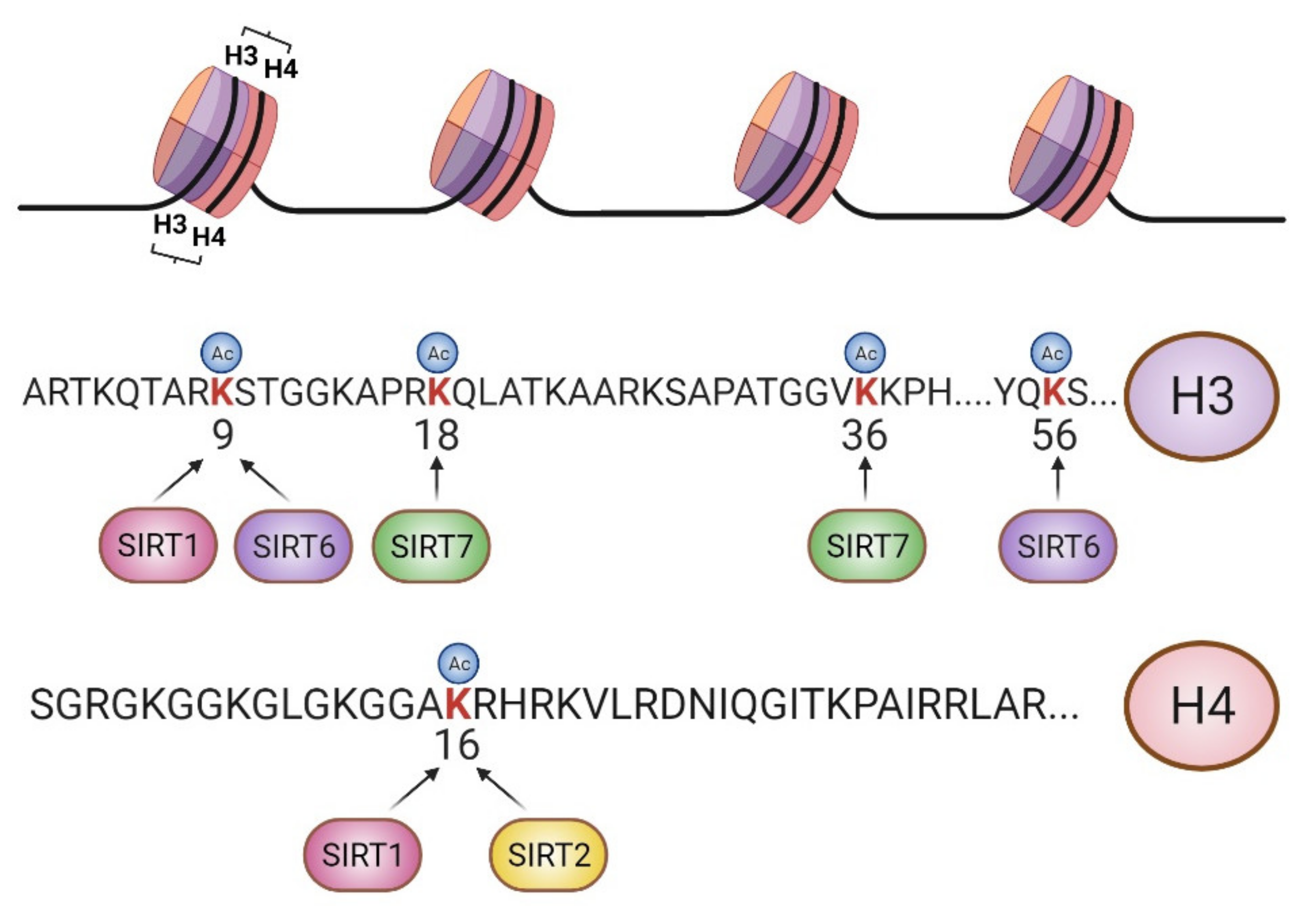
:1. Introduction
2. HSCs
| Compound | Function | Role in Immune Cells | Species Described |
|---|---|---|---|
| Resveratrol | Sirtuin activator |
| Mouse |
| Mouse | ||
| Mouse | ||
| Human and mouse | ||
| Human and mouse | ||
| Nicotinamide | Sirtuin inhibitor |
| Mouse |
| Mouse | ||
| Mouse | ||
| Human and mouse | |||
| Human and mouse | ||
| Metformin & SRT-1720 | SIRT1 agonists |
| Human and mouse |
| Human and mouse | ||
| Mouse | ||
| Sirtinol | SIRT1 antagonist |
| Mouse |
| Mouse | ||
| Cambinol | SIRT1/2 inhibitor |
| Human |
| S6 | SIRT6 inhibitor |
| Human |
3. Innate Immunity
4. Monocytes and Macrophages
5. Eosinophils
6. NK Cells
7. Dendritic Cells
8. Adaptive Immunity
9. T Cells

10. B Cells
11. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef] [Green Version]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.A.; McGowan, S.J.; et al. An Aged Immune System Drives Senescence and Ageing of Solid Organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Žugich, J. The Twilight of Immunity: Emerging Concepts in Aging of the Immune System Review-Article. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef]
- Haynes, L. Aging of the Immune System: Research Challenges to Enhance the Health Span of Older Adults. Front. Aging 2020, 1, 2. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the Ageing Microenvironment Influences Tumour Progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhao, Y.; Ruan, L.; Zhu, L.; Jin, K.; Zhuge, Q.; Su, D.M.; Zhao, Y. Impact of Aging Immune System on Neurodegeneration and Potential Immunotherapies. Prog. Neurobiol. 2017, 157, 2–28. [Google Scholar] [CrossRef]
- Kovtonyuk, L.v.; Fritsch, K.; Feng, X.; Manz, M.G.; Takizawa, H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Front. Immunol. 2016, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Harry, G.J.; Kraft, A.D. Neuroinflammation and Microglia: Considerations and Approaches for Neurotoxicity Assessment. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1265. [Google Scholar] [CrossRef]
- Vaquero, A. The Conserved Role of Sirtuins in Chromatin Regulation. Int. J. Dev. Biol. 2009, 53, 303–322. [Google Scholar] [CrossRef]
- Bosch-Presegué, L.; Vaquero, A. Sirtuin-Dependent Epigenetic Regulation in the Maintenance of Genome Integrity. FEBS J. 2015, 282, 1745–1767. [Google Scholar] [CrossRef]
- Vaquero, A.; Scher, M.; Erdjument-Bromage, H.; Tempst, P.; Serrano, L.; Reinberg, D. SIRT1 Regulates the Histone Methyl-Transferase SUV39H1 during Heterochromatin Formation. Nature 2007, 450, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; Martínez-Redondo, P.; Marazuela-Duque, A.; Vazquez, B.; Dooley, S.; Voigt, P.; Beck, D.; Kane-Goldsmith, N.; Tong, Q.; Rabanal, R.; et al. The Tumor Suppressor SirT2 Regulates Cell Cycle Progression and Genome Stability by Modulating the Mitotic Deposition of H4K20 Methylation. Genes Dev. 2013, 27, 639–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, T.; Michishita, E.; Adler, A.; Damian, M.; Berber, E.; Lin, M.; McCord, R.; Ongaigui, K.; Boxer, L.; Chang, H.; et al. SIRT6 Links Histone H3 Lysine 9 Deacetylation to NF-KappaB-Dependent Gene Expression and Organismal Life Span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toiber, D.; Erdel, F.; Bouazoune, K.; Silberman, D.; Zhong, L.; Mulligan, P.; Sebastian, C.; Cosentino, C.; Martinez-Pastor, B.; Giacosa, S.; et al. SIRT6 Recruits SNF2H to DNA Break Sites, Preventing Genomic Instability through Chromatin Remodeling. Mol. Cell 2013, 51, 454–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasselli, L.; Xi, Y.; Zheng, W.; Tennen, R.; Odrowaz, Z.; Simeoni, F.; Li, W.; Chua, K. SIRT6 Deacetylates H3K18ac at Pericentric Chromatin to Prevent Mitotic Errors and Cellular Senescence. Nat. Struct. Mol. Biol. 2016, 23, 434–440. [Google Scholar] [CrossRef] [Green Version]
- Simonet, N.G.; Thackray, J.K.; Vazquez, B.N.; Ianni, A.; Espinosa-Alcantud, M.; Morales-Sanfrutos, J.; Hurtado-Bagès, S.; Sabidó, E.; Buschbeck, M.; Tischfield, J.; et al. SirT7 Auto-ADP-Ribosylation Regulates Glucose Starvation Response through MH2A1. Sci. Adv. 2020, 6, eaaz2590. [Google Scholar] [CrossRef]
- Vazquez, B.; Thackray, J.; Simonet, N.; Chahar, S.; Kane-Goldsmith, N.; Newkirk, S.; Lee, S.; Xing, J.; Verzi, M.; An, W.; et al. SIRT7 Mediates L1 Elements Transcriptional Repression and Their Association with the Nuclear Lamina. Nucleic Acids Res. 2019, 47, 7870–7885. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, B.; Thackray, J.; Simonet, N.; Kane-Goldsmith, N.; Martinez-Redondo, P.; Nguyen, T.; Bunting, S.; Vaquero, A.; Tischfield, J.; Serrano, L. SIRT7 Promotes Genome Integrity and Modulates Non-Homologous End Joining DNA Repair. EMBO J. 2016, 35, 1488–1503. [Google Scholar] [CrossRef]
- Maissan, P.; Mooij, E.J.; Barberis, M. Sirtuins-Mediated System-Level Regulation of Mammalian Tissues at the Interface between Metabolism and Cell Cycle: A Systematic Review. Syst. Rev. Biol. 2021, 10, 194. [Google Scholar] [CrossRef]
- Donlon, T.; Morris, B.; Chen, R.; Masaki, K.; Allsopp, R.; Willcox, D.; Tiirikainen, M.; Willcox, B. Analysis of Polymorphisms in 59 Potential Candidate Genes for Association With Human Longevity. J. Gerontol. Series A Biol. Sci. Med. Sci. 2018, 73, 1459–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korotkov, A.; Seluanov, A.; Gorbunova, V. Sirtuin 6: Linking Longevity with Genome and Epigenome Stability. Trends Cell Biol. 2021, 31, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Hadar, A.; Milanesi, E.; Walczak, M.; Puzianowska-Kuźnicka, M.; Kuźnicki, J.; Squassina, A.; Niola, P.; Chillotti, C.; Attems, J.; Gozes, I.; et al. SIRT1, MiR-132 and MiR-212 Link Human Longevity to Alzheimer’s Disease. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The Sirtuin SIRT6 Regulates Lifespan in Male Mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S.I. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef] [Green Version]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic Instability and Aging-like Phenotype in the Absence of Mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental Defects and P53 Hyperacetylation in Sir2 Homolog (SIRT1)-Deficient Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Vassilopoulos, A.; Wang, R.H.; Lahusen, T.; Xiao, Z.; Xu, X.; Li, C.; Veenstra, T.D.; Li, B.; Yu, H.; et al. SIRT2 Maintains Genome Integrity and Suppresses Tumorigenesis through Regulating APC/C Activity. Cancer Cell 2011, 20, 487–499. [Google Scholar] [CrossRef] [Green Version]
- Mejia-Ramirez, E.; Florian, M.C. Understanding Intrinsic Hematopoietic Stem Cell Aging. Haematologica 2020, 105, 22. [Google Scholar] [CrossRef]
- Verovskaya, E.v.; Dellorusso, P.v.; Passegué, E. Losing Sense of Self and Surroundings: Hematopoietic Stem Cell Aging and Leukemic Transformation. Trends Mol. Med. 2019, 25, 494–515. [Google Scholar] [CrossRef]
- Xie, M.; Lu, C.; Wang, J.; McLellan, M.D.; Johnson, K.J.; Wendl, M.C.; McMichael, J.F.; Schmidt, H.K.; Yellapantula, V.; Miller, C.A.; et al. Age-Related Mutations Associated with Clonal Hematopoietic Expansion and Malignancies. Nat. Med. 2014, 20, 1472–1478. [Google Scholar] [CrossRef]
- Mohrin, M.; Shin, J.; Liu, Y.; Brown, K.; Luo, H.; Xi, Y.; Haynes, C.M.; Chen, D. A Mitochondrial UPR-Mediated Metabolic Checkpoint Regulates Hematopoietic Stem Cell Aging. Science 2015, 347, 1374–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, S.M.; Shaw, C.A.; Gatza, C.; Fisk, C.J.; Donehower, L.A.; Goodell, M.A. Aging Hematopoietic Stem Cells Decline in Function and Exhibit Epigenetic Dysregulation. PLoS Biol. 2007, 5, e201. [Google Scholar] [CrossRef] [PubMed]
- Petrini, S.; Borghi, R.; D’Oria, V.; Restaldi, F.; Moreno, S.; Novelli, A.; Bertini, E.; Compagnucci, C. Aged Induced Pluripotent Stem Cell (IPSCs) as a New Cellular Model for Studying Premature Aging. Aging 2017, 9, 1453–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florian, M.C.; Dörr, K.; Niebel, A.; Daria, D.; Schrezenmeier, H.; Rojewski, M.; Filippi, M.D.; Hasenberg, A.; Gunzer, M.; Scharffetter-Kochanek, K.; et al. Cdc42 Activity Regulates Hematopoietic Stem Cell Aging and Rejuvenation. Cell Stem Cell 2012, 10, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Matsui, K.; Ezoe, S.; Oritani, K.; Shibata, M.; Tokunaga, M.; Fujita, N.; Tanimura, A.; Sudo, T.; Tanaka, H.; McBurney, M.W.; et al. NAD-Dependent Histone Deacetylase, SIRT1, Plays Essential Roles in the Maintenance of Hematopoietic Stem Cells. Biochem. Biophys. Res. Commun. 2012, 418, 811–817. [Google Scholar] [CrossRef]
- Luo, H.; Mu, W.-C.; Karki, R.; Chiang, H.-H.; Mohrin, M.; Shin, J.J.; Ohkubo, R.; Ito, K.; Kanneganti, T.-D.; Chen, D. Mitochondrial Stress-Initiated Aberrant Activation of the NLRP3 Inflammasome Regulates the Functional Deterioration of Hematopoietic Stem Cell Aging. Cell Rep. 2019, 26, 945–954. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Diao, D.; Shi, Z.; Zhu, X.; Gao, Y.; Gao, S.; Liu, X.; Wu, Y.; Rudolph, K.L.; Liu, G.; et al. SIRT6 Controls Hematopoietic Stem Cell Homeostasis through Epigenetic Regulation of Wnt Signaling. Cell Stem Cell 2016, 18, 495–507. [Google Scholar] [CrossRef] [Green Version]
- McGuire, P.J. Mitochondrial Dysfunction and the Aging Immune System. Biology 2019, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Rimmelé, P.; Bigarella, C.L.; Liang, R.; Izac, B.; Dieguez-Gonzalez, R.; Barbet, G.; Donovan, M.; Brugnara, C.; Blander, J.M.; Sinclair, D.A.; et al. Aging-like Phenotype and Defective Lineage Specification in SIRT1-Deleted Hematopoietic Stem and Progenitor Cells. Stem Cell Rep. 2014, 3, 44. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Yang, Y.; Xia, F.; Huang, A.; Gao, X.; Fang, D.; Xiong, S.; Zhang, J. Resveratrol Inhibits CD4+ T Cell Activation by Enhancing the Expression and Activity of Sirt1. PLoS ONE 2013, 8, e75139. [Google Scholar] [CrossRef] [Green Version]
- Limagne, E.; Thibaudin, M.; Euvrard, R.; Apetoh, L. Sirtuin-1 Activation Controls Tumor Growth by Impeding Th17 Differentiation via STAT3 Deacetylation. Cell Rep. 2017, 19, 746–759. [Google Scholar] [CrossRef] [Green Version]
- Imperatore, F.; Maurizio, J.; Vargas Aguilar, S.; Busch, C.J.; Favret, J.; Kowenz-Leutz, E.; Cathou, W.; Gentek, R.; Perrin, P.; Leutz, A.; et al. SIRT1 Regulates Macrophage Self-renewal. EMBO J. 2017, 36, 2353–2372. [Google Scholar] [CrossRef]
- Van Loosdregt, J.; Vercoulen, Y.; Guichelaar, T.; Gent, Y.Y.J.; Beekman, J.M.; van Beekum, O.; Brenkman, A.B.; Hijnen, D.-J.; Mutis, T.; Kalkhoven, E.; et al. Regulation of Treg Functionality by Acetylation-Mediated Foxp3 Protein Stabilization. Blood 2010, 115, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-S.; Lim, H.W.; Wu, J.; Schnölzer, M.; Verdin, E.; Ott, M. Three Novel Acetylation Sites in the Foxp3 Transcription Factor Regulate the Suppressive Activity of Regulatory T Cells. J. Immunol. 2012, 188, 2712–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darrigues, J.; van Meerwijk, J.P.M.; Romagnoli, P. Age-Dependent Changes in Regulatory T Lymphocyte Development and Function: A Mini-Review. Gerontology 2018, 64, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Gan, H.; Shen, T.; Chupp, D.P.; Taylor, J.R.; Sanchez, H.N.; Li, X.; Xu, Z.; Zan, H.; Casali, P. B Cell Sirt1 Deacetylates Histone and Non-Histone Proteins for Epigenetic Modulation of AID Expression and the Antibody Response. Sci. Adv. 2020, 6, eaay2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Garcia-Gomez, A.; Morante-Palacios, O.; Ciudad, L.; Özkaramehmet, S.; van Dijck, E.; Rodríguez-Ubreva, J.; Vaquero, A.; Ballestar, E. SIRT1/2 Orchestrate Acquisition of DNA Methylation and Loss of Histone H3 Activating Marks to Prevent Premature Activation of Inflammatory Genes in Macrophages. Nucleic Acids Res. 2020, 48, 665–681. [Google Scholar] [CrossRef]
- Lasigliè, D.; Boero, S.; Bauer, I.; Morando, S.; Damonte, P.; Cea, M.; Monacelli, F.; Odetti, P.; Ballestrero, A.; Uccelli, A.; et al. Sirt6 Regulates Dendritic Cell Differentiation, Maturation, and Function. Aging 2016, 8, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 Is Downregulated by Autophagy in Senescence and Ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef]
- Fleming, H.E.; Janzen, V.; Celso, C.; lo Guo, J.; Leahy, K.M.; Kronenberg, H.M.; Scadden, D.T. Wnt Signaling in the Niche Enforces Hematopoietic Stem Cell Quiescence and Is Necessary to Preserve Self-Renewal in Vivo. Cell Stem Cell 2008, 2, 274. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, S.; Kincade, P.W. Wnt-Related Molecules and Signaling Pathway Equilibrium in Hematopoiesis. Cell Stem Cell 2009, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian Sir2 Homolog SIRT7 Is an Activator of RNA Polymerase I Transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monie, T.P. The Innate Immune System: A Compositional and Functional Perspective; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780081007587. [Google Scholar]
- Castelo-Branco, C.; Soveral, I. The Immune System and Aging: A Review. Gynecol. Endocrinol. 2013, 30, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ka, S.O.; Cha, H.N.; Chae, Y.N.; Kim, M.K.; Park, S.Y.; Bae, E.J.; Park, B.H. Myeloid Sirtuin 6 Deficiency Causes Insulin Resistance in High-Fat Diet–Fed Mice by Eliciting Macrophage Polarization toward an M1 Phenotype. Diabetes 2017, 66, 2659–2668. [Google Scholar] [CrossRef] [Green Version]
- Pais, T.F.; Szego, É.M.; Marques, O.; Miller-Fleming, L.; Antas, P.; Guerreiro, P.; de Oliveira, R.M.H.; Kasapoglu, B.; Outeiro, T.F. The NAD-Dependent Deacetylase Sirtuin 2 Is a Suppressor of Microglial Activation and Brain Inflammation. EMBO J. 2013, 32, 2603–2616. [Google Scholar] [CrossRef] [Green Version]
- Schug, T.T.; Xu, Q.; Gao, H.; Peres-da-Silva, A.; Draper, D.W.; Fessler, M.B.; Purushotham, A.; Li, X. Myeloid Deletion of SIRT1 Induces Inflammatory Signaling in Response to Environmental Stress. Mol. Cell. Biol. 2010, 30, 4712–4721. [Google Scholar] [CrossRef] [Green Version]
- Lo Sasso, G.; Menzies, K.J.; Mottis, A.; Piersigilli, A.; Perino, A.; Yamamoto, H.; Schoonjans, K.; Auwerx, J. SIRT2 Deficiency Modulates Macrophage Polarization and Susceptibility to Experimental Colitis. PLoS ONE 2014, 9, e103573. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Van Beek, A.A.; van den Bossche, J.; Mastroberardino, P.G.; de Winther, M.P.J.; Leenen, P.J.M. Metabolic Alterations in Aging Macrophages: Ingredients for Inflammaging? Trends Immunol. 2019, 40, 113–127. [Google Scholar] [CrossRef]
- Bouchlaka, M.N.; Sckisel, G.D.; Chen, M.; Mirsoian, A.; Zamora, A.E.; Maverakis, E.; Wilkins, D.E.C.; Alderson, K.L.; Hsiao, H.-H.; Weiss, J.M.; et al. Aging Predisposes to Acute Inflammatory Induced Pathology after Tumor Immunotherapy. J. Exp. Med. 2013, 210, 2223–2237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Klein, D.; Kerscher, S.; West, B.L.; Weis, J.; Katona, I.; Martini, R. Macrophage Depletion Ameliorates Peripheral Neuropathy in Aging Mice. J. Neurosci. 2018, 38, 4610–4620. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Chiang, H.H.; Luo, H.; Zheng, Z.; Qiao, Q.; Wang, L.; Tan, M.; Ohkubo, R.; Mu, W.C.; Zhao, S.; et al. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. 2020, 31, 580–591. [Google Scholar] [CrossRef]
- Kaiser, A.; Schmidt, M.; Huber, O.; Frietsch, J.J.; Scholl, S.; Heidel, F.H.; Hochhaus, A.; Müller, J.P.; Ernst, T. SIRT7: An Influence Factor in Healthy Aging and the Development of Age-Dependent Myeloid Stem-Cell Disorders. Leukemia 2020, 34, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-ΚB-Dependent Transcription and Cell Survival by the SIRT1 Deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Rothgiesser, K.M.; Erener, S.; Waibel, S.; Lüscher, B.; Hottiger, M.O. SIRT2 Regulates NF-ΚB-Dependent Gene Expression through Deacetylation of P65 Lys310. J. Cell Sci. 2010, 123, 4251–4258. [Google Scholar] [CrossRef] [Green Version]
- Santos-Barriopedro, I.; Bosch-Presegué, L.; Marazuela-Duque, A.; de la Torre, C.; Colomer, C.; Vazquez, B.N.; Fuhrmann, T.; Martínez-Pastor, B.; Lu, W.; Braun, T.; et al. SIRT6-Dependent Cysteine Monoubiquitination in the PRE-SET Domain of Suv39h1 Regulates the NF-ΚB Pathway. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 Protects against Microglia-Dependent Amyloid-β Toxicity through Inhibiting NF-ΚB Signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef] [Green Version]
- Soucie, E.L.; Weng, Z.; Geirsdóttir, L.; Molawi, K.; Maurizio, J.; Fenouil, R.; Mossadegh-Keller, N.; Gimenez, G.; Vanhille, L.; Beniazza, M.; et al. Lineage-Specific Enhancers Activate Self-Renewal Genes in Macrophages and Embryonic Stem Cells. Science 2016, 351, 6274. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, A.J.; Kale, A.; Perrone, R.; Lopez-Dominguez, J.A.; Pisco, A.O.; Kasler, H.G.; Schmidt, M.S.; Heckenbach, I.; Kwok, R.; Wiley, C.D.; et al. Senescent Cells Promote Tissue NAD+ Decline during Ageing via the Activation of CD38+ Macrophages. Nature Metab. 2020, 2, 1265–1283. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Willebrand, R.; Voehringer, D. Regulation of Eosinophil Development and Survival. Curr. Opin. Hematol. 2017, 24, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Brigger, D.; Riether, C.; van Brummelen, R.; Mosher, K.I.; Shiu, A.; Ding, Z.; Zbären, N.; Gasser, P.; Guntern, P.; Yousef, H.; et al. Eosinophils Regulate Adipose Tissue Inflammation and Sustain Physical and Immunological Fitness in Old Age. Nat. Metab. 2020, 2, 688–702. [Google Scholar] [CrossRef]
- Bang, I.H.; Park, D.; Lee, Y.; Cho, H.; Park, B.H.; Bae, E.J. Sirtuin 6 Promotes Eosinophil Differentiation by Activating GATA-1 Transcription Factor. Aging Cell 2021, 20, e13418. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, B.N.; Thackray, J.K.; Serrano, L. Sirtuins and DNA Damage Repair: SIRT7 Comes to Play. Nucleus 2017, 8, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Salati, S.; Bianchi, E.; Zini, R.; Tenedini, E.; Quaglino, D.; Manfredini, R.; Ferrari, S. Eosinophils, but Not Neutrophils, Exhibit an Efficient DNA Repair Machinery and High Nucleolar Activity. Haematologica 2007, 92, 1311–1318. [Google Scholar] [CrossRef] [Green Version]
- Zoico, E.; Rubele, S.; de Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Xu, M.; Zhu, C.; Wang, H.; Zhao, Q.; Zhou, F. Sirtuin2 Enhances the Tumoricidal Function of Liver Natural Killer Cells in a Mouse Hepatocellular Carcinoma Model. Cancer Immunol. Immunother. 2019, 68, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bi, Y.; Xue, L.; Zhang, Y.; Yang, H.; Chen, X.; Lu, Y.; Zhang, Z.; Liu, H.; Wang, X.; et al. Dendritic Cell SIRT1–HIF1α Axis Programs the Differentiation of CD4+ T Cells through IL-12 and TGF-Β1. Proc. Nat. Acad. Sci. USA 2015, 112, E957–E965. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lee, S.M.; Gao, B.; Zhang, J.; Fang, D. Histone Deacetylase Sirtuin 1 Deacetylates IRF1 Protein and Programs Dendritic Cells to Control Th17 Protein Differentiation during Autoimmune Inflammation. J. Biol. Chem. 2013, 288, 37256–37266. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, Y.; Rodríguez, M.; Municio, C.; Hugo, E.; Alonso, S.; Ibarrola, N.; Fernández, N.; Crespo, M.S. Sirtuin 1 Is a Key Regulator of the Interleukin-12 P70/Interleukin-23 Balance in Human Dendritic Cells * □ S. J. Biol. Chem. 2012, 287, 35689–35701. [Google Scholar] [CrossRef] [Green Version]
- Hazeldine, J.; Lord, J.M. The Impact of Ageing on Natural Killer Cell Function and Potential Consequences for Health in Older Adults. Ageing Res. Rev. 2013, 12, 1069. [Google Scholar] [CrossRef]
- Kaszubowska, L.; Foerster, J.; Kaczor, J.J.; Schetz, D.; Ślebioda, T.J.; Kmieć, Z. Expression of Cellular Protective Proteins SIRT1, HSP70 and SOD2 Correlates with Age and Is Significantly Higher in NK Cells of the Oldest Seniors. Immun. Ageing I A 2017, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, T.; Hamazaki, J.; Hirayama, S.; McBurney, M.W.; Yashiroda, H.; Murata, S. Sirt1-Deficiency Causes Defective Protein Quality Control. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of Dendritic Cells in Inflammation and Loss of Tolerance in the Elderly. Front. Immunol. 2017, 8, 896. [Google Scholar] [CrossRef]
- Geginat, J.; Paroni, M.; Maglie, S.; Alfen, J.S.; Kastirr, I.; Gruarin, P.; de Simone, M.; Pagani, M.; Abrignani, S. Plasticity of Human CD4 T Cell Subsets. Front. Immunol. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and Memory T-Cell Differentiation: Implications for Vaccine Development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Palmer, S.; Albergante, L.; Blackburn, C.C.; Newman, T.J. Thymic Involution and Rising Disease Incidence with Age. Proc. Nat. Acad. Sci. USA 2018, 115, 1883. [Google Scholar] [CrossRef] [Green Version]
- Goronzy, J.J.; Fang, F.; Cavanagh, M.M.; Qi, Q.; Weyand, C.M. Naive T Cell Maintenance and Function in Human Aging. J. Immunol. 2015, 194, 4073–4080. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T Cell Aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef]
- Viola, A.; Lanzavecchia, A. T Cell Activation Determined by T Cell Receptor Number and Tunable Thresholds. Science 1996, 273, 104–106. [Google Scholar] [CrossRef]
- Guram, K.; Kim, S.S.; Wu, V.; Sanders, P.D.; Patel, S.; Schoenberger, S.P.; Cohen, E.E.W.; Chen, S.-Y.; Sharabi, A.B. A Threshold Model for T-Cell Activation in the Era of Checkpoint Blockade Immunotherapy. Front. Immunol. 2019, 10, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lee, S.-M.; Shannon, S.; Gao, B.; Chen, W.; Chen, A.; Divekar, R.; Mcburney, M.W.; Braley-Mullen, H.; Zaghouani, H.; et al. The Type III Histone Deacetylase Sirt1 Is Essential for Maintenance of T Cell Tolerance in Mice. J. Clin. Investig. 2009, 119, 3048–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Chen, H.Z.; Liu, J.J.; Jia, Y.Y.; Zhang, Z.Q.; Yang, R.F.; Zhang, Y.; Xu, J.; Wei, Y.S.; Liu, D.P.; et al. SIRT1 Suppresses Activator Protein-1 Transcriptional Activity and Cyclooxygenase-2 Expression in Macrophages. J. Biol. Chem. 2010, 285, 7097–7110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Ye, J. Inhibition of Transcriptional Activity of C-JUN by SIRT1. Biochem. Biophys. Res. Commun. 2008, 376, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Kong, Q.; Kemp, K.; Zhao, Y.-S.; Fang, D. Analysis of Sirtuin 1 Expression Reveals a Molecular Explanation of IL-2–Mediated Reversal of T-Cell Tolerance. Proc. Nat. Acad. Sci. USA 2012, 109, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, S.; Yamazaki, M.; Abe, M.; Sakimura, K.; Takayanagi, H.; Iwai, Y. Basic Leucine Zipper Transcription Factor, ATF-like (BATF) Regulates Epigenetically and Energetically Effector CD8 T-Cell Differentiation via Sirt1 Expression. Proc. Nat. Acad. Sci. USA 2011, 108, 14885–14889. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Li, G.; Kim, C.; Hu, B.; Jadhav, R.R.; Weyand, C.M.; Goronzy, J.J. Regulation of MiR-181a Expression in T Cell Aging. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Kang, S.G.; Ryu, J.K.; Schilling, B.; Fei, M.; Lee, I.S.; Kehasse, A.; Shirakawa, K.; Yokoyama, M.; Schnölzer, M.; et al. SIRT1 Deacetylates RORγt and Enhances Th17 Cell Generation. J. Exp. Med. 2015, 212, 607–617. [Google Scholar] [CrossRef]
- Jeng, M.Y.; Hull, P.A.; Fei, M.; Kwon, H.-S.; Tsou, C.-L.; Kasler, H.; Ng, C.-P.; Gordon, D.E.; Johnson, J.; Krogan, N.; et al. Metabolic Reprogramming of Human CD8+ Memory T Cells through Loss of SIRT1. J. Exp. Med. 2018, 215, 51. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, Y.; Chen, X.; Li, C.; Li, Y.; Zhang, Z.; Wang, J.; Lu, Y.; Yu, Q.; Su, H.; et al. Histone Deacetylase SIRT1 Negatively Regulates the Differentiation of Interleukin-9-Producing CD4+ T Cells. Immunity 2016, 44, 1337–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-R.; Han, Y.; Shin, J.; Kang, J. Sirt1 Is a Requisite for the Antigen Presentation by B Cells. J. Immunol. 2020, 204 Suppl. 1, 217.15. [Google Scholar]
- Moskowitz, D.M.; Zhang, D.W.; Hu, B.; le Saux, S.; Yanes, R.E.; Ye, Z.; Buenrostro, J.D.; Weyand, C.M.; Greenleaf, W.J.; Goronzy, J.J. Epigenomics of Human CD8 T Cell Differentiation and Aging. Sci. Immunol. 2017, 2, eaag0192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Li, G.; Ye, Z.; Gustafson, C.E.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Transcription Factor Networks in Aged Naïve CD4 T Cells Bias Lineage Differentiation. Aging Cell 2019, 18, e12957. [Google Scholar] [CrossRef]
- Sequeira, J.; Boily, G.; Bazinet, S.; Saliba, S.; He, X.; Jardine, K.; Kennedy, C.; Staines, W.; Rousseaux, C.; Mueller, R.; et al. Sirt1-Null Mice Develop an Autoimmune-like Condition. Exp. Cell Res. 2008, 314, 3069–3074. [Google Scholar] [CrossRef]
- Owczarz, M.; Budzinska, M.; Domaszewska-Szostek, A.; Borkowska, J.; Polosak, J.; Gewartowska, M.; Slusarczyk, P.; Puzianowska-Kuznicka, M. MiR-34a and MiR-9 Are Overexpressed and SIRT Genes Are Downregulated in Peripheral Blood Mononuclear Cells of Aging Humans. Exp. Biol. Med. 2017, 242, 1453. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Jin, J.; Ye, Z.; Jadhav, R.R.; Gustafson, C.E.; Hu, B.; Cao, W.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Histone Deficiency and Accelerated Replication Stress in T Cell Aging. J. Clin. Investig. 2021, 131, e143632. [Google Scholar] [CrossRef]
- Chua, K.F.; Mostoslavsky, R.; Lombard, D.B.; Pang, W.W.; Saito, S.; Franco, S.; Kaushal, D.; Cheng, H.-L.; Fischer, M.R.; Stokes, N.; et al. Mammalian SIRT1 Limits Replicative Life Span in Response to Chronic Genotoxic Stress. Cell Metab. 2005, 2, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Fagnoni, F.F.; Vescovini, R.; Mazzola, M.; Bologna, G.; Nigro, E.; Lavagetto, G.; Franceschi, C.; Passeri, M.; Sansoni, P. Expansion of Cytotoxic CD8+ CD28- T Cells in Healthy Ageing People, Including Centenarians. Immunology 1996, 88, 501–507. [Google Scholar] [CrossRef]
- Effros, R.B.; Boucher, N.; Porter, V.; Zhu, X.; Spaulding, C.; Walford, R.L.; Kronenberg, M.; Cohen, D.; Schächter, F. Decline in CD28+ T Cells in Centenarians and in Long-Term T Cell Cultures: A Possible Cause for Both in Vivo and in Vitro Immunosenescence. Exp. Gerontol. 1994, 29, 601–609. [Google Scholar] [CrossRef]
- Effros, R.B. The Role of CD8 T Cell Replicative Senescence in Human Aging. Discov. Med. 2009, 5, 293–297. [Google Scholar] [CrossRef]
- Delpoux, A.; Marcel, N.; Hess, R.; Lappas, M.; Hedrick, S.M.; Doedens, A.L.; Michelini, R.H.; Katayama, C.D.; Allison, K.A.; Glass, C.K.; et al. Article FOXO1 Constrains Activation and Regulates Senescence in CD8 T Cells FOXO1 Constrains Activation and Regulates Senescence in CD8 T Cells. Cell Rep. 2021, 34, 108674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, X.; Zhao, A.; Cai, H.; Wang, X.; Li, J. Increased Activated Regulatory T Cell Subsets and Aging Treg-like Cells in Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: A Case Control Study. Cancer Cell Int. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jagger, A.T.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. T Regulatory Cells and the Immune Aging Process. Gerontology 2014, 60, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Beier, U.H.; Wang, L.; Bhatti, T.R.; Liu, Y.; Han, R.; Ge, G.; Hancock, W.W. Sirtuin-1 Targeting Promotes Foxp3 + T-Regulatory Cell Function and Prolongs Allograft Survival. Mol. Cell. Biol. 2011, 31, 1022–1029. [Google Scholar] [CrossRef] [Green Version]
- Sidler, C.; Wóycicki, R.; Ilnytskyy, Y.; Metz, G.; Kovalchuk, I.; Kovalchuk, O. Immunosenescence Is Associated with Altered Gene Expression and Epigenetic Regulation in Primary and Secondary Immune Organs. Front. Genet. 2013, 4, 211. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Chow, M.Z.Y.; Wang, Z.; Zhang, L.; Liu, B.; Liu, X.; Zhou, Z. Histone H4 Lysine 16 Hypoacetylation Is Associated with Defective DNA Repair and Premature Senescence in Zmpste24-Deficient Mice. Proc. Nat. Acad. Sci. USA 2011, 108, 12325–12330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, L.; Goudot, C.; Zueva, E.; Gueguen, P.; Burgdorf, N.; Waterfall, J.J.; Quivy, J.-P.; Almouzni, G.; Amigorena, S. The Epigenetic Control of Stemness in CD8+ T Cell Fate Commitment. Science 2018, 359, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Wang, R.-H.; Lahusen, T.J.; Park, O.; Bertola, A.; Maruyama, T.; Reynolds, D.; Chen, Q.; Xu, X.; Young, H.A.; et al. Progression of Chronic Liver Inflammation and Fibrosis Driven by Activation of C-JUN Signaling in Sirt6 Mutant Mice * □ S. J. Biol. Chem. 2012, 287, 41903–41913. [Google Scholar] [CrossRef] [Green Version]
- Huo, Q.; Li, Z.; Cheng, L.; Yang, F.; Xie, N. SIRT7 Is a Prognostic Biomarker Associated With Immune Infiltration in Luminal Breast Cancer. Front. Oncol. 2020, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B Cell Dysfunction Associated With Aging and Autoimmune Diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuzhu, G.; Komai-Koma, M.; Leung, B.P.; Howe, H.S.; McSharry, C.; McInnes, I.B.; Xu, D. Resveratrol Modulates Murine Collagen-Induced Arthritis by Inhibiting Th17 and B-Cell Function. Ann. Rheum. Dis. 2012, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Ma, G.; Li, W.; Wu, J.; Lai, T.; Huang, D.; Zhao, X.; Lv, Q.; Chen, M.; et al. Increases in Peripheral SIRT1: A New Biological Characteristic of Asthma. Respirology 2015, 20, 1066–1072. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, Y.; Li, Y.; Shi, H.; Teng, J.; Liu, H.; Cheng, X.; Ye, J.; Su, Y.; Yin, Y.; et al. Anti-SIRT1 Autoantibody Is Elevated in Ankylosing Spondylitis: A Potential Disease Biomarker. BMC Immunol. 2018, 19, 1–8. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, C.; Xin, M.; Han, L.; Zhang, Y.; Sun, M. Sirtuin 1 (Sirt1) Overexpression in BaF3 Cells Contributes to Cell Proliferation Promotion, Apoptosis Resistance and pro-Inflammatory Cytokine Production. Med. Sci. Monit. 2017, 23, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Kurupati, R.K.; Haut, L.H.; Schmader, K.E.; Ertl, H.C.J. Age-Related Changes in B Cell Metabolism. Aging 2019, 11, 4367–4381. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gámez-García, A.; Vazquez, B.N. Nuclear Sirtuins and the Aging of the Immune System. Genes 2021, 12, 1856. https://doi.org/10.3390/genes12121856
Gámez-García A, Vazquez BN. Nuclear Sirtuins and the Aging of the Immune System. Genes. 2021; 12(12):1856. https://doi.org/10.3390/genes12121856
Chicago/Turabian StyleGámez-García, Andrés, and Berta N. Vazquez. 2021. "Nuclear Sirtuins and the Aging of the Immune System" Genes 12, no. 12: 1856. https://doi.org/10.3390/genes12121856
APA StyleGámez-García, A., & Vazquez, B. N. (2021). Nuclear Sirtuins and the Aging of the Immune System. Genes, 12(12), 1856. https://doi.org/10.3390/genes12121856





