The Influence of CYP2D6 and CYP2C19 Genetic Variation on Diabetes Mellitus Risk in People Taking Antidepressants and Antipsychotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Phenotype Data
2.2. Genetic Data and Quality Control
2.3. Assigning CYP Metabolic Phenotype
2.4. Statistical Analysis
3. Results
3.1. Dataset
3.2. Psychotropics Prescribed in the UK Biobank
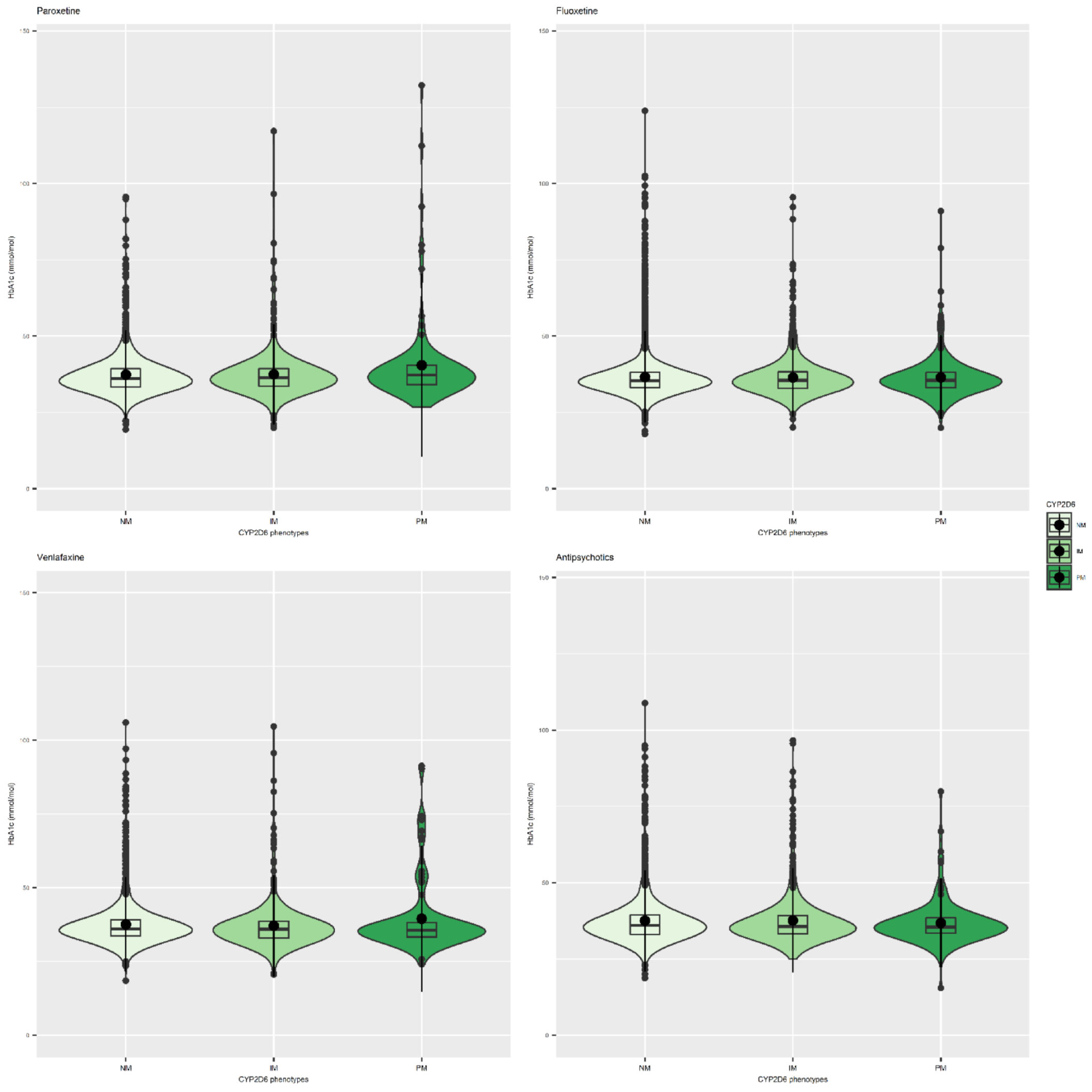
3.3. Antidepressants and CYP Metabolic Status
3.4. Antipsychotics and CYP Metabolic Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, S.; Annand, F.; Burkinshaw, P.; Greaves, F.; Kelleher, M.; Knight, J.; Perkins, C.; Tran, A.; White, M.; Marsden, J. Dependence and Withdrawal Associated with Some Prescribed Medicines: An Evidence Review; Public Health England: London, UK, 2019; p. 152. [Google Scholar]
- Prescription Cost Analysis—England. 2018. NHS Digital. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed on 24 August 2020).
- Iacobucci, G. NHS prescribed record number of antidepressants last year. BMJ 2019, 364, 1508. [Google Scholar] [CrossRef] [PubMed]
- Andersohn, F.; Schade, R.; Suissa, S.; Garbe, E. Long-Term Use of Antidepressants for Depressive Disorders and the Risk of Diabetes Mellitus. Am. J. Psychiatry 2009, 166, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.I.G. Association Between Antipsychotic Medication Use and Diabetes. Curr. Diabetes Rep. 2019, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 2004, 27, 596–601. [Google Scholar] [CrossRef]
- Gill, S.P. Stable monotherapy with clozapine or olanzapine increases the incidence of diabetes mellitus in people with schizophrenia. Evid. Based Ment. Health 2005, 8, 24. [Google Scholar] [CrossRef][Green Version]
- Leslie, D.L.; Rosenheck, R.A. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am. J. Psychiatry 2004, 161, 1709–1711. [Google Scholar] [CrossRef]
- Schwenkreis, P.; Assion, H.-J. Atypical antipsychotics and diabetes mellitus. World J. Biol. Psychiatry 2004, 5, 73–82. [Google Scholar] [CrossRef]
- Taylor, D.; Barnes, T.; Young, A. The Maudsley Prescribing Guidelines in Psychiatry, 13th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Kivimäki, M.; Hamer, M.; Batty, G.D.; Geddes, J.R.; Tabak, A.G.; Pentti, J.; Virtanen, M.; Vahtera, J. Antidepressant medication use, weight gain, and risk of type 2 diabetes: A population-based study. Diabetes Care 2010, 33, 2611–2616. [Google Scholar] [CrossRef]
- Lustman, P.J.; Griffith, L.S.; Clouse, R.E.; Freedland, K.E.; Eisen, S.A.; Rubin, E.H.; Carney, R.M.; McGill, J.B. Effects of Nortriptyline on Depression and Glycemic Control in Diabetes: Results of a Double-blind, Placebo-controlled Trial. Psychosom. Med. 1997, 59, 241–250. [Google Scholar] [CrossRef]
- Mumoli, N.; Cocciolo, M.; Vitale, J.; Mantellassi, M.; Sabatini, S.; Gambaccini, L.; Cei, M. Diabetes mellitus associated with clomipramine treatment: A retrospective analysis. Acta Diabetol. 2014, 51, 167–168. [Google Scholar] [CrossRef]
- Burcu, M.; Zito, J.M.; Safer, D.J.; Magder, L.S.; dos Reis, S.; Shaya, F.T.; Rosenthal, G.L. Association of Antidepressant Medications with Incident Type 2 Diabetes Among Medicaid-Insured Youths. JAMA Pediatr. 2017, 171, 1200–1207. [Google Scholar] [CrossRef]
- Gagnon, J.; Lussier, M.-T.; MacGibbon, B.; Daskalopoulou, S.S.; Bartlett, G. The Impact of Antidepressant Therapy on Glycemic Control in Canadian Primary Care Patients with Diabetes Mellitus. Front. Nutr. 2018, 5, 47. [Google Scholar] [CrossRef]
- Hall, J.A.; Wang, F.; Oakes, T.M.M.; Utterback, B.G.; Crucitti, A.; Acharya, N. Safety and tolerability of duloxetine in the acute management of diabetic peripheral neuropathic pain: Analysis of pooled data from three placebo-controlled clinical trials. Expert Opin. Drug Saf. 2010, 9, 525–537. [Google Scholar] [CrossRef]
- Barnard, K.; Peveler, R.C.; Holt, R.I.G. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: Systematic review. Diabetes Care 2013, 36, 3337–3345. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): Clinical consequences, evolutionary aspects and functional diversity. Pharm. J. 2005, 5, 6–13. [Google Scholar] [CrossRef]
- Sim, S.C.; Ingelman-Sundberg, M. The Human Cytochrome P450 (CYP) Allele Nomenclature website: A peer-reviewed database of CYP variants and their associated effects. Hum. Genom. 2010, 4, 278–281. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M.; Sim, S.C.; Gomez, A.; Rodriguez-Antona, C. Influence of cytochrome P450 polymorphisms on drug therapies: Pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol. Ther. 2007, 116, 496–526. [Google Scholar] [CrossRef]
- Gaedigk, A.; Sangkuhl, K.; Whirl-Carrillo, M.; Klein, T.; Leeder, J.S. Prediction of CYP2D6 phenotype from genotype across world populations. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 69–76. [Google Scholar] [CrossRef]
- Martis, S.; Peter, I.; Hulot, J.-S.; Kornreich, R.; Desnick, R.J.; Scott, S.A. Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharm. J. 2013, 13, 369–377. [Google Scholar] [CrossRef]
- Grasmäder, K.; Verwohlt, P.L.; Rietschel, M.; Dragicevic, A.; Müller, M.; Hiemke, C.; Freymann, F.N.; Zobel, A.; Maier, W.; Rao, M.L. Impact of polymorphisms of cytochrome-P450 isoenzymes 2C9, 2C19 and 2D6 on plasma concentrations and clinical effects of antidepressants in a naturalistic clinical setting. Eur. J. Clin. Pharmacol. 2004, 60, 329–336. [Google Scholar] [CrossRef]
- Chang, M.; Tybring, G.; Dahl, M.-L.; Lindh, J.D. Impact of cytochrome P450 2C19 polymorphisms on citalopram/escitalopram exposure: A systematic review and meta-analysis. Clin. Pharmacokinet. 2014, 53, 801–811. [Google Scholar] [CrossRef]
- Huezo-Diaz, P.; Perroud, N.; Spencer, E.P.; Smith, R.; Sim, S.; Virding, S.; Uher, R.; Gunasinghe, C.; Gray, C.; Campbell, D.; et al. CYP2C19 genotype predicts steady state escitalopram concentration in GENDEP. J. Psychopharmacol. 2012, 26, 398–407. [Google Scholar] [CrossRef]
- Chen, R.; Wang, H.; Shi, J.; Shen, K.; Hu, P. Cytochrome P450 2D6 genotype affects the pharmacokinetics of controlled-release paroxetine in healthy Chinese subjects: Comparison of traditional phenotype and activity score systems. Eur. J. Clin. Pharmacol. 2015, 71, 835–841. [Google Scholar] [CrossRef]
- Rudberg, I.; Hermann, M.; Refsum, H.; Molden, E. Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. Eur. J. Clin. Pharmacol. 2008, 64, 1181–1188. [Google Scholar] [CrossRef]
- Schenk, P.W.; van Vliet, M.; Mathot, R.A.; van Gelder, T.; Vulto, A.G.; van Fessem, M.A.C.; Rij, S.V.-V.; Lindemans, J.; Bruijn, J.A.; van Schaik, R.H.N. The CYP2C19*17 genotype is associated with lower imipramine plasma concentrations in a large group of depressed patients. Pharm. J. 2010, 10, 219–225. [Google Scholar] [CrossRef]
- Milosavljevic, F.; Bukvic, N.; Pavlovic, Z.; Miljevic, C.; Pešic, V.; Molden, E.; Ingelman-Sundberg, M.; Leucht, S.; Jukić, M.M. Association of CYP2C19 and CYP2D6 Poor and Intermediate Metabolizer Status with Antidepressant and Antipsychotic Exposure: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 270–280. [Google Scholar] [CrossRef]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharm. Ther. 2016, 102, 37–44. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Muller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef]
- Swen, J.J.; Nijenhuis, M.; de Boer, A.; Grandia, L.; Maitland-van der Zee, A.H.; Mulder, H.; Rongen, G.A.; van Schaik, R.H.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From bench to byte—An update of guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef]
- Turner, R.M.; Newman, W.G.; Bramon, E.; McNamee, C.J.; Wong, W.L.; Misbah, S.; Hill, S.; Caulfield, M.; Pirmohamed, M. Pharmacogenomics in the UK National Health Service: Opportunities and challenges. Pharmacogenomics 2020, 21, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- van Westrhenen, R.; Aitchison, K.J.; Ingelman-Sundberg, M.; Jukić, M.M. Pharmacogenomics of Antidepressant and Antipsychotic Treatment: How Far Have We Got and Where Are We Going? Front. Psychiatry 2020, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Conomos, M.P.; Reiner, A.P.; Weir, B.S.; Thornton, T.A. Model-free Estimation of Recent Genetic Relatedness. Am. J. Hum. Genet. 2016, 98, 127–148. [Google Scholar] [CrossRef]
- Conomos, M.P.; Miller, M.B.; Thornton, T.A. Robust inference of population structure for ancestry prediction and correction of stratification in the presence of relatedness. Genet. Epidemiol. 2015, 39, 276–293. [Google Scholar] [CrossRef]
- Manichaikul, A.; Mychaleckyj, J.C.; Rich, S.S.; Daly, K.; Sale, M.; Chen, W.-M. Robust relationship inference in genome-wide association studies. Bioinformatics 2010, 26, 2867–2873. [Google Scholar] [CrossRef]
- Gogarten, S.M.; Bhangale, T.; Conomos, M.P.; Laurie, C.A.; McHugh, C.P.; Painter, I.; Zheng, X.; Crosslin, D.R.; Levine, D.; Lumley, T.; et al. GWASTools: An R/Bioconductor package for quality control and analysis of genome-wide association studies. Bioinformatics 2012, 28, 3329–3331. [Google Scholar] [CrossRef]
- Morris, J.A.; Randall, J.C.; Maller, J.B.; Barrett, J.C. Evoker: A visualization tool for genotype intensity data. Bioinformatics 2010, 26, 1786–1787. [Google Scholar] [CrossRef][Green Version]
- Browning, B.; Zhou, Y.; Browning, S. A one-penny imputed genome from next generation reference panels. Am. J. Hum. Genet. 2016, 103, 338–348. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef]
- Delaneau, O.; Marchini, J.L.; McVeanh, G.A.; Donnelly, P.; Lunter, G.; Marchini, J.L.; Myers, S.; Gupta-Hinch, A.; Iqbal, Z.; Mathieson, I.; et al. Integrating sequence and array data to create an improved 1000 Genomes Project haplotype reference panel. Nat. Commun. 2014, 5, 3934. [Google Scholar] [CrossRef]
- Pharmacogene Variation Consortium (PharmVar), (Gaedigk et al. 2018, CPT 103:399; Gaedigk et al. 2019, CPT 105:29. Available online: www.PharmVar.org (accessed on 1 September 2019).
- Gaedigk, A.; Simon, S.D.; Pearce, R.E.; Bradford, L.D.; Kennedy, M.J.; Leeder, J.S. The CYP2D6 activity score: Translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 2008, 83, 234–242. [Google Scholar] [CrossRef]
- Gaedigk, A.; Dinh, J.C.; Jeong, H.; Prasad, B.; Leeder, J.S. Ten years’ experience with the CYP2D6 activity score: A perspective on future investigations to improve clinical predictions for precision therapeutics. J. Pers. Med. 2018, 8, 15. [Google Scholar] [CrossRef]
- Roopan, S.; Larsen, E.R. Use of antidepressants in patients with depression and comorbid diabetes mellitus: A systematic review. Acta Neuropsychiatr. 2017, 29, 127–139. [Google Scholar] [CrossRef]
- Stingl, J.; Oesterheld, J.; Turpeinen, M. Metabolism of Psychotropic drugs. In Encyclopedia of Drug Metabolism and Interaction, 1st ed.; Lyubimov, A.V., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
- Li, M.X.; Yeung, J.M.Y.; Cherny, S.S.; Sham, P.C. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 2012, 131, 747–756. [Google Scholar] [CrossRef]
- R Core Team. R:. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 September 2019).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis Media; Springer: New York, NY, USA, 2016; Available online: http://ggplot2.org (accessed on 1 September 2019).
- Dowle, M.; Srinivasan, A. data.table: Extension of data.frame. 2019. Available online: https://CRAN.R-project.org/package=data.table (accessed on 1 September 2019).
- Zhang, J.P.; Lencz, T.; Zhang, R.X.; Nitta, M.; Maayan, L.; John, M.; Robinson, D.G.; Fleischhacker, W.W.; Kahn, R.S.; Ophoff, R.A.; et al. Pharmacogenetic Associations of Antipsychotic Drug-Related Weight Gain: A Systematic Review and Meta-analysis. Schizophr. Bull. 2016, 42, 1418–1437. [Google Scholar] [CrossRef]
- Kumar, Y.; Kung, S.; Shinozaki, G. CYP2C19 variation, not citalopram dose nor serum level, is associated with QTc prolongation. J. Psychopharmacol. 2014, 28, 1143–1148. [Google Scholar] [CrossRef]
- Tay, J.K.X.; Tan, C.H.; Chong, S.A.; Tan, E.C. Functional polymorphisms of the cytochrome P450 1A2 (CYP1A2) gene and prolonged QTc interval in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1297–1302. [Google Scholar] [CrossRef]
- Lane, H.-Y.; Liu, Y.-C.; Huang, C.-L.; Chang, Y.-C.; Wu, P.-L.; Lu, C.-T.; Chang, W.-H. Risperidone-related weight gain: Genetic and nongenetic predictors. J. Clin. Psychopharmacol. 2006, 26, 128–134. [Google Scholar] [CrossRef]
- Correia, C.T.; Almeida, J.P.; Santos, P.E.; Sequeira, A.F.; Marques, C.E.; Miguel, T.S.; Abreu, R.L.; Oliveira, G.G.; Vicente, A.M. Pharmacogenetics of risperidone therapy in autism: Association analysis of eight candidate genes with drug efficacy and adverse drug reactions. Pharm. J. 2010, 10, 418–430. [Google Scholar] [CrossRef]
- Sukasem, C.; Hongkaew, Y.; Ngamsamut, N.; Puangpetch, A.; Vanwong, N.; Chamnanphon, M.; Chamkrachchangpada, B.; Sinrachatanant, A.; Limsila, P. Impact of Pharmacogenetic Markers of CYP2D6 and DRD2 on Prolactin Response in Risperidone-Treated Thai Children and Adolescents with Autism Spectrum Disorders. J. Clin. Psychopharmacol. 2016, 36, 141–146. [Google Scholar] [CrossRef]
- Fleeman, N.; Dundar, Y.; Dickson, R.; Jorgensen, A.; Pushpakom, S.; McLeod, C.; Pirmohamed, M.; Walley, T. Cytochrome P450 testing for prescribing antipsychotics in adults with schizophrenia: Systematic review and meta-analyses. Pharm. J. 2011, 11, 1–14. [Google Scholar] [CrossRef]
- Calafato, M.S.; Austin-Zimmerman, I.; Thygesen, J.H.; Sairam, M.; Metastasio, A.; Marston, L.; Abad-Santos, F.; Bhat, A.; Harju-Seppänen, J.; Irizar, H.; et al. The effect of CYP2D6 variation on antipsychotic-induced hyperprolactinaemia: A systematic review and meta-analysis. Pharm. J. 2020, 20, 629–637. [Google Scholar] [CrossRef]
- Chávez-Castillo, M.; Ortega, Á.; Nava, M.; Fuenmayor, J.; Lameda, V.; Velasco, M.; Bermúdez, V.; Rojas-Quintero, J. Metabolic Risk in Depression and Treatment with Selective Serotonin Reuptake Inhibitors: Are the Metabolic Syndrome and an Increase in Cardiovascular Risk Unavoidable? Vessel Plus. 2018. Available online: https://vpjournal.net/article/view/2486 (accessed on 17 March 2021).
- Pan, A.; Sun, Q.; Okereke, O.I.; Rexrode, K.M.; Rubin, R.R.; Lucas, M.; Willett, W.C.; Manson, J.E.; Hu, F.B. Use of antidepressant medication and risk of type 2 diabetes: Results from three cohorts of US adults. Diabetologia 2012, 55, 63–72. [Google Scholar] [CrossRef]
- Yoon, J.M.; Cho, E.-G.; Lee, H.-K.; Park, S.M. Antidepressant use and diabetes mellitus risk: A meta-analysis. Korean J. Fam. Med. 2013, 34, 228–240. [Google Scholar] [CrossRef]
- Heald, A.H.; Stedman, M.; Davies, M.; Livingston, M.; Taylor, D.; Gadsby, R. Antidepressant Prescribing in England: Patterns and Costs. Prim. Care Companion CNS Disord. 2020, 16, 22. [Google Scholar] [CrossRef]
- Sindrup, S.H.; Brøsen, K.; Gram, L.F.; Hallas, J.; Skjelbo, E.; Allen, A.; Allen, G.D.; Cooper, S.M.; Mellows, G.; Tasker, T.C.; et al. The relationship between paroxetine and the sparteine oxidation polymorphism. Clin. Pharmacol. Ther. 1992, 51, 278–287. [Google Scholar] [CrossRef]
- Solai, L.K.; Pollock, B.G.; Mulsant, B.H.; Frye, R.F.; Miller, M.D.; Sweet, R.A.; Kirshner, M.; Sorisio, D.; Begley, A.; Reynolds, C.F. Effect of nortriptyline and paroxetine on CYP2D6 activity in depressed elderly patients. J. Clin. Psychopharmacol. 2002, 22, 481–486. [Google Scholar] [CrossRef]
- Zourková, A.; Hadasová, E. Paroxetine-induced conversion of cytochrome P450 2D6 phenotype and occurence of adverse effects. Gen. Physiol. Biophys. 2003, 22, 103–113. [Google Scholar] [PubMed]
- Khoza, S.; Barner, J.C.; Bohman, T.M.; Rascati, K.; Lawson, K.; Wilson, J.P. Use of antidepressant agents and the risk of type 2 diabetes. Eur. J. Clin. Pharmacol. 2012, 68, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, B.; Corcoy, R. Hypoglycemia associated with fluoxetine treatment in a patient with type 1 diabetes. World J. Clin. Cases 2013, 1, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Rebai, R.; Jasmin, L.; Boudah, A. The antidepressant effect of melatonin and fluoxetine in diabetic rats is associated with a reduction of the oxidative stress in the prefrontal and hippocampal cortices. Brain Res. Bull. 2017, 134, 142–150. [Google Scholar] [CrossRef]
- Baumeister, H.; Hutter, N.; Bengel, J. Psychological and Pharmacological Interventions for Depression in Patients with Diabetes Mellitus and Depression. Cochrane Database Syst. Rev. 2012, 12, CD008381. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008381.pub2/full (accessed on 26 March 2021). [CrossRef]
- Baumann, P.; Jonzier-Perey, M.; Koeb, L.; Küpfer, A.; Tinguely, D.; Schöpf, J. Amitriptyline pharmacokinetics and clinical response: II. Metabolic polymorphism assessed by hydroxylation of debrisoquine and mephenytoin. Int. Clin. Psychopharmacol. 1986, 1, 102–112. [Google Scholar] [CrossRef]
- Dean, L. Amitriptyline Therapy and CYP2D6 and CYP2C19 Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kane, M.S., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK425165/ (accessed on 4 June 2021).
- Gaziano, J.M.; Concato, J.; Brophy, M.; Fiore, L.; Pyarajan, S.; Breeling, J.; Whitbourne, S.; Deen, J.; Shannon, C.; Humphries, D.; et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 2016, 70, 214–223. [Google Scholar] [CrossRef]
- Rehman, A.; Setter, S.M.; Vue, M.H. Drug-induced glucose alteraions part 2: Drug-induced hyperglycemia. Diabetes Spectr. 2011, 24, 234–238. [Google Scholar] [CrossRef]
- Xue, A.; Wu, Y.; Zhu, Z.; Zhang, F.; Kemper, K.E.; Zheng, Z.; Yengo, L.; Lloyd-Jones, L.R.; Sidorenko, J.S.; Wu, Y.; et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 2018, 9, 2941. [Google Scholar] [CrossRef]
- Zhou, Y.; Ingelman-Sundberg, M.; Lauschke, V.M. Worldwide Distribution of Cytochrome P450 Alleles: A Meta-analysis of Population-scale Sequencing Projects. Clin. Pharmacol. Ther. 2017, 102, 688–700. [Google Scholar] [CrossRef]
- McInnes, G.; Lavertu, A.; Sangkuhl, K.; Klein, T.E.; Whirl-Carrillo, M.; Altman, R.B. Pharmacogenetics at Scale: An Analysis of the UK Biobank. Clin. Pharmacol. Ther. 2020, 109, 1528–1537. [Google Scholar] [CrossRef]
- Müller, D.J.; Kekin, I.; Kao, A.C.C.; Brandl, E.J. Towards the implementation of CYP2D6 and CYP2C19 genotypes in clinical practice: Update and report from a pharmacogenetic service clinic. Int. Rev. Psychiatry Abingdon Engl. 2013, 25, 554–571. [Google Scholar] [CrossRef]
- Altar, C.A.; Carhart, J.M.; Allen, J.D.; Hall-Flavin, D.K.; Dechairo, B.M.; Winner, J.G. Clinical validity: Combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharm. J. 2015, 15, 443–451. [Google Scholar] [CrossRef]
- Arranz, M.J.; Gonzalez-Rodriguez, A.; Perez-Blanco, J.; Penadés, R.; Gutierrez, B.; Ibañez, L.; Arias, B.; Brunet, M.; Cervilla, J.; Salazar, J.; et al. A pharmacogenetic intervention for the improvement of the safety profile of antipsychotic treatments. Transl. Psychiatry 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Greden, J.F.; Parikh, S.V.; Rothschild, A.J.; Thase, M.E.; Dunlop, B.W.; DeBattista, C.; Conway, C.R.; Forester, B.P.; Mondimore, F.M.; Shelton, R.C.; et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J. Psychiatr. Res. 2019, 111, 59–67. [Google Scholar] [CrossRef]
- Herbild, L.; Andersen, S.E.; Werge, T.; Rasmussen, H.B.; Jürgens, G. Does pharmacogenetic testing for CYP450 2D6 and 2C19 among patients with diagnoses within the schizophrenic spectrum reduce treatment costs? Basic Clin. Pharmacol. Toxicol. 2013, 113, 266–272. [Google Scholar] [CrossRef]
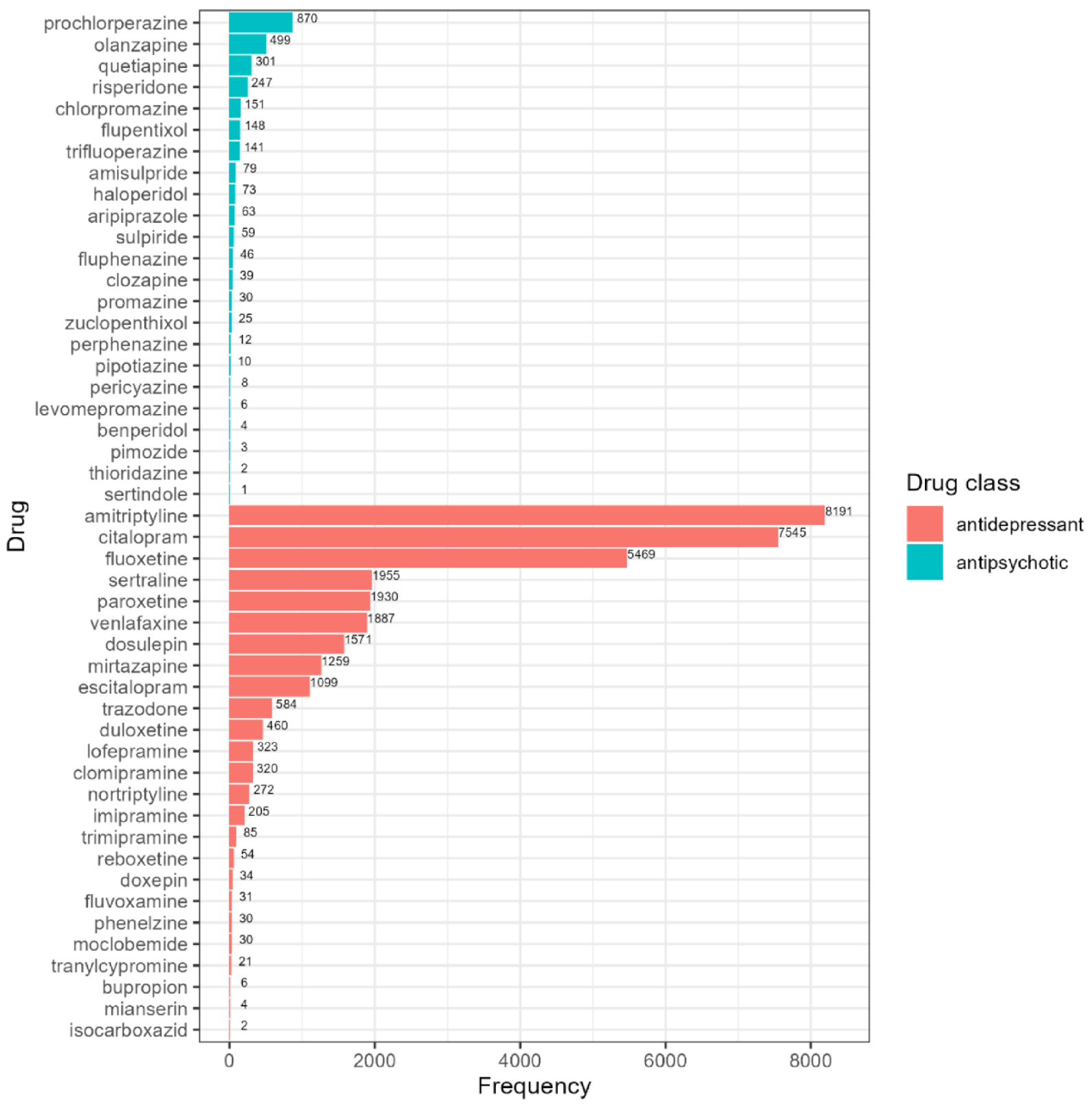
| Antidepressants (n = 31,579) | Antipsychotics (n = 2699) | |
|---|---|---|
| CYP2D6 metabolic phenotype | ||
| Normal metabolizers | 22,486 (71.2%) | 1914 (70.9%) |
| Intermediate metabolizers | 7433 (23.5%) | 650 (24.1%) |
| Poor metabolizers | 1660 (5.3%) | 135 (5.0%) |
| CYP2C19 metabolic phenotype | ||
| Normal metabolizers | 12,001 (38.0%) | 1004 (37.2%) |
| Intermediate metabolizers | 9367 (29.7%) | 789 (29.2%) |
| Poor metabolizers | 1065 (3.4%) | 100 (3.7%) |
| Rapid metabolizers | 7805 (24.7%) | 686 (25.4%) |
| Ultra-rapid metabolizers | 1341 (4.2%) | 120 (4.4%) |
| Takes CYP2D6 inhibitors A | ||
| No | 29,713 (94.1%) | 2548 (94.4%) |
| Yes | 1866 (5.9%) | 151 (5.6%) |
| Takes CYP2C19 inhibitors A | ||
| No | 23,608 (74.8%) | 2091 (77.5%) |
| Yes | 7971 (25.2%) | 608 (22.5%) |
| Sex | ||
| Female | 21,752 (68.9%) | 1553 (57.5%) |
| Male | 9827 (31.1%) | 1146 (42.5%) |
| Age | ||
| Mean (SD) (years) | 56.6 (7.78) | 56.4 (8.12) |
| Range (median) (years) | 40–70 (58) | 40–70 (57) |
| Ethnicity | ||
| Caucasian | 29,628 (93.8%) | 2403 (89.0%) |
| Admix Caucasian | 795 (2.5%) | 72 (2.7%) |
| African | 289 (0.9%) | 90 (3.3%) |
| East Asian | 43 (0.1%) | 12 (0.4%) |
| Other | 450 (1.4%) | 57 (2.1%) |
| South Asian | 374 (1.2%) | 65 (2.4%) |
| Hb1Ac | ||
| Mean (SD) (mmol/mol) | 37.1 (7.75) | 37.5 (8.31) |
| Diabetes status | ||
| No | 28,776 (91.1%) | 2415 (89.5%) |
| Yes | 2803 (8.9%) | 284 (10.5%) |
| Takes antidiabetic medications B | ||
| No | 29,573 (93.6%) | 2491 (92.3%) |
| Yes | 2006 (6.4%) | 208 (7.7%) |
| BMI | ||
| Mean (SD) (kg/m2) | 28.8 (5.66) | 29.1 (5.94) |
| Predictors | Paroxetine | |||
|---|---|---|---|---|
| n | Estimates | CI | p | |
| Diabetes | 174 | 6.85 | 5.11, 8.59 | <0.001 |
| CYP2D6 IM | 457 | 0.23 | −0.42, 0.87 | 0.489 |
| CYP2D6 PM | 106 | 2.43 | 1.23, 3.63 | <0.001 |
| Observations | 1930 | |||
| R2/R2 adjusted | 0.454/0.450 | |||
| Fluoxetine | |||||
|---|---|---|---|---|---|
| Predictors | n | Estimates | CI | p | |
| Diabetes | 426 | 7.22 | 6.20, 8.23 | <0.001 | |
| CYP2D6 IM | 1282 | 0.06 | −0.29, 0.41 | 0.728 | |
| CYP2D6 PM | 299 | 0.04 | −0.62, 0.69 | 0.916 | |
| Diabetes: CYP2D6 IM | −3.78 | −5.03, −2.53 | <0.001 | ||
| Diabetes: CYP2D6 PM | −1.81 | −4.11, 0.49 | 0.124 | ||
| Observations | 5469 | ||||
| R2/R2 adjusted | 0.467/0.465 | ||||
| Diabetes | No Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | n | Estimates | CI | p | n | Estimates | CI | p |
| CYP2D6 IM | 100 | −3.74 | −6.82, −0.67 | 0.017 | 1182 | 0.05 | −0.21, 0.31 | 0.696 |
| CYP2D6 PM | 24 | −0.94 | −6.61, 4.73 | 0.745 | 275 | 0.04 | −0.43, 0.52 | 0.859 |
| Observations | 426 | 5043 | ||||||
| R2/R2 adjusted | 0.196/0.175 | 0.130/0.128 | ||||||
| Venlafaxine | |||||
|---|---|---|---|---|---|
| Predictors | n | Estimates | CI | p | |
| Diabetes | 182 | 5.68 | 4.04, 7.33 | 1.77 × 10−11 | |
| CYP2D6 IM | 430 | −0.23 | −0.89, 0.43 | 0.495 | |
| CYP2D6 PM | 103 | −0.46 | −1.73, 0.80 | 0.473 | |
| Diabetes: CYP2D6 IM | 3.62 | 1.27, 5.98 | 0.003 | ||
| Diabetes: CYP2D6 PM | 11.44 | 8.05, 14.84 | 4.79 × 10−11 | ||
| Observations | 1887 | ||||
| R2/R2 adjusted | 0.528/0.524 | ||||
| Diabetes | No Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | n | Estimates | CI | p | n | Estimates | CI | p |
| CYP2D6 IM | 32 | 3.55 | −1.75, 8.85 | 0.188 | 398 | −0.22 | −0.71, 0.26 | 0.367 |
| CYP2D6 PM | 15 | 10.15 | 2.63, 17.67 | 0.008 | 88 | −0.44 | −1.36, 0.49 | 0.356 |
| Observations | 182 | 1703 | ||||||
| R2/R2 adjusted | 0.280/0.233 | 0.122/0.116 | ||||||
| Predictors | n/mean |
HbA1c mmol/mol Estimates |
95% CI mmol/mol | p |
|---|---|---|---|---|
| CYP2D6 IM | 650 | −0.02 | −0.58, 0.53 | 0.930 |
| CYP2D6 PM | 135 | −0.93 | −2.01, 0.16 | 0.093 |
| Takes CYP2D6 inhibitor | 151 | 0.59 | −0.43, 1.61 | 0.260 |
| Diabetes | 284 | 4.55 | 3.13, 5.97 | <0.001 |
| Observations | 2699 | |||
| R2 / R2 adjusted | 0.449/0.446 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austin-Zimmerman, I.; Wronska, M.; Wang, B.; Irizar, H.; Thygesen, J.H.; Bhat, A.; Denaxas, S.; Fatemifar, G.; Finan, C.; Harju-Seppänen, J.; et al. The Influence of CYP2D6 and CYP2C19 Genetic Variation on Diabetes Mellitus Risk in People Taking Antidepressants and Antipsychotics. Genes 2021, 12, 1758. https://doi.org/10.3390/genes12111758
Austin-Zimmerman I, Wronska M, Wang B, Irizar H, Thygesen JH, Bhat A, Denaxas S, Fatemifar G, Finan C, Harju-Seppänen J, et al. The Influence of CYP2D6 and CYP2C19 Genetic Variation on Diabetes Mellitus Risk in People Taking Antidepressants and Antipsychotics. Genes. 2021; 12(11):1758. https://doi.org/10.3390/genes12111758
Chicago/Turabian StyleAustin-Zimmerman, Isabelle, Marta Wronska, Baihan Wang, Haritz Irizar, Johan H. Thygesen, Anjali Bhat, Spiros Denaxas, Ghazaleh Fatemifar, Chris Finan, Jasmine Harju-Seppänen, and et al. 2021. "The Influence of CYP2D6 and CYP2C19 Genetic Variation on Diabetes Mellitus Risk in People Taking Antidepressants and Antipsychotics" Genes 12, no. 11: 1758. https://doi.org/10.3390/genes12111758
APA StyleAustin-Zimmerman, I., Wronska, M., Wang, B., Irizar, H., Thygesen, J. H., Bhat, A., Denaxas, S., Fatemifar, G., Finan, C., Harju-Seppänen, J., Giannakopoulou, O., Kuchenbaecker, K., Zartaloudi, E., McQuillin, A., & Bramon, E. (2021). The Influence of CYP2D6 and CYP2C19 Genetic Variation on Diabetes Mellitus Risk in People Taking Antidepressants and Antipsychotics. Genes, 12(11), 1758. https://doi.org/10.3390/genes12111758






