Advances and Limitations of Next Generation Sequencing in Animal Diet Analysis
Abstract
:1. Introduction to Diet Analysis
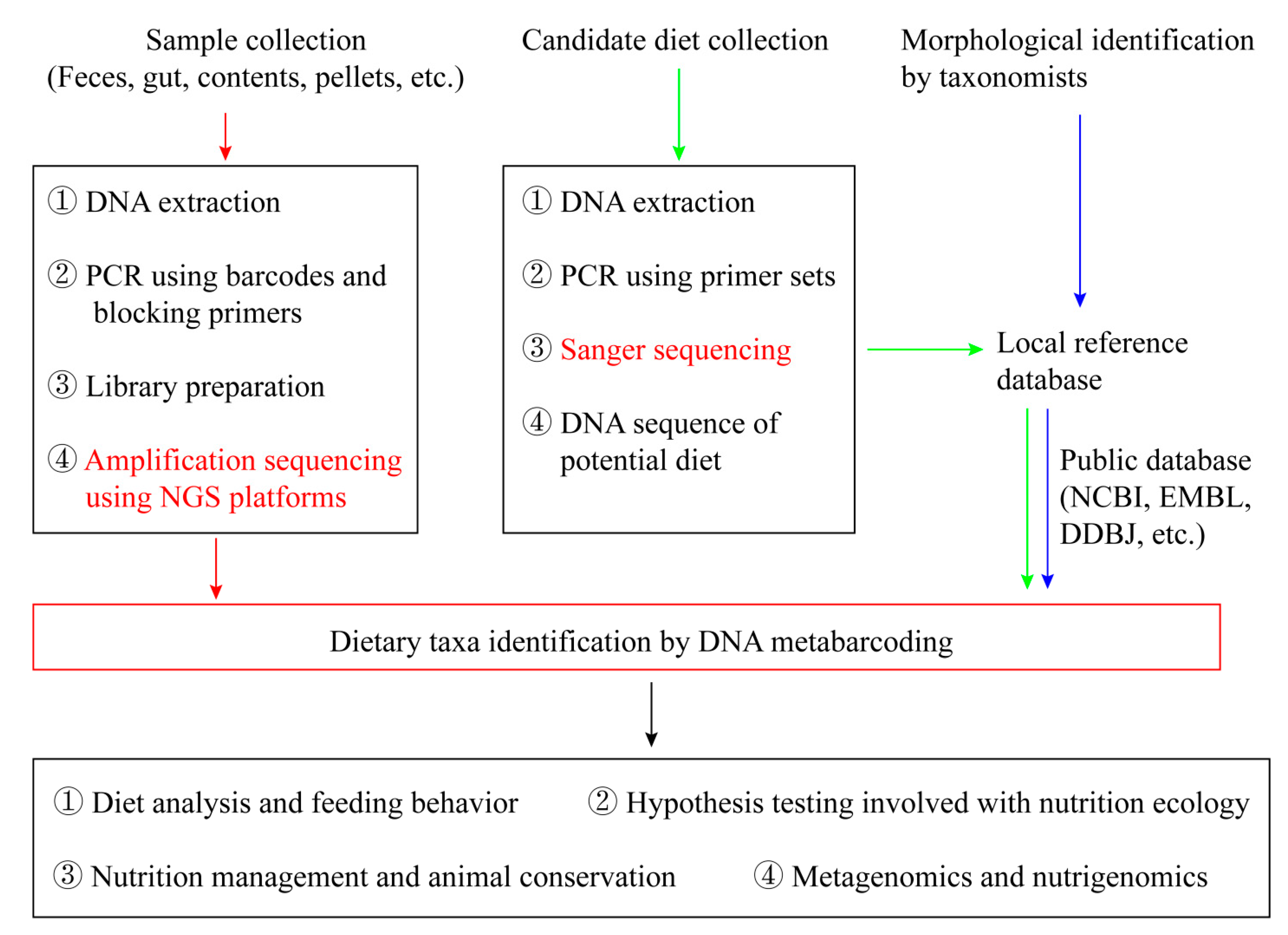
2. Conceptual Framework of Diet Analysis Using NGS
2.1. Sample Collection and DNA Extraction
2.2. PCR Amplification and NGS Processing
2.3. Building a Local Reference Database
2.4. Data Filtering and Analysis
3. Case Studies of NGS Based Diet Analysis
3.1. Investigating Effects of Animal Feeding on Environmental Changes
3.2. Identifying Detailed Diet Taxonomies
3.3. Inferring Predator-Prey-Environment Relationship
3.4. Ecosystem Monitoring
4. Current Limitations
4.1. Technical Errors
4.2. Biological Factors
5. Conclusions and Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raubenheimer, D.; Simpson, S.J. Nutritional ecology and foraging theory. Curr. Opin. Insect. Sci. 2018, 27, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shafer, A.B.A.; Hu, X.; Li, L.; Ning, Y.; Gong, M.; Cui, L.; Li, H.; Hu, D.; Qi, L.; et al. Meta-barcoding insights into the spatial and temporal dietary patterns of the threatened Asian Great Bustard (Otis tarda dybowskii) with potential implications for diverging migratory strategies. Ecol. Evol. 2018, 8, 1736–1745. [Google Scholar] [CrossRef] [Green Version]
- Szoboszlai, A.I.; Thayer, J.A.; Wood, S.A.; Sydeman, W.J.; Koehn, L.E. Forage species in predator diets: Synthesis of data from the California Current. Ecol. Inform. 2015, 29, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Demi, L.M.; Taylor, B.W.; Reading, B.J.; TordoffR, M.G.; Dunn, R. Understanding the evolution of nutritive taste in animals: Insights from biological stoichiometry and nutritional geometry. Ecol. Evol. 2021, 11, 8441–8455. [Google Scholar] [CrossRef]
- Carreon-Martinez, L.; Heath, D. Revolution in food web analysis and trophic ecology: Diet analysis by DNA and stable isotope analysis. Mol. Ecol. 2010, 19, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.K.; Harwood, J.D. Advances in molecular ecology: Tracking trophic links through predator–prey food-webs. Funct. Ecol. 2005, 19, 751–762. [Google Scholar] [CrossRef]
- Khanam, S.; Howitt, R.; Mushtaq, M.; Russell, C. Diet analysis of small mammal pests: A comparison of molecular and microhistological methods. Integr. Zool. 2016, 11, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Traugott, M.; Thalinger, B.; Wallinger, C.; Sint, D. Fish as predators and prey: DNA-based assessment of their role in food webs. J. Fish Biol. 2021, 98, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.L.F.; Koprivnikar, J. Your infections are what you eat: How host ecology shapes the helminth parasite communities of lizards. J. Anim. Ecol. 2019, 88, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Dingemanse, N.J. You are what you eat: Diet shapes body composition, personality and behavioural stability. BMC Evol. Biol. 2017, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Aryal, A.; Panthi, S.; Barraclough, R.K.; Bencini, R.; Adhikari, B.; Ji, W. Raubenheimer. Habitat selection and feeding ecology of dhole (Cuon alpinus) in the Himalayas. J. Mammal. 2015, 96, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Severud, W.J.; Windels, S.K.; Belant, J.L.; Bruggink, G. The role of forage availability on diet choice and body condition in American beavers (Castor canadensis). Mamm. Biol.—Z. Säugetierkunde 2013, 78, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Maixner, F. Molecular Reconstruction of the Diet in Human Stool Samples. mSystems 2019, 4, e00634-19. [Google Scholar] [CrossRef] [Green Version]
- Reese, A.T.; Kartzinel, T.R.; Petrone, B.L.; Turnbaugh, P.J.; Pringle, R.M.; David, A. Using DNA Metabarcoding To Evaluate the Plant Component of Human Diets: A Proof of Concept. mSystems 2019, 4, e00458-19. [Google Scholar] [CrossRef] [Green Version]
- Pierce, G.J.; Boyle, P.R. A review of methods for diet analysis in piscivorous marine mammals. Oceanogr. Mar. Biol. 1991, 29, 409–486. [Google Scholar]
- Figueiredo, A.M.; Valente, A.M.; Barros, T.; Carvalho, J.; Silva, D.A.M.; Fonseca, C.; Carvalho, L.M.; Torres, R.T. What does the wolf eat? Assessing the diet of the endangered Iberian wolf (Canis lupus signatus) in northeast Portugal. PLoS ONE 2020, 15, e0230433. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J. The cafeteria diet as a tool for studies of thermogenesis. J. Nutr. 1988, 118, 925–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amundsen, P.A.; Sánchez-Hernández, J. Feeding studies take guts—Critical review and recommendations of methods for stomach contents analysis in fish. J. Fish Biol. 2019, 95, 1364–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westoby, M.; Rost, G.R.; Weis, J.A. Problems with Estimating Herbivore Diets by Microscopically Identifying Plant Fragments from Stomachs. J. Mammal. 1976, 57, 167–172. [Google Scholar] [CrossRef]
- Bugalho, M.N.; Milne, J.A.; Mayes, R.W.; Rego, F.C. Plant-wax alkanes as seasonal markers of red deer dietary components. Can. J. Zool. 2005, 83, 465–473. [Google Scholar]
- Inger, R.; Bearhop, S. Applications of stable isotope analyses to avian ecology. Ibis 2008, 150, 447–461. [Google Scholar] [CrossRef]
- Han, H.; Wei, W.; Hu, Y.; Nie, Y.; Ji, X.; Yan, L.; Zhang, Z.; Shi, X.; Zhu, L.; Luo, Y.; et al. Diet Evolution and Habitat Contraction of Giant Pandas via Stable Isotope Analysis. Curr. Biol. 2019, 29, 664–669.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelms, S.E.; Parry, H.E.; Bennett, K.A.; Galloway, T.S.; Godley, B.J.; Santillo, D.; Lindeque, P.K. What goes in, must come out: Combining scat-based molecular diet analysis and quantification of ingested microplastics in a marine top predator. Methods Ecol. Evol. 2019, 10, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, D.R.; Mangan, M.T.; Mangan, K.E.; Bokulich, N.A.; Foster, J.T. Lord of the Diptera (and Moths and a Spider): Molecular Diet Analyses and Foraging Ecology of Indiana Bats in Illinois. Front. Ecol. Evol. 2021, 9, 623655. [Google Scholar] [CrossRef]
- Braley, M.; Goldsworthy, S.D.; Page, B.; Steer, M.; Austin, J.J. Assessing morphological and DNA-based diet analysis techniques in a generalist predator, the arrow squid Nototodarus gouldi. Mol. Ecol. Resour. 2010, 10, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Z.; Hu, S.M.; Liu, S.; Huang, H. Comparison between traditional sequencing and high-throughput sequencing on the dietary analysis of juvenile fish. Ying Yong Sheng Tai Xue Bao 2018, 29, 3093–3101. [Google Scholar]
- Gong, M.; Ning, Y.; Han, M.; Zhao, C.; Tian, J.; Li, L.; Xiao, H.; Liu, G. A comparison of next-generation sequencing with clone sequencing in the diet analysis of Asian great bustard. Conserv. Genet. Resour. 2019, 11, 15–17. [Google Scholar] [CrossRef]
- Clare, E.L.; Symondson, W.O.; Broders, H.; Fabianek, F.; Fraser, E.E.; Mackenzie, A.; Boughen, A.; Hamilton, R.; Willis, C.K.; Martinez-Nuñez, F. The diet of Myotis lucifugus across Canada: Assessing foraging quality and diet variability. Mol. Ecol. 2014, 23, 3618–3632. [Google Scholar] [CrossRef] [PubMed]
- Emami-Khoyi, A.; Hartley, D.A.; Paterson, A.M.; Boren, L.J.; Cruickshank, R.H.; Ross, J.G.; Murphy, E.C.; Else, T.A. Identifying prey items from New Zealand fur seal (Arctocephalus forsteri) faeces using massive parallel sequencing. Conserv. Genet. Resour. 2016, 8, 1–10. [Google Scholar]
- Oehm, J.; Thalinger, B.; Eisenkölbl, S.; Traugott, M. Diet analysis in piscivorous birds: What can the addition of molecular tools offer? Ecol. Evol. 2017, 7, 1984–1995. [Google Scholar] [CrossRef]
- Alberdi, A.; Aizpurua, O.; Bohmann, K.; Gopalakrishnan, S.; Lynggaard, C.; Nielsen, M.; Gilbert, M.T. Promises and pitfalls of using high-throughput sequencing for diet analysis. Mol. Ecol. Resour. 2019, 19, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Mcclenaghan, B.; Gibson, J.F.; Shokralla, S.; Hajibabaei, M. Discrimination of grasshopper (Orthoptera: Acrididae) diet and niche overlap using next-generation sequencing of gut contents. Ecol. Evol. 2015, 5, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Waraniak, J.M.; Baker, E.A.; Scribner, K.T. Molecular diet analysis reveals predator-prey community dynamics and environmental factors affecting predation of larval lake sturgeon Acipenser fulvescens in a natural system. J. Fish 2018, 93, 616–629. [Google Scholar] [CrossRef]
- Gosselin, E.N.; Lonsinger, R.C.; Waits, L.P. Comparing morphological and molecular diet analyses and fecal DNA sampling protocols for a terrestrial carnivore. Wildl. Soc. Bull. 2017, 41, 362–369. [Google Scholar] [CrossRef]
- Erickson, D.L.; Reed, E.; Ramachandran, P.; Bourg, N.A.; Mcshea, W.J.; Ottesen, A. Reconstructing a herbivore’s diet using a novel rbcL DNA mini-barcode for plants. AoB Plants 2017, 9, plx015. [Google Scholar] [CrossRef] [Green Version]
- Panteli, N.; Mastoraki, M.; Nikouli, E.; Lazarina, M.; Antonopoulou, E.; Kormas, K.A. Imprinting statistically sound conclusions for gut microbiota in comparative animal studies: A case study with diet and teleost fishes. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 36, 100738. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zang, S.; Li, L.; Hu, X.; Zhao, S.; Li, K.; Hu, D. Evaluation of fecal DNA preservation and extraction methods in Przewalski’s horse. Conserv. Genet. Resour. 2014, 6, 511–513. [Google Scholar] [CrossRef]
- Lopes, C.M.; De Barba, M.; Boyer, F.; Mercier, C.; Da, S.F.; Heidtmann, L.M.; Galiano, D.; Kubiak, B.B.; Langone, P.; Garcias, F.M.; et al. DNA metabarcoding diet analysis for species with parapatric vs sympatric distribution: A case study on subterranean rodents. Heredity 2015, 114, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Barba, M.; Miquel, C.; Boyer, F.; Mercier, C.; Rioux, D.; Coissac, E.; Taberlet, P. DNA metabarcoding multiplexing and validation of data accuracy for diet assessment: Application to omnivorous diet. Mol. Ecol. Resour. 2014, 14, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Kartzinel, T.R.; Pringle, R.M. Molecular detection of invertebrate prey in vertebrate diets: Trophic ecology of Caribbean island lizards. Mol. Ecol. Resour. 2015, 15, 903–914. [Google Scholar] [CrossRef]
- Bohmann, K.; Monadjem, A.; Lehmkuhl, N.C.; Rasmussen, M.; Zeale, M.R.; Clare, E.; Jones, G.; Willerslev, E.; Gilbert, M.T. Molecular diet analysis of two african free-tailed bats (molossidae) using high throughput sequencing. PLoS ONE 2011, 6, e21441. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Jarman, S.N.; Haman, K.H.; Trites, A.W.; Deagle, B.E. Improving accuracy of DNA diet estimates using food tissue control materials and an evaluation of proxies for digestion bias. Mol. Ecol. 2014, 23, 3706–3718. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, X.; Shafer, A.B.A.; Gong, M.; Han, M.; Yu, C.; Zhou, J.; Bai, J.; Meng, D.; Yu, G.; et al. Genetic structure and population history of wintering Asian Great Bustard (Otis tarda dybowskii) in China: Implications for conservation. J. Ornithol. 2017, 158, 761–772. [Google Scholar] [CrossRef]
- Vesterinen, E.J.; Ruokolainen, L.; Wahlberg, N.; Peña, C.; Roslin, T.; Laine, V.N.; Vasko, V.; Sääksjärvi, I.E.; Norrdahl, K.; Lilley, T. What you need is what you eat? Prey selection by the batMyotis daubentonii. Mol. Ecol. 2016, 25, 1581–1594. [Google Scholar] [CrossRef]
- Shehzad, W.; Riaz, T.; Nawaz, M.A.; Miquel, C.; Poillot, C.; Shah, S.A.; Pompanon, F.; Coissac, E.; Taberlet, P. Carnivore diet analysis based on next-generation sequencing: Application to the leopard cat (Prionailurus bengalensis) in Pakistan. Mol. Ecol. 2012, 21, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.S.; Ebenezer, K.L.; Symondson, W.O. Molecular analysis of the diets of snakes: Changes in prey exploitation during development of the rare smooth snake Coronella austriaca. Mol. Ecol. 2014, 23, 3734–3743. [Google Scholar] [CrossRef]
- Valentini, A.; Pompanon, F.; Taberlet, P. DNA barcoding for ecologists. Trends Ecol. Evol. 2009, 24, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Oehm, J.; Juen, A.; Nagiller, K.; Neuhauser, S.; Traugott, M. Molecular scatology: How to improve prey DNA detection success in avian faeces? Mol. Ecol. Resour. 2011, 11, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Jedlicka, J.A.; Sharma, A.M.; Almeida, R.P.P. Molecular tools reveal diets of insectivorous birds from predator fecal matter. Conserv. Genet. Resour. 2013, 5, 879–885. [Google Scholar] [CrossRef] [Green Version]
- Coissac, E. OligoTag: A program for designing sets of tags for next-generation sequencing of multiplexed samples. Data Prod. Anal. Popul. Genom. Methods Protoc. 2012, 888, 13–31. [Google Scholar]
- Kumari, P.; Dong, K.; Eo, K.Y.; Lee, W.S.; Kimura, J.; Yamamoto, N. DNA metabarcoding-based diet survey for the Eurasian otter (Lutra lutra): Development of a Eurasian otter-specific blocking oligonucleotide for 12S rRNA gene sequencing for vertebrates. PLoS ONE 2019, 14, e0226253. [Google Scholar] [CrossRef] [PubMed]
- Vestheim, H.; Jarman, S.N. Blocking primers to enhance PCR amplification of rare sequences in mixed—A case study on prey DNA in Antarctic krill stomachs. Front. Zool. 2008, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñol, J.; San Andrés, V.; Clare, E.; Mir, G.; Symondson, W. A pragmatic approach to the analysis of diets of generalist predators: The use of next-generation sequencing with no blocking probes. Mol. Ecol. Resour. 2014, 14, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.E.; Menning, D.M.; Wald, E.J.; Talbot, S.L.; Rattenbury, K.L.; Prugh, L.R. Using next generation sequencing of alpine plants to improve fecal metabarcoding diet analysis for Dall’s sheep. BMC Res. Notes 2021, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Leray, M.; Yang, J.Y.; Meyer, C.; Mills, S.C.; Agudelo, N.; Ranwez, V.; Boehm, J.T.; Machida, R.J. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: Application for characterizing coral reef fish gut contents. Front Zool. 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, T.; Shehzad, W.; Viari, A.; Pompanon, F.; Taberlet, P.; Coissac, E. ecoPrimers: Inference of new DNA barcode markers from whole genome sequence analysis. Nucleic Acids Res. 2011, 39, e145. [Google Scholar] [CrossRef] [PubMed]
- Burgar, J.M.; Murray, D.C.; Craig, M.D.; Haile, J.; Houston, J.; Stokes, V.; Bunce, M. Who’s for dinner? High-throughput sequencing reveals bat dietary differentiation in a biodiversity hotspot where prey taxonomy is largely undescribed. Mol. Ecol. 2014, 23, 3605–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siriwardena, G.M.; Stevens, D.K.; Anderson, G.Q.A.; Vickery, J.A.; Calbrade, N.A.; Dodd, S. The effect of supplementary winter seed food on breeding populations of farmland birds: Evidence from two large-scale experiments. J. Appl. Ecol. 2007, 44, 920–932. [Google Scholar] [CrossRef]
- Soininen, E.M.; Valentini, A.; Coissac, E.; Miquel, C.; Gielly, L.; Brochmann, C.; Brysting, A.K.; Sønstebø, J.H.; Ims, A.R.; Yoccoz, N.G.; et al. Analysing diet of small herbivores: The efficiency of DNA barcoding coupled with high-throughput pyrosequencing for deciphering the composition of complex plant mixtures. Front. Zool. 2009, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Pompanon, F.; Deagle, B.; Symondson, W.O.C.; Brown, D.S.; Jarman, S.; Taberlet, P. Who is eating what: Diet assessment using next generation sequencing. Mol. Ecol. 2011, 21, 1931–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahaye, R.; Bank, M.V.D.; Bogarin, D.; Warner, J.; Pupulin, F.; Gigot, G.; Maurin, O.; Duthoit, S.; Barraclough, T.G.; Savolainen, V. From the Cover: DNA barcoding the floras of biodiversity hotspots. Proc. Natl. Acad. Sci. USA 2008, 105, 2923–2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, F.; Clare, E.L.; Symondson, W.O.C.; Keišs, O.; Pētersons, G. Diet of the insectivorous bat Pipistrellus nathusii during autumn migration and summer residence. Mol. Ecol. 2014, 23, 3672–3683. [Google Scholar] [CrossRef]
- Pegard, A.; Miquel, C.; Valentini, A.; Coissac, E.; Bouvier, F.; François, D.; Taberlet, P.; Engel, E.; Pompanon, F. Universal DNA-based methods for assessing the diet of grazing livestock and wildlife from feces. J. Agric. Food Chem. 2009, 57, 5700–5706. [Google Scholar]
- Espunyes, J.; Bartolomé, J.; Garel, M.; Gálvez-Cerón, A.; Fernández Aguilar, X.; Colom-Cadena, A.; Calleja, J.A.; Gassó, D.; Jarque, L.; Lavín, S.; et al. Seasonal diet composition of Pyrenean chamois is mainly shaped by primary production waves. PLoS ONE 2019, 14, e0210819. [Google Scholar] [CrossRef] [PubMed]
- Šturm, M.B.; Smith, S.; Ganbaatar, O.; Buuveibaatar, B.; Balint, B.; Payne, J.C.; Voigt, C.C.; Kaczensky, P. Isotope analysis combined with DNA barcoding provide new insights into the dietary niche of khulan in the Mongolian Gobi. PLoS ONE 2021, 16, e0248294. [Google Scholar] [CrossRef] [PubMed]
- Egeter, B.; Bishop, P.J.; Robertson, B.C. Detecting frogs as prey in the diets of introduced mammals: A comparison between morphological and DNA-based diet analyses. Mol. Ecol. Resour. 2015, 15, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Spitzer, S.M.; Thomas, A.C.; Kohnert, C.M.; Keates, T.R.; Acevedo-Gutierrez, A. Large-scale molecular diet analysis in a generalist marine mammal reveals male preference for prey of conservation concern. Ecol. Evol. 2018, 8, 9889–9905. [Google Scholar] [CrossRef]
- Jang-Liaw, N.H. A barcoding-based scat-analysis assessment of Eurasian otter Lutra lutra diet on Kinmen Island. Ecol. Evol. 2021, 11, 8795–8813. [Google Scholar] [CrossRef]
- Soininen, E.M.; Gauthier, G.; Bilodeau, F.; Berteaux, D.; Gielly, L.; Taberlet, P.; Gussarova, G.; Bellemain, E.; Hassel, K.; Stenøien, H.K.; et al. Highly Overlapping Winter Diet in Two Sympatric Lemming Species Revealed by DNA Metabarcoding. PLoS ONE 2015, 10, e0115335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orly, R.; Clare, E.L.; Zeale, M.R.K.; Julia, H.; Bærholm, S.I.; Morten, R.; Gilbert, T.; Gareth, J. High-throughput sequencing offers insight into mechanisms of resource partitioning in cryptic bat species. Ecol. Evol. 2011, 1, 556–570. [Google Scholar]
- Schmitt, R.J.; Coyer, J.A. Variation in surfperch diets between allopatry and sympatry: Circumstantial evidence for competition. Oecologia 1983, 58, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef] [Green Version]
- Weimerskirch, H.; Cherel, Y.; Cuenot-Chaillet, F.; Ridoux, V. Alternative Foraging Strategies and Resource Allocation by Male and Female Wandering Albatrosses. Ecology 1997, 78, 2051–2063. [Google Scholar] [CrossRef]
- Shao, X.; Lu, Q.; Xiong, M.; Bu, H.; Shi, X.; Wang, D.; Zhao, J.; Li, S.; Yao, M. Prey partitioning and livestock consumption in the world’s richest large carnivore assemblage. Curr. Biol. 2021, 31, 4887–4897.e5. [Google Scholar] [CrossRef]
- Olsson, I.C.; Greenberg, L.A.; Bergman, E.; Wysujack, K. Environmentally induced migration: The importance of food. Ecol. Lett. 2006, 9, 645–651. [Google Scholar] [CrossRef]
- Bounas, A.; Sotiropoulos, K. Change of feeding strategy prior to migration: A comparative diet analysis in the Lesser Kestrel (Falco naumanni). Avian Biol. Res. 2017, 10, 27–35. [Google Scholar] [CrossRef]
- Trevelline, B.K.; Latta, S.C.; Marshall, L.C.; Nuttle, T.; Porter, B.A. Molecular analysis of nestling diet in a long-distance Neotropical migrant, the Louisiana Waterthrush (Parkesia motacilla). Auk 2016, 133, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Jarman, S.N.; McInnes, J.; Faux, C.; Polanowski, A.; Marthick, J.; Deagle, B.; Southwell, C.; Emmerson, L. Adélie Penguin Population Diet Monitoring by Analysis of Food DNA in Scats. PLoS ONE 2013, 8, e82227. [Google Scholar] [CrossRef] [PubMed]
- Bourret, V.; Gutiérrez López, R.; Melo, M.; Loiseau, C. Metabarcoding options to study eukaryotic endoparasites of birds. Ecol. Evol. 2021, 11, 10821–10833. [Google Scholar] [CrossRef]
- Jo, H.; Ventura, M.; Vidal, N.; Gim, J.; Buchaca, T.; Barmuta, L.A.; Jeppesen, E.; Joo, G. Discovering hidden biodiversity: The use of complementary monitoring of fish diet based on DNA barcoding in freshwater ecosystems. Ecol. Evol. 2015, 6, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deagle, B.E.; Thomas, A.C.; Shaffer, A.K.; Trites, A.W.; Jarman, S.N. Quantifying sequence proportions in a DNA-based diet study using Ion Torrent amplicon sequencing: Which counts count? Mol. Ecol. Resour. 2013, 13, 620–633. [Google Scholar] [CrossRef]
- Leray, M.; Meyer, C.P.; Mills, S.C. Metabarcoding dietary analysis of coral dwelling predatory fish demonstrates the minor contribution of coral mutualists to their highly partitioned, generalist diet. PeerJ 2015, 3, e1047. [Google Scholar] [CrossRef] [PubMed]
- Bowser, A.K.; Diamond, A.W.; Addison, J.A. From puffins to plankton: A DNA-based analysis of a seabird food chain in the northern Gulf of Maine. PLoS ONE 2013, 8, e83152. [Google Scholar] [CrossRef] [Green Version]
- Czernik, M.; Taberlet, P.; Świsłocka, M.; Czajkowska, M.; Duda, N.; Ratkiewicz, M. Fast and efficient DNA-based method for winter diet analysis from stools of three cervids: Moose, red deer, and roe deer. Mammal Res. 2013, 58, 379–386. [Google Scholar]
- Murray, D.C.; Bunce, M.; Cannell, B.; Oliver, R.; Houston, J.; White, N.; Barrero, R.; Bellgard, M.; Haile, J. DNA-Based Faecal Dietary Analysis: A Comparison of qPCR and High Throughput Sequencing Approaches. PLoS ONE 2011, 6, e25776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivathsan, A.; Sha, J.; Vogler, A.; Meier, R. Comparing the effectiveness of metagenomics and metabarcoding for diet analysis of a leaf-feeding monkey (Pygathrix nemaeus). Mol. Ecol. Resour. 2015, 15, 250–261. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Aizen, M.A.; Alcántara, J.M.; Arroyo, J.; Cocucci, A.; Galetti, M.; García, M.B.; García, D.; Gómez Jordano, J.M. Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 2015, 29, 299–307. [Google Scholar] [CrossRef]
- Carreira, B.M.; Segurado, P.; Orizaola, G.; Gonçalves, N.; Pinto, V.; Laurila, A.; Rebelo, R. Warm vegetarians? Heat waves and diet shifts in tadpoles. Ecology 2016, 97, 2964–2974. [Google Scholar]
- Hambäck, P.A.; Weingartner, E.; Dalén, L.; Wirta, H.; Roslin, T. Spatial subsidies in spider diets vary with shoreline structure: Complementary evidence from molecular diet analysis and stable isotopes. Ecol. Evol. 2016, 6, 8431–8439. [Google Scholar] [CrossRef]
- Matley, J.K.; Maes, G.E.; Devloo-Delva, F.; Huerlimann, R.; Chua, G.; Tobin, A.J.; Fisk, A.T.; Simpfendorfer, C.A.; Heupel, M.R. Integrating complementary methods to improve diet analysis in fishery-targeted species. Ecol. Evol. 2018, 8, 9503–9515. [Google Scholar] [CrossRef]
- Witt, K.E.; Yarlagadda, K.; Allen, J.M.; Bader, A.C.; Simon, M.L.; Kuehn, S.R.; Swanson, K.S.; Cross, T.L.; Hedman, K.M.; Ambrose, S.H.; et al. Integrative analysis of DNA, macroscopic remains and stable isotopes of dog coprolites to reconstruct community diet. Sci. Rep. 2021, 11, 3113. [Google Scholar] [CrossRef] [PubMed]
- Grillo, K.M.; Dunne, J.; Marshall, F.; Prendergast, M.E.; Casanova, E.; Gidna, A.O.; Janzen, A.; Munene, K.; Keute, J.; Mabulla, A.Z.P.; et al. Molecular and isotopic evidence for milk, meat, and plants in prehistoric eastern African herder food systems. Proc. Natl. Acad. Sci. USA 2020, 117, 9793–9799. [Google Scholar] [CrossRef] [PubMed]
- Chua, P.Y.S.; Crampton-Platt, A.; Lammers, Y.; Alsos, I.G.; Boessenkool, S.; Bohmann, K. Metagenomics: A viable tool for reconstructing herbivore diet. Mol. Ecol. Resour. 2021, 21, 2249–2263. [Google Scholar] [CrossRef] [PubMed]
- Marcum, J.A. Nutrigenetics/Nutrigenomics, Personalized Nutrition, and Precision Healthcare. Curr. Nutr. Rep. 2020, 9, 338–345. [Google Scholar] [CrossRef]
- Nasir, A.; Bullo, M.M.H.; Ahmed, Z.; Imtiaz, A.; Yaqoob, E.; Jadoon, M.; Ahmed, H.; Afreen, A.; Yaqoob, S. Nutrigenomics: Epigenetics and cancer prevention: A comprehensive review. Crit. Rev. Food. Sci. Nutr. 2020, 60, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
| Prey Types | Prey Taxa | Target | Primer Name | Primer Sequence (5′–3′) | Blocking Nucleotides | References |
|---|---|---|---|---|---|---|
| Vertebrata | Metazoan | COI | mlCOIint-F: | GGWACWGGWTGAACWGTWTAYCCYCC | Human blocking: CTATGCTTAGCCCTAAACCTCAACAGTTAAATCAACAAAACTGCT-C3 Mammal blocking: CTAGGGATAACAGCGCAATCCTATT-C3 or GATAGCTTACATAACAAAACTATCTGC-C3 | [55] |
| jgHCO2198-R: | TAIACYTCIGGRTGICCRAARAAYCA | |||||
| Mammal | 12S V5 | 12SV5-F: | TTAGATACCCCACTATGC | [39,45] | ||
| 12SV5-R: | TAGAACAGGCTCCTCTAG | |||||
| Amphibian | Cytb | RT-F | TACAGCCGATACCTCCCTC | [46,51] | ||
| RT-R | TTCATGTCTCTTTGTAGAGG | |||||
| Fish | 16S | Chord_16S_F | CGAGAAGACCCTRTGGAGCT | [42,56] | ||
| Chord16S_R | CCTNGGTCGCCCCAAC | |||||
| Invertebrate | Arthropoda | COI | ZBJ-Art-F | AGATATTGGAACWTTATATTTTATTTTTGG | [28,57] | |
| ZBJ-Art-R | WACTAATCAATTWCCAAATCCTCC | |||||
| Arthropoda | 16S | IN16STK-F | TGAACTCAGATCATGTAA | [40] | ||
| IN16STK-R | TTAGGGATAACAGCGTAA | |||||
| Mollusca | 16S | 16SMAV-F | CCAACATCGAGGTCRYAA | [39] | ||
| 16SMAV-R | ARTTACYNTAGGGATAACAG | |||||
| Annelida | 12S | 185F | TGTGTACTGCCGTCGTAAGCA | [46] | ||
| 14233R | AAGAGCGACGGGCGATGTGT | |||||
| Plant | Universal | trnL P6 | G | GGGCAATCCTGAGCCAA | [38,58] | |
| H | CCATTGAGTCTCTGCACCTATC | |||||
| Universal | rbcL | rbcLa-F | ATGTCACCACAAACAGAGACTAAAGCGTAAAATCAAGTCCACCRCG | [32] | ||
| rbcLa-R | ||||||
| Universal | rbcL | rbcL-F | CTTACCAGYCTTGATCGTTACAAAGGGTAAAATCAAGTCCACCRCG | [35] | ||
| rbcL-R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zhang, S.; Zhao, X.; Li, C.; Gong, M. Advances and Limitations of Next Generation Sequencing in Animal Diet Analysis. Genes 2021, 12, 1854. https://doi.org/10.3390/genes12121854
Liu G, Zhang S, Zhao X, Li C, Gong M. Advances and Limitations of Next Generation Sequencing in Animal Diet Analysis. Genes. 2021; 12(12):1854. https://doi.org/10.3390/genes12121854
Chicago/Turabian StyleLiu, Gang, Shumiao Zhang, Xinsheng Zhao, Chao Li, and Minghao Gong. 2021. "Advances and Limitations of Next Generation Sequencing in Animal Diet Analysis" Genes 12, no. 12: 1854. https://doi.org/10.3390/genes12121854
APA StyleLiu, G., Zhang, S., Zhao, X., Li, C., & Gong, M. (2021). Advances and Limitations of Next Generation Sequencing in Animal Diet Analysis. Genes, 12(12), 1854. https://doi.org/10.3390/genes12121854






