Genomic Tools for the Identification of Loci Associated with Facial Eczema in New Zealand Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Genotyping
2.2. Imputation
2.3. Genome-Wide Association Studies
2.4. Validation of Markers of Interest
3. Results
3.1. Imputation
3.2. Genome-Wide Association Studies
3.3. Regions of Interest
3.4. Proteomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Menna, M.E.; Smith, B.L.; Miles, C.O. A history of facial eczema (pithomycotoxicosis) research. N. Z. J. Agric. Res. 2009, 52, 345–376. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Hyde, K.D.; Jayasiri, S.C.; Buyck, B.; Chethana, K.W.T.; Dai, D.Q.; Dai, Y.C.; Daranagama, D.A.; Jayawardena, R.S.; Lücking, R.; et al. Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015, 75, 27–274. [Google Scholar] [CrossRef]
- Smith, B.L.; Towers, N.R. Mycotoxicoses of grazing animals in New Zealand. N. Z. Vet. J. 2002, 50, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Towers, N.R. Mycotoxin poisoning in grazing livestock in New Zealand. Proc. N. Z. Soc. Anim. Prod. 2006, 66, 300. [Google Scholar]
- Smith, B.L. Effects of low dose rates of sporidesmin given orally to sheep. N. Z. Vet. J. 2000, 48, 176–181. [Google Scholar] [CrossRef]
- Moore, R.W.; Sumner, R.M.W.; Bass, J.J.; Hockey, H.-U.P. Hogget lambing and its effect on the subsequent two-tooth performance of three breeds. Proc. N. Z. Soc. Anim. Prod. 1983, 43, 21–24. [Google Scholar]
- McMillan, W.H.; Dockrill, G.; Towers, N.R. Sporidesmin poisoning in ewes during late pregnancy. Proc. N. Z. Soc. Anim. Prod. 1988, 48, 131–134. [Google Scholar]
- Morris, C.A.; Towers, N.R.; Wesselink, C.; Southey, B.R. Effects of facial eczema on ewe reproduction and postnatal lamb survival in Romney sheep. N. Z. J. Agric. Res. 1991, 34, 407–412. [Google Scholar] [CrossRef]
- Smith, B.L.; Towers, N.R. Pithomycotoxicosis (facial eczema) in New Zealand and the use of zinc salts for its prevention. In Proceedings of the Australia–USA Poisonous Plants Symposium, Brisbane, Australia, 14–18 May 1984; pp. 70–79. [Google Scholar]
- Beef + Lamb New Zealand. Facing up to Facial Eczema, 3rd ed.; Beef + Lamb New Zealand: Wellington, New Zealand, 2019. [Google Scholar]
- Clare, N.T. Photosensitivity diseases in New Zealand. 3. The photosensitizing agent in facial eczema. N. Z. J. Sci. Technol. 1944, 25, 202–220. [Google Scholar]
- Flynn, D.M. Facial eczema. 1. History of the disease in Victoria. J. Agric. Vic. Dep. Agric. 1962, 60, 49–50. [Google Scholar]
- Marasas, W.F.; Adelaar, T.F.; Kellerman, T.S.; Minné, J.A.; Van Rensburg, I.B.; Burroughs, G.W. First report of facial eczema in sheep in South Africa. Onderstepoort J. Vet. Res. 1972, 39, 107–112. [Google Scholar] [PubMed]
- Brewer, D.; Russell, D.W.; de Melio Amaral, R.E.; Aycardi, E.R. An examination of North and South American isolates of Pithomyces chartarum for production of sporidesmin and sporidesmolides. Proc. Nova Scotian Inst. Sci. 1989, 38, 73–81. [Google Scholar]
- Hansen, D.E.; McCoy, R.D.; Hedstrom, O.R.; Snyder, S.P.; Ballerstedt, P.B. Photosensitization associated with exposure to Pithomyces chartarum in lambs. J. Am. Vet. Med. Assoc. 1994, 204, 1668–1671. [Google Scholar] [PubMed]
- Bezille, P.; Braun, J.P.; Le Bars, J. First identification of facial eczema in Europe. Epidemiological, clinical and biological aspects. Rec. Med. Vet. Ec. Alfort. 1984, 160, 339–347. [Google Scholar]
- Liu, L.; Zhang, Y.; Sun, Z.; Ou, K.; Sun, H.; Yan, G. Isolation and PCR-DGGE studies of the fungal pathogens from pastures with high incidence of sheep facial eczema on the northern slope of Tianshan Mountains. Acta Vet. Zootech. Sin. 2014, 45, 1718–1725. [Google Scholar]
- Brook, P.J. Ecology of the fungus Pithomyces chartarum (Berk. & Curt.) M. B. Ellis in pasture in relation to facial eczema disease of sheep. N. Z. J. Agric. Res. 1963, 6, 147–228. [Google Scholar]
- Mitchell, K.J.; Walshe, T.O.; Robertson, N.G. Weather Conditions Associated with Outbreaks of Facial Eczema. N. Z. J. Agric. Res. 1959, 2, 584–604. [Google Scholar] [CrossRef]
- di Menna, M.E.; Bailey, J.R. Pithomyces chartarum spore counts in pasture. N. Z. J. Agric. Res. 1973, 16, 343–351. [Google Scholar] [CrossRef]
- Dennis, N.A.; Amer, P.R.; Meier, S. BRIEF COMMUNICATION: Predicting the impact of climate change on the risk of facial eczema outbreaks throughout New Zealand. Proc. N. Z. Soc. Anim. Prod. 2014, 74, 1–6. [Google Scholar]
- Smith, B.L.; Embling, P.P.; Towers, N.R.; Wright, D.E.; Payne, E. The protective effect of zinc sulphate in experimental sporidesmin poisoning of sheep. N. Z. Vet. J. 1977, 25, 124–127. [Google Scholar] [CrossRef]
- Parle, J.N.; di Menna, M.E. Fungicides and the control of Pithomyces chartarum. N. Z. J. Agric. Res. 1972, 15, 54–63. [Google Scholar] [CrossRef][Green Version]
- Towers, N.R. Facial Eczema—Problems and Successes in Control. Proc. N. Z. Grassl. Assoc. 1986, 47, 121–127. [Google Scholar] [CrossRef]
- Morris, C.A.; Towers, N.R.; Campbell, A.G.; Meyer, H.H.; Wesselink, C.; Wheeler, M. Responses achieved in Romney flocks selected for or against susceptibility to facial eczema, 1975–1987. N. Z. J. Agric. Res. 1989, 32, 379–388. [Google Scholar] [CrossRef]
- Campbell, A.G.; Meyer, H.H.; Henderson, H.V.; Wesselink, C. Breeding for Facial Eczema Resistance—A Progress Report. Proc. N. Z. Soc. Anim. Prod. 1981, 41, 273–278. [Google Scholar]
- Towers, N.R.; Stratton, G.C. Serum gamma-glutamyltransferase as a measure of sporidesmin-induced liver damage in sheep. N. Z. Vet. J. 1978, 26, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.A.; Towers, N.R.; Wheeler, M.; Wesselink, C. Selection for or against facial eczema susceptibility in Romney sheep, as monitored by serum concentrations of a liver enzyme. N. Z. J. Agric. Res. 1995, 38, 211–219. [Google Scholar] [CrossRef]
- Amyes, N.C.; Hawkes, A.D. Others Ramguard-increasing the tolerance to facial eczema in New Zealand sheep. Proc. N. Z. Soc. Anim. Prod. 2014, 74, 154–157. [Google Scholar]
- McRae, K.M.; Cullen, N.G.; Amyes, N.C.; Johnson, P.L. Brief Communication: An update on genetic parameters for facial eczema tolerance in sheep. Proc. N. Z. Soc. Anim. Prod. 2016, 76, 43–45. [Google Scholar]
- Phua, S.H.; Dodds, K.G.; Morris, C.A.; Paterson, K.A.; McEwan, J.C.; Garmonsway, H.G.; Towers, N.R.; Crawford, A.M. Catalase gene is associated with facial eczema disease resistance in sheep. Anim. Genet. 1999, 30, 286–295. [Google Scholar] [CrossRef]
- Duncan, E.J.; Dodds, K.G.; Henry, H.M.; Thompson, M.P.; Phua, S.H. Cloning, mapping and association studies of the ovine ABCG2 gene with facial eczema disease in sheep. Anim. Genet. 2007, 38, 126–131. [Google Scholar] [CrossRef]
- Phua, S.H.; Dodds, K.G.; Morris, C.A.; Henry, H.M.; Beattie, A.E.; Garmonsway, H.G.; Towers, N.R.; Crawford, A.M. A genome-screen experiment to detect quantitative trait loci affecting resistance to facial eczema disease in sheep. Anim. Genet. 2009, 40, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Phua, S.H.; Dodds, K.G. Others Different methods detected different loci involved in resistance to facial eczema disease of sheep. In Proceedings of the 19th Conference of the Association for the Advancement of Animal Breeding and Genetics, Perth, Australia, 19–21 July 2011; Volume 19, pp. 171–174. [Google Scholar]
- Phua, S.H.; Brauning, R.; Baird, H.J.; Dodds, K.G. Identifying chromosomal selection-sweep regions in facial eczema selection-line animals using an ovine 50K-SNP array. Anim. Genet. 2014, 45, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Phua, S.H.; Hyndman, D.L.; Baird, H.J.; Auvray, B.; McEwan, J.C.; Lee, M.A.; Dodds, K.G. Towards genomic selection for facial eczema disease tolerance in the New Zealand sheep industry. Anim. Genet. 2014, 45, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xie, M.; Chen, W.; Talbot, R.; Maddox, J.F.; Faraut, T.; Wu, C.; Muzny, D.M.; Li, Y.; Zhang, W.; et al. The sheep genome illuminates biology of the rumen and lipid metabolism. Science 2014, 344, 1168–1173. [Google Scholar] [CrossRef]
- Sargolzaei, M.; Chesnais, J.P.; Schenkel, F.S. A new approach for efficient genotype imputation using information from relatives. BMC Genom. 2014, 15, 478. [Google Scholar] [CrossRef]
- Hayes, B.J.; Bowman, P.J.; Daetwyler, H.D.; Kijas, J.W.; van der Werf, J.H.J. Accuracy of genotype imputation in sheep breeds. Anim. Genet. 2012, 43, 72–80. [Google Scholar] [CrossRef]
- Gilmour, A.R.; Gogel, B.J.; Cullis, B.R.; Welham, S.J.; Thompson, R. ASReml User Guide Release 4.1 Structural Specification; VSN International: Hemel Hempstead, UK, 2015; pp. 1–375. [Google Scholar]
- Jiang, Y.; Wang, X.; Kijas, J.W.; Dalrymple, B.P. β-globin gene evolution in the ruminants: Evidence for an ancient origin of sheep haplotype B. Anim. Genet. 2015, 46, 506–514. [Google Scholar] [CrossRef]
- Garner, K.J.; Lingrel, J.B. Structural organization of the β-globin locus of B-haplotype sheep. Mol. Biol. Evol. 1988, 5, 134–140. [Google Scholar]
- Garner, K.J.; Lingrel, J.B. A comparison of the β A-and β B-globin gene clusters of sheep. J. Mol. Evol. 1989, 28, 175–184. [Google Scholar] [CrossRef]
- Boyer, S.H.; Hathaway, P.; Pascasio, F.; Orton, C.; Bordley, J.; Naughton, M.A. Hemoglobins in sheep: Multiple differences in amino acid sequences of three β-chains and possible origins. Science 1966, 153, 1539–1543. [Google Scholar] [CrossRef]
- Wilson, J.B.; Edwards, W.C.; McDaniel, M.; Dobbs, M.M.; Huisman, T.H.J. The structure of sheep hemoglobins. II. The amino acid composition of the tryptic peptides of the non-α chains of hemoglobins A, B, C, and F. Arch. Biochem. Biophys. 1966, 115, 385–400. [Google Scholar] [CrossRef]
- Boyer, S.H.; Hathaway, P.; Pascasio, F.; Bordley, J.; Orton, C.; Naughton, M.A. Differences in the amino acid sequences of tryptic peptides from three sheep hemoglobin β chains. J. Biol. Chem. 1967, 242, 2211–2232. [Google Scholar] [CrossRef]
- Rosen, B.D. Personal Communication, 2021.
- Kijas, J.W.; Porto-Neto, L.; Dominik, S.; Reverter, A.; Bunch, R.; McCulloch, R.; Hayes, B.J.; Brauning, R.; McEwan, J. International Sheep Genomics Consortium Linkage disequilibrium over short physical distances measured in sheep using a high-density SNP chip. Anim. Genet. 2014, 45, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Martinez de la Torre, Y.; Fabbri, M.; Jaillon, S.; Bastone, A.; Nebuloni, M.; Vecchi, A.; Mantovani, A.; Garlanda, C. Evolution of the pentraxin family: The new entry PTX4. J. Immunol. 2010, 184, 5055–5064. [Google Scholar] [CrossRef]
- Clark, E.L.; Bush, S.J.; McCulloch, M.E.B.; Farquhar, I.L.; Young, R.; Lefevre, L.; Pridans, C.; Tsang, H.G.; Wu, C.; Afrasiabi, C.; et al. A high resolution atlas of gene expression in the domestic sheep (Ovis aries). PLoS Genet. 2017, 13, e1006997. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zou, H.; Dai, Z.; Feng, S.; Zhang, M.; Xiao, G.; Liu, Z.; Cheng, Q. The Basic Characteristics of the Pentraxin Family and Their Functions in Tumor Progression. Front. Immunol. 2020, 11, 1757. [Google Scholar] [CrossRef]
- Townes, T.M.; Fitzgerald, M.C.; Lingrel, J.B. Triplication of a four-gene set during evolution of the goat β-globin locus produced three genes now expressed differentially during development. Proc. Natl. Acad. Sci. USA 1984, 81, 6589–6593. [Google Scholar] [CrossRef]
- Schon, E.A.; Cleary, M.L.; Haynes, J.R.; Lingrel, J.B. Structure and evolution of goat γ-, β C- and β A-globin genes: Three developmentally regulated genes contain inserted elements. Cell 1981, 27, 359–369. [Google Scholar] [CrossRef]
- Schimenti, J.C.; Duncan, C.H. Structure and organization of the bovine β-globin genes. Mol. Biol. Evol. 1985, 2, 514–525. [Google Scholar]
- Cohen, B.L.; Evans, J.V.; Harris, H.; King, J.W.; Warren, F.L. Genetics of haemoglobin and blood potassium differences in sheep. Nature 1956, 178, 849–850. [Google Scholar]
- Huisman, T.H.; Van, V.; Sebens, T. Some genetic and physiological aspects of two different adult haemoglobins in sheep. Nature 1958, 182, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.; Warren, F.L. Occurrence of electrophoretically distinct haemoglobins in ruminants. Biochem. J 1955, 60, xxix. [Google Scholar] [PubMed]
- Treangen, T.J.; Salzberg, S.L. Repetitive DNA and next-generation sequencing: Computational challenges and solutions. Nat. Rev. Genet. 2011, 13, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.J.; Evans, J.V. Effect of anaemia on exygen transport in sheep with different haemoglobin types. Aust. J. Exp. Biol. Med. Sci. 1967, 45, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.J.; Evans, J.V. Haemoglobin and Erythrocyte Potassium Types in Sheep and their Influence on Oxygen Dissociation and Haemoglobin Denaturation. Aust. J. Bio. Sci. 1962, 15, 371–378. [Google Scholar] [CrossRef]
- Dawson, T.J.; Evans, J.V. Effect of hemoglobin type on the cardiorespiratory system of sheep. Am. J. Physiol. 1965, 209, 593–598. [Google Scholar] [CrossRef]
- Manca, L.; Pirastru, M.; Mereu, P.; Multineddu, C.; Olianas, A.; el Sherbini, E.S.; Franceschi, P.; Pellegrini, M.; Masala, B. Barbary sheep (Ammotragus lervia): The structure of the adult β-globin gene and the functional properties of its hemoglobin. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 145, 214–219. [Google Scholar] [CrossRef]
- Dawson, T.J.; Evans, J.V. Effect hypoxia on oxygen transport in sheep with different hemoglobin types. Am. J. Physiol. 1966, 210, 1021–1025. [Google Scholar] [CrossRef]
- Battaglia, F.C.; Behrman, R.E.; De Lannoy, C.W.; Hathaway, W.; Makowski, E.L.; Meschia, G.; Seeds, A.E.; Schruefer, J.J.P. Exposure to high altitude of sheep with different hæmoglobins. Q. J. Exp. Physiol. Cogn. Med. Sci. 1969, 54, 423–431. [Google Scholar] [CrossRef]
- Blunt, M.H.; Evans, J.V. Changes in the concentration of potassium in the erythrocytes and in hæmoglobin type in merino sheep under a severe anæmic stress. Nature 1963, 200, 1215–1216. [Google Scholar] [CrossRef]
- van Vliet, G.; Huisman, T.H. Changes in the haemoglobin types of sheep as a response to anaemia. Biochem. J 1964, 93, 401–409. [Google Scholar] [CrossRef]
- Huisman, T.H. The in vivo production of hemoglobin C in ruminants. Ann. N. Y. Acad. Sci. 1974, 241, 549–555. [Google Scholar] [CrossRef]
- Huisman, T.H.; Lewis, J.P.; Blunt, M.H.; Adams, H.R.; Miller, A.; Dozy, A.M.; Boyd, E.M. Hemoglobin C in newborn sheep and goats: A possible explanation for its function and biosynthesis. Pediatr. Res. 1969, 3, 189–198. [Google Scholar] [CrossRef][Green Version]
- Huisman, T.H.; Kitchens, J. Oxygen equilibria studies of the hemoglobins from normal and anemic sheep and goats. Am. J. Physiol. 1968, 215, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-J.; Yang, J.; Xie, X.-L.; Lv, F.-H.; Cao, Y.-H.; Li, W.-R.; Liu, M.-J.; Wang, Y.-T.; Li, J.-Q.; Liu, Y.-G.; et al. The Genome Landscape of Tibetan Sheep Reveals Adaptive Introgression from Argali and the History of Early Human Settlements on the Qinghai-Tibetan Plateau. Mol. Biol. Evol. 2019, 36, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Dally, M.R.; Hohenboken, W.; Thomas, D.L.; Craig, A.M. Relationships between hemoglobin type and reproduction, lamb, wool and milk production and health-related traits in crossbred ewes. J. Anim. Sci. 1980, 50, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Laksesvela, B.; Dishington, I.W. Bog asphodel (Narthecium ossifragum) as a cause of photosensitisation in lambs in Norway. Vet. Rec. 1983, 112, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Coetzer, J.A.; Kellerman, T.S.; Sadler, W.; Bath, G.F. Photosensitivity in South Africa. V. A comparative study of the pathology of the ovine hepatogenous photosensitivity diseases, facial eczema and geeldikkop (Tribulosis ovis), with special reference to their pathogenesis. Onderstepoort J. Vet. Res. 1983, 50, 59–71. [Google Scholar] [PubMed]
- Neethling, L.P.; Brown, J.M.M.; Osterhoff, D.R.; De Wet, P.J.; Ward-Cox, I.S. The functional advantage of haemoglobin type A in haemolytic syndromes in sheep. Phenylhydrazine, organic selenium and partial exsanguination as external agents in the production of anaemias. J. S. Afr. Vet. Assoc. 1969, 40, 121–128. [Google Scholar]
- Osterhoff, D.R. Haemoglobin types and the geeldikkop-enzootic icterus disease complex in sheep. Anim. Blood Groups Biochem. Genet. 2009, 2, 181–184. [Google Scholar] [CrossRef]
- Millar, K.R. Haemoglobin types, blood parameters and erythrocyte glutathione in the New Zealand Romney and other breeds. N. Z. Vet. J. 1980, 28, 251–252, 261–262. [Google Scholar] [CrossRef]
- Morris, C.A.; Towers, N.R.; Wesselink, C.; Amyes, N.C. Susceptibility of Finnish landrace, Romney, and Finn × Romney lambs to a sporidesmin challenge. N. Z. J. Agric. Res. 1994, 37, 547–552. [Google Scholar] [CrossRef]
- Munday, R. Studies on the mechanism of toxicity of the mycotoxin, sporidesmin. I. Generation of superoxide radical by sporidesmin. Chem. Biol. Interact. 1982, 41, 361–374. [Google Scholar] [CrossRef]
- Jordan, T.W. The cellular and molecular toxicity of sporidesmin. N. Z. Vet. J. 2020, 68, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Inagi, R.; Kato, H.; Tanemoto, M.; Kojima, I.; Son, D.; Fujita, T.; Nangaku, M. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J. Am. Soc. Nephrol. 2008, 19, 1500–1508. [Google Scholar] [CrossRef]
- Biagioli, M.; Pinto, M.; Cesselli, D.; Zaninello, M.; Lazarevic, D.; Roncaglia, P.; Simone, R.; Vlachouli, C.; Plessy, C.; Bertin, N.; et al. Unexpected expression of α- and β-globin in mesencephalic dopaminergic neurons and glial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15454–15459. [Google Scholar] [CrossRef]
- Liu, W.; Baker, S.S.; Baker, R.D.; Nowak, N.J.; Zhu, L. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS ONE 2011, 6, e24363. [Google Scholar] [CrossRef]
- Hohenboken, W.D.; Morris, C.A.; Munday, R.; De Nicolo, G.; Amyes, N.C.; Towers, N.R.; Phua, S.H. Antioxidants in blood from sheep lines divergently selected for facial eczema resistance. N. Z. J. Agric. Res. 2004, 47, 119–127. [Google Scholar] [CrossRef]
- Wiener, G.; Hall, J.G.; Hayter, S. An association between the concentration of copper in whole blood and haemoglobin type in sheep. Anim. Sci. 1973, 17, 1–7. [Google Scholar] [CrossRef]
- Wiener, G.; Field, A.C. Seasonal changes, breed differences and repeatability of plasma copper levels of sheep at pasture. J. Agric. Sci. 1974, 83, 403–408. [Google Scholar] [CrossRef]
- Wiener, G.; Herbert, J.G. Variation in liver and plasma copper concentrations of sheep in relation to breed and haemoglobin type. J. Comp. Pathol. 1976, 86, 101–109. [Google Scholar] [CrossRef]
- Johnson, P.L.; Amyes, N.C. An association between circulating copper concentrations and gammaglutamyl transferase activity in sheep after exposure to the toxin sporidesmin. N. Z. J. Anim. Sci. Prod. 2020, 80, 34–38. [Google Scholar]
- Neary, D.M.; Sutcliffe, E.; Haley, C.S.; Woolliams, J.A. Single marker QTL mapping for copper in the plasma of sheep. In Proceedings of the 6th World Congress on Genetics Applied to Livestock Production, Armidale, Australia, 11–16 January 1998; Volume 27, pp. 437–440. [Google Scholar]
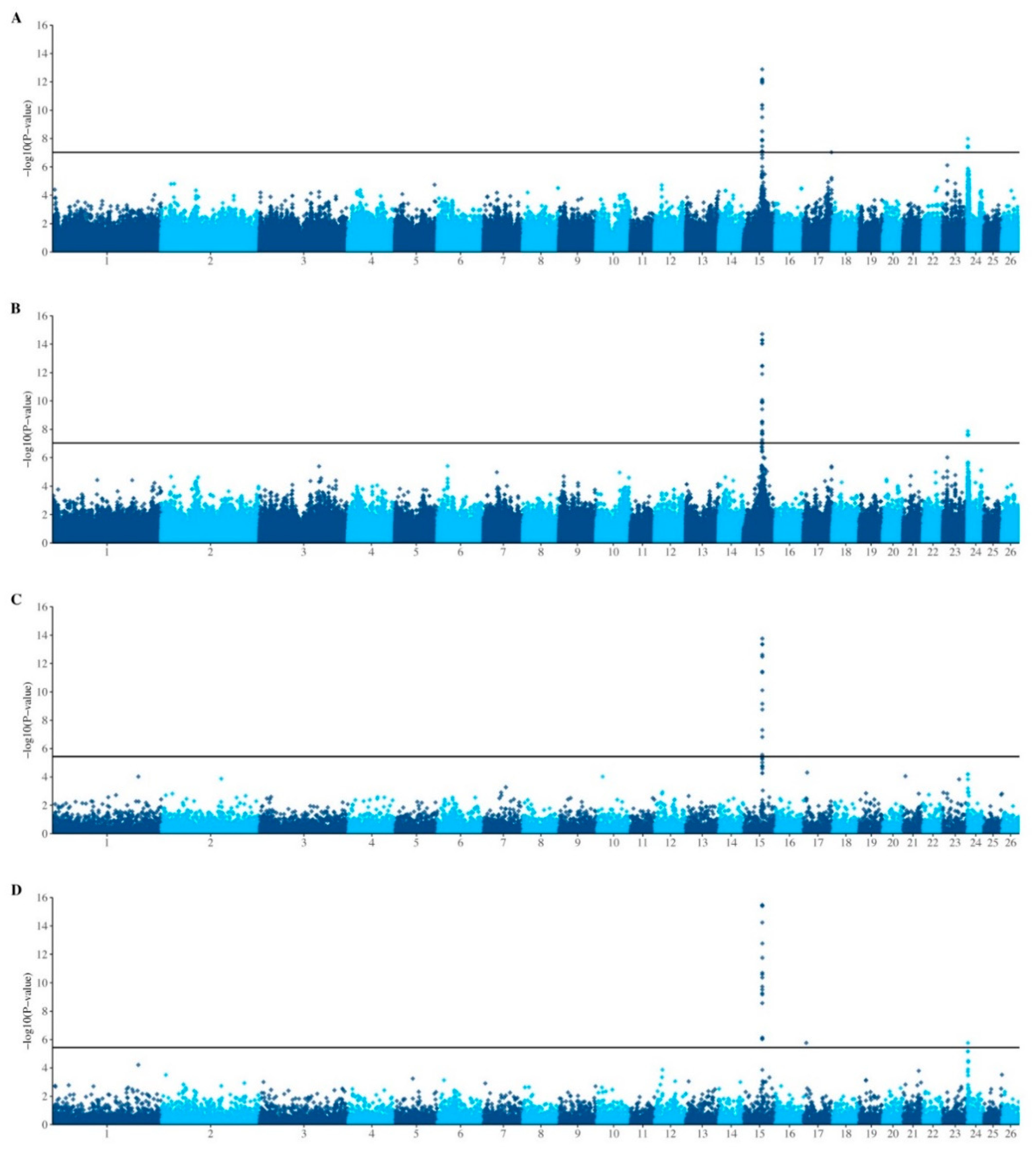
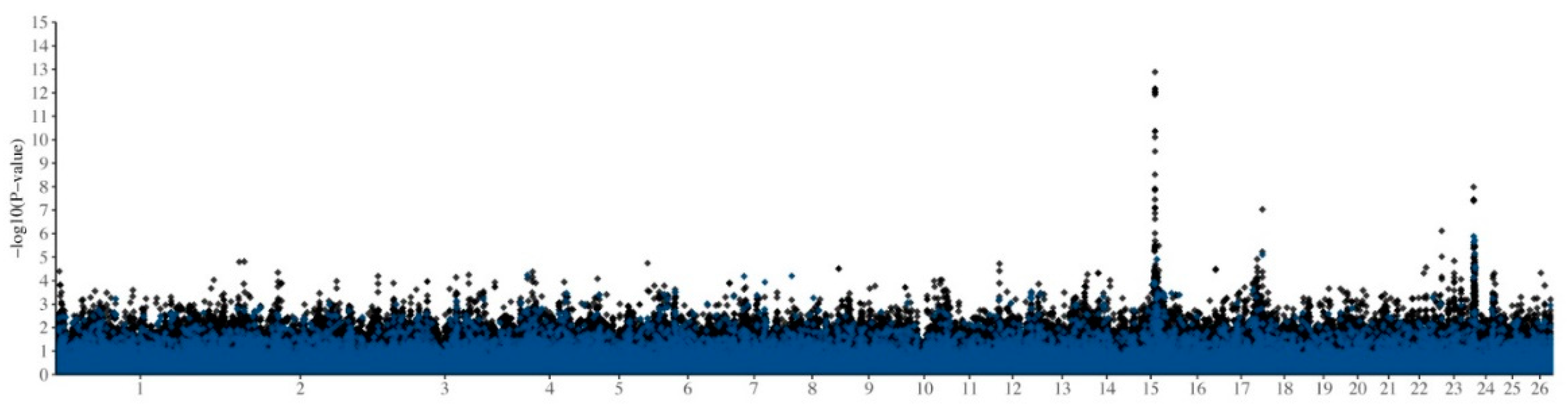
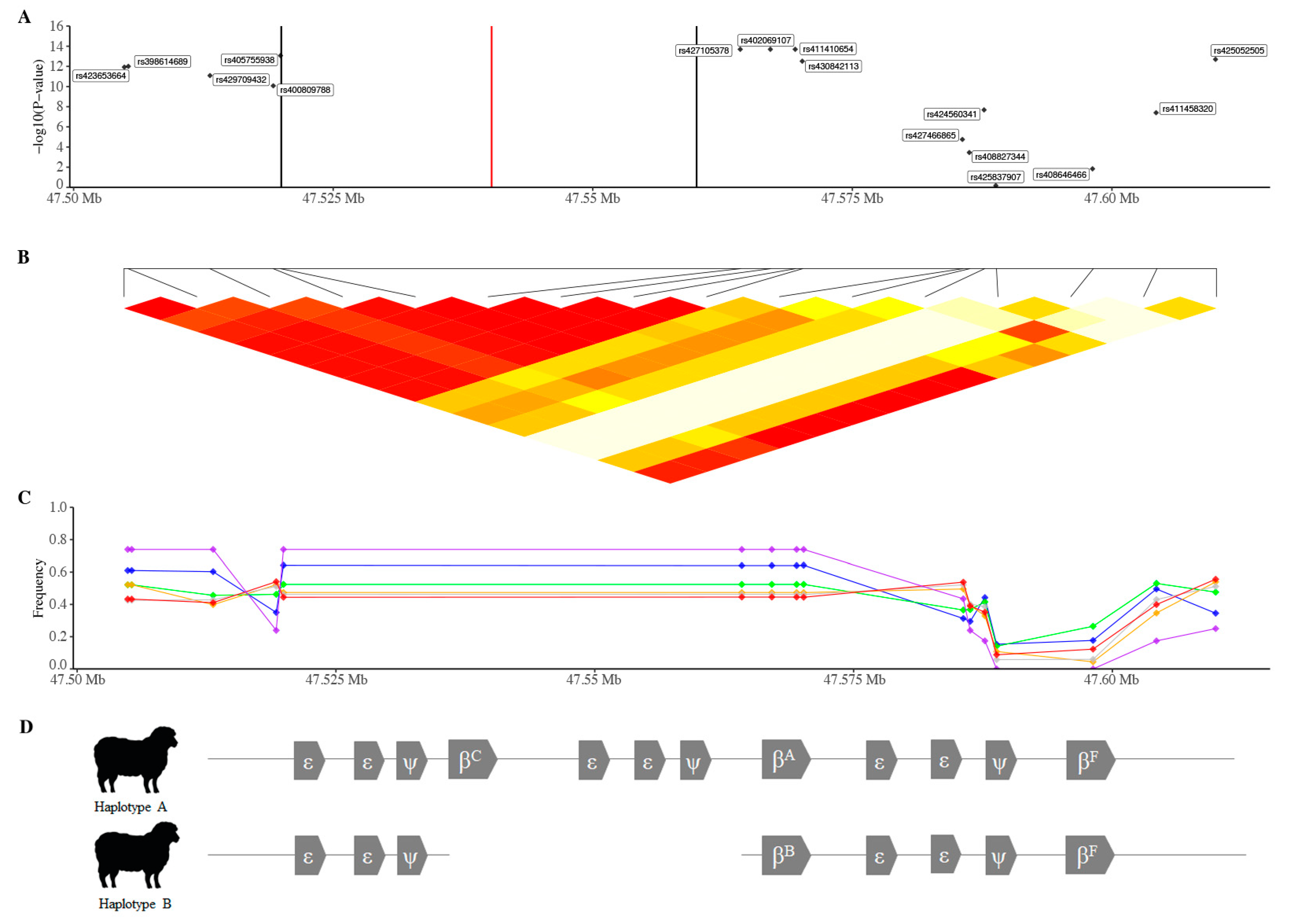
| RSID | Chr | Position 1 | −log10 p-Value 2 | MAF (Allele) 3 | Predicted GGT21 Values 4 | α | d | Prop VA | Prop VP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (±SE) | AB (±SE) | BB (±SE) | |||||||||
| rs398930318 | 15 | 47,489,709 | 6.09 | 0.45 (A) | 5.04 (0.07) | 4.9 (0.06) | 4.63 (0.07) | −0.21 | 0.07 | 0.04 | 0.02 |
| rs423653664 | 15 | 47,504,887 | 11.93 | 0.48 (A) | 5.09 (0.07) | 4.92 (0.06) | 4.54 (0.07) | −0.28 | 0.10 | 0.07 | 0.04 |
| rs398614689 | 15 | 47,505,272 | 12.00 | 0.48 (A) | 5.09 (0.07) | 4.92 (0.06) | 4.54 (0.07) | −0.28 | 0.11 | 0.07 | 0.04 |
| rs429709432 | 15 | 47,513,135 | 11.09 | 0.46 (A) | 5.08 (0.07) | 4.96 (0.06) | 4.51 (0.07) | −0.29 | 0.16 | 0.08 | 0.04 |
| rs400809788 | 15 | 47,519,231 | 10.07 | 0.49 (B) | 5.09 (0.07) | 4.94 (0.06) | 4.49 (0.07) | 0.30 | 0.14 | 0.08 | 0.04 |
| rs405755938 | 15 | 47,519,931 | 13.07 | 0.50 (A) | 5.09 (0.07) | 4.92 (0.06) | 4.49 (0.07) | −0.30 | 0.13 | 0.08 | 0.05 |
| rs427105378 | 15 | 47,564,204 | 13.69 | 0.50 (B) | 4.49 (0.07) | 4.92 (0.06) | 5.09 (0.07) | 0.30 | 0.13 | 0.08 | 0.05 |
| rs402069107 | 15 | 47,567,105 | 13.69 | 0.50 (A) | 5.09 (0.07) | 4.92 (0.06) | 4.49 (0.07) | −0.30 | 0.13 | 0.08 | 0.05 |
| rs411410654 | 15 | 47,569,499 | 13.69 | 0.50 (B) | 4.49 (0.07) | 4.92 (0.06) | 5.09 (0.07) | 0.30 | 0.13 | 0.08 | 0.05 |
| rs430842113 | 15 | 47,570,178 | 12.51 | 0.50 (A) | 5.10 (0.07) | 4.94 (0.06) | 4.50 (0.07) | −0.30 | 0.14 | 0.08 | 0.05 |
| rs425052505 | 15 | 47,609,978 | 12.70 | 0.50 (B) | 5.09 (0.07) | 4.91 (0.06) | 4.48 (0.07) | 0.30 | 0.12 | 0.09 | 0.05 |
| rs425270036 | 24 | 1,155,234 | 5.70 | 0.33 (B) | 4.94 (0.06) | 4.83 (0.06) | 4.56 (0.08) | 0.19 | 0.08 | 0.04 | 0.01 |
| rs409675199 | 24 | 1,156,263 | 5.76 | 0.33 (A) | 4.56 (0.08) | 4.83 (0.06) | 4.94 (0.06) | −0.19 | 0.08 | 0.04 | 0.01 |
| Genome Assembly | Genbank Accession | Chromosome | rs405755938 | rs402069107 | Region Distance |
|---|---|---|---|---|---|
| OAR_v3.1 | GCA_000298735.2 | 15 | 47,519,931 | 47,567,105 | 65,291 |
| Oar_v4.0 | GCA_000298735.2 | 15 | 47,414,827 | 47,462,139 | 65,429 |
| Oar_rambouillet_v1.0 | GCA_002742125.1 | 15 | 51,898,128 | 51,984,579 | 104,993 |
| ARS-UI_Ramb_v2.0 | GCA_016772045.1 | 15 | 47,951,894 | 48,038,330 | 104,963 |
| White Dorper 1 | 15 | 47,750,476 | 47,797,960 | 65,855 | |
| Romanov 1 | 15 | 48,107,973 | 48,194,400 | 104,969 |
| Markers | Fixed Effects 1 | PPMCC 2 (± SD) | h2 | GBLUP Accuracy 3 |
|---|---|---|---|---|
| All SNPs (n = 12,500) | Contemporary group | 0.335 ± 0.007 | 0.44 | 0.505 |
| Removing markers in regions of interest (n = 12,477) | Contemporary group | 0.326 ± 0.007 | 0.491 |
| Predicted β-Globin Type 1 | βA | βB |
|---|---|---|
| βA (n = 3) | 001 MLTAEEKAAV TGFWGKVKVD EVGAEALGRL LVVYPWTQRF ********** ********** ********** ********** 041 FEHFGDLSSA DAVMNNAKVK AHGKKVLDSF SNGVQHLDDL ******** * ****** *** ********** *** ***** 081 KGTFAQLSEL HCDKLHVDPE NFRLLGNVLV VVLARHHGSE ********** ********** ********** ******** * 121 FTPVLQAEFQ KVVAGVANAL AHRYH ******* ** ********** ** ** | |
| βAB (n = 3) | 001 MLTAEEKAAV TGFWGKVKVD EVGAEALGRL LVVYPWTQRF ********** ********** ********** ********** 041 FEHFGDLSSA DAVMNNAKVK AHGKKVLDSF SNGVQHLDDL ******** * ****** *** ********** *** ***** 081 KGTFAQLSEL HCDKLHVDPE NFRLLGNVLV VVLARHHGSE ********** ********** ********** ******** * 121 FTPVLQAEFQ KVVAGVANAL AHRYH ******* ** ********** ** ** | 001 MLTAEEKAAV TGFWGKVKVD EVGAEALGRL LVVYPWTQRF ********** ********** ********** ********** 041 FEHFGDLSNA DAVMNNPKVK AHGKKVLDSF SNGMKHLDDL ******** * ****** *** ********** *** ***** 081 KGTFAQLSEL HCDKLHVDPE NFRLLGNVLV VVLARHHGNE ********** ********** ********** ******** * 121 FTPVLQADFQ KVVAGVANAL AHKYH ******* ** ********** ** ** |
| βB (n = 1) | 001 MLTAEEKAAV TGFWGKVKVD EVGAEALGRL LVVYPWTQRF ********** ********** ********** ********** 041 FEHFGDLSSA DAVMNNAKVK ******** * ****** *** | 001 MLTAEEKAAV TGFWGKVKVD EVGAEALGRL LVVYPWTQRF ********** ********** ********** ********** 041 FEHFGDLSNA DAVMNNPKVK AHGKKVLDSF SNGMKHLDDL ******** * ****** *** ********** *** ***** 081 KGTFAQLSEL HCDKLHVDPE NFRLLGNVLV VVLARHHGNE ********** ********** ********** ******** * 121 FTPVLQADFQ KVVAGVANAL AHKYH ******* ** ********** ** ** |
| βB (n = 2) | 001 MLTAEEKAAV TGFWGKVKVD EVGAEALGRL LVVYPWTQRF ********** ********** ********** ********** 041 FEHFGDLSNA DAVMNNPKVK AHGKKVLDSF SNGMKHLDDL ******** * ****** *** ********** *** ***** 081 KGTFAQLSEL HCDKLHVDPE NFRLLGNVLV VVLARHHGNE ********** ********** ********** ******** * 121 FTPVLQADFQ KVVAGVANAL AHKYH ******* ** ********** ** ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McRae, K.M.; Rowe, S.J.; Johnson, P.L.; Baird, H.J.; Cullen, N.G.; Bixley, M.J.; Plowman, J.E.; Deb-Choudhury, S.; Brauning, R.; Amyes, N.C.; et al. Genomic Tools for the Identification of Loci Associated with Facial Eczema in New Zealand Sheep. Genes 2021, 12, 1560. https://doi.org/10.3390/genes12101560
McRae KM, Rowe SJ, Johnson PL, Baird HJ, Cullen NG, Bixley MJ, Plowman JE, Deb-Choudhury S, Brauning R, Amyes NC, et al. Genomic Tools for the Identification of Loci Associated with Facial Eczema in New Zealand Sheep. Genes. 2021; 12(10):1560. https://doi.org/10.3390/genes12101560
Chicago/Turabian StyleMcRae, Kathryn M., Suzanne J. Rowe, Patricia L. Johnson, Hayley J. Baird, Neil G. Cullen, Matthew J. Bixley, Jeffrey E. Plowman, Santanu Deb-Choudhury, Rudiger Brauning, Neville C. Amyes, and et al. 2021. "Genomic Tools for the Identification of Loci Associated with Facial Eczema in New Zealand Sheep" Genes 12, no. 10: 1560. https://doi.org/10.3390/genes12101560
APA StyleMcRae, K. M., Rowe, S. J., Johnson, P. L., Baird, H. J., Cullen, N. G., Bixley, M. J., Plowman, J. E., Deb-Choudhury, S., Brauning, R., Amyes, N. C., Dodds, K. G., Newman, S.-A. N., McEwan, J. C., & Clarke, S. M. (2021). Genomic Tools for the Identification of Loci Associated with Facial Eczema in New Zealand Sheep. Genes, 12(10), 1560. https://doi.org/10.3390/genes12101560








