Abstract
Molecular mechanisms underlying neuropsychiatric and neurodegenerative diseases are insufficiently elucidated. A detailed understanding of these mechanisms may help to further improve medical intervention. Recently, intellectual abilities, creativity, and amnesia have been associated with neuroplastin, a cell recognition glycoprotein of the immunoglobulin superfamily that participates in synapse formation and function and calcium signaling. Data from animal models suggest a role for neuroplastin in pathways affected in neuropsychiatric and neurodegenerative diseases. Neuroplastin loss or disruption of molecular pathways related to neuronal processes has been linked to various neurological diseases, including dementia, schizophrenia, and Alzheimer’s disease. Here, we review the molecular features of the cell recognition molecule neuroplastin, and its binding partners, which are related to neurological processes and involved in learning and memory. The emerging functions of neuroplastin may have implications for the treatment of diseases, particularly those of the nervous system.
1. Introduction
The prevalence of mental disorders, including autism spectrum disorder (ASD) and schizophrenia (SZ) [1,2,3], related to neurodevelopmental deficits and neurodegenerative diseases, is predicted to increase in future decades because of a growing and ageing world population. In addition to its severe effects on cognitive and social communication, its economic burden is a major challenge for patients and for economies at an international level [4,5,6,7]. To understand the pathogenesis of neuropsychiatric disorders, it is important to consider that both genetic and environmental factors can act separately or in combination to play crucial roles in these diseases. Based on genome-wide association studies (GWAS) with large groups of patients, potential mechanisms underlying different psychiatric disorders can be elucidated and directly investigated. As GWAS and next generation sequencing (NGS) have rapidly developed recently, many gene loci have been associated with different neuropsychiatric disorders [8]. For instance, numerous genetic variants have been associated with SZ [9,10,11,12,13] and ASD [14,15,16,17]. Furthermore, a significant genetic correlation exists between ASD and SZ [17,18]. In combination with transgenic mouse models targeting the potential risk genes, the association of different psychiatric disorders has been confirmed for explicit genes such as the SHANK genes [19,20,21,22,23]. Furthermore, gene variants leading to synaptic dysfunction play a critical role as causal factors for these psychiatric disorders [24].
The abnormal expression or function of several proteins can affect synaptic transmission and further impair network activities, such as the excitatory and inhibitory balance, contributing to different neuropsychiatric or neurodegenerative diseases [25,26]. Therefore, numerous efforts have focused on exploring interventions with identified pathogenic mechanisms, although many attempts did not achieve the desired clinical outcome [27,28,29,30,31]. As psychiatric disorders frequently exhibit overlapping symptoms, such as the association of cognitive impairment with ASD and SZ [32], it is essential to understand their underlying cellular mechanisms.
In this review, we will focus on recent studies of the cell recognition molecule neuroplastin (Np) and its gene (Nptn/NPTN) in relation to psychiatric and neurodegenerative diseases (Figure 1). We propose that targeting neuroplastin may make it possible to reverse network dysfunctions and contribute to ameliorating the onset and progression of neuropsychiatric diseases.
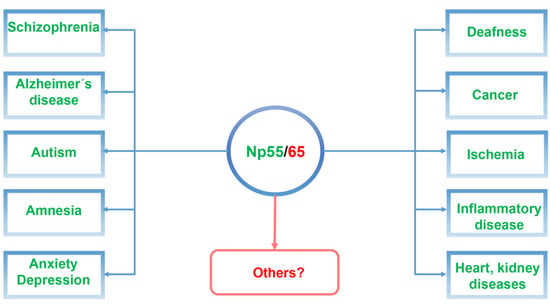
Figure 1.
Schematic illustration of neuroplastin Np55/65 as a central component related to neuropsychiatric and neurodegenerative diseases as well as in other diseases associated with neuroplastin malfunctions.
2. Molecular Characteristics of Neuroplastin
2.1. Structure and Expression of Neuroplastin
In humans and rodents, the small basigin gene family comprises three paralogs: basigin (BSG/Bsg; also designated CD147 or EMMPRIN), embigin (EMB/Emb), and neuroplastin (NPTN/Nptn) [33]. Neuroplastin isoforms were identified as glycoprotein components with molecular weights of 65 kDa (Np65) and 55 kDa (Np55) in isolated synaptic membranes from brain [34,35] (Table 1). The neuroplastin isoforms Np65 and Np55 are encoded by a single gene (Nptn in rodents, NPTN in humans) and result from alternative splicing of the mRNA [36]. Both isoforms are single-spanned transmembrane proteins belonging to the immunoglobulin (Ig) superfamily with two (Np55) and three (Np65) Ig domains, respectively. The intracellular carboxy-terminal tail of neuroplastin may also differ due to alternative splicing, resulting in variants that contain four additional amino acids Asp-Asp-Glu-Pro (DDEP) [36]. Glycosylation at several sites in the Ig2 and Ig3 domains results in a shift from the predicted molecular weight of 28 and 40 kDa to 55 and 65 kDa for the apparent molecular weight of the glycoproteins. Np55 is widely expressed with different glycosylated forms in many tissues such as the brain, liver, lung, and kidney [37], whereas Np65 is restricted to the brain, although it was also recently detected in cultured keratinocytes [38]. Both neuroplastin isoforms are expressed synaptically and extra-synaptically in excitatory and inhibitory neurons but have not been detected in glia [35,39,40]. In human and rodent brain, Np65 is strongly expressed in cortex, hippocampus, striatum, cerebellum, thalamus, and hypothalamus [35,39,40]. Np65 was also detected in both the inner and outer plexiform layers of the rat retina [41]. Np55 is expressed in all brain regions and is the major isoform in rodent cerebellum [42]. In the inner ear of the mouse, Np55 is expressed in the stereocilia of the outer hair cells, in the cell bodies of inner hair cells, and in spiral ganglia cells [43].

Table 1.
History of discoveries in neuroplastin research.
2.2. Interactions and Binding Partners of Neuroplastin in the Nervous System
2.2.1. Neuroplastin Homophilic Binding and AMPA Receptor Subunit GluA1
The adhesive capacity of the Np65-specific Ig1 domain to undergo trans-homophilic binding was first described using an aggregation assay of microspheres coated with neuroplastin-Fc chimeric proteins [39]. Later, crystallographic studies combined with Surface Plasmon Resonance confirmed that the Ig1 F-G loop of Np65 contains an adhesive binding site, and that this loop binds to the corresponding loop of an opposing recombinant Np65 with a KD value of 0.52 ± 0.08 μM [46] (Figure 2).
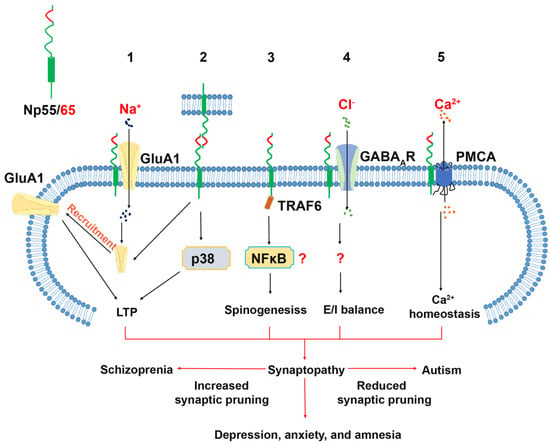
Figure 2.
Neuroplastin binding proteins and their related cellular function in the central nervous system. 1. The Ig1 domain of Np65 interacts with GluA1 supporting targeting of GluA1 to the plasma membrane and is required for the LTP maintenance. 2. Neuroplastin trans-homophilic binding is involved in LTP maintenance. The binding motif resides in the Ig1 domain indicating that only Np65 can engage in this interaction. Homophilic binding of neuroplastin can be disrupted by the peptide "enplastin". Np55 and Np65 were proposed to bind homophilically in cis, however there are no explicit data supporting dimer formation. 3. The intracellular tail of neuroplastin contains a TRAF6 binding motif, which is important for spinogenesis. 4. Neuroplastin interacts with GABAAR. This interaction is critical for the balance of excitatory and inhibitory transmission. However, the binding domains are not identified. 5. The transmembrane domain of neuroplastin is responsible for the interaction with the plasma membrane anchor domain TM10 of PMCA which regulates the extrusion of Ca2+ ions.
This interaction was blocked by enplastin, which is a dendromeric peptide derived from the Np65 trans-homophilic binding site itself [46]. The application of Np65-specific antibodies, able to block the aggregation of Np65-Np65, severely impaired the maintenance, but not the induction, of long-term potentiation (LTP) in CA1 neurons in rat hippocampal slices [39]. Furthermore, treatment with these antibodies resulted in a reduced cell surface expression of AMPA Receptor Subunit GluA1 and an increased phosphorylation of p38 MAPK [64]. These studies suggest that the blockade of potentially exciting trans-synaptic Np65-Np65 binding could result in electrophysiological deficits. Furthermore, incubation with the recombinant extracellular domain of Np65 caused loss of synaptic contacts in cultured hippocampal neurons [40]. Nevertheless, it remains to be confirmed whether in vivo trans-synaptic Np65-Np65 interactions exist and whether they confer structural stabilization to synaptic contacts. Alternatively, a recent study by Jiang et al. showed that the Ig1 domain of Np65 is specifically required for interaction with the extracellular N-terminal domain of GluA1. The absence of GluA1, or of its binding to Np65 as a receptor, resulted in impaired LTP maintenance [62]. GluA1 is critically important for LTP and is associated with various neurological diseases [65,66]. In GluA1-deficient mice, hippocampal LTP was absent without spatial reference memory deficits [67], but working memory deficits [68], schizophrenia-like behaviors [69,70] and increased locomotor activity, accompanied by reduced clearance of striatal dopamine, was displayed [69]. While GWAS identified GluA1 dysfunction as a risk factor for schizophrenia [12], molecular and pharmacological studies over the past decade have linked GluA1 to depression, anxiety, stress-related behavior, and Alzheimer‘s disease [71,72,73,74]. Interestingly, the levels of GluA1 expression and phosphorylation appear to be important for these neurological conditions [71,73,75,76]. In the future, it would be interesting to determine whether the phosphorylation state of GluA1 is modified by its interaction with Np65 or by the loss of neuroplastin.
2.2.2. Neuroplastin and GABAA Receptor (GABAAR)
GABAA receptors were identified as potential binding partners of neuroplastin [47]. Indicative of a close interaction between Np65 and alpha1/alpha2 subunit-containing GABAAR (GABAAα1/α2) are fluorescence resonance energy transfer experiments performed in HEK cells, co-precipitation assays using rodent brain material, and co-localization analysis in cultured neurons and brain sections [47]. Loss of the association of neuroplastin and GABAAR may underlie the different GABAAα1/ GABAAα2 ratio in synapses and the altered inhibitory transmission in cultured neuroplastin-deficient hippocampal neurons [40], as well as in the hippocampus of neuroplastin-deficient mouse models [50]. The neuroplastin-GABAA receptor association has not been determined on the atomic level, but the recently resolved structure of the GABAA receptor [77] may contribute to resolving this issue.
The dysfunction of GABAergic transmission contributes to several neurological conditions, such as depression, anxiety, epilepsy (for a review, see: [78]), SZ [79], and ASD [80,81]. Based on the considerable combinatorial possibilities, a novel and more specific pharmacological intervention has been proposed as a potential advancement for clinical treatment over the use of nonselective GABAA receptor agonists (for a review, see: [78,82]. Furthermore, accessory molecules that interact with GABAA receptors may be new potential targets. Interestingly, neuroplastin expression has been linked to anxiety, depression, and 5-HT levels (see below, Depression and Anxiety Disorder). In neuroplastin-deficient mice, altered excitatory and inhibitory synaptic transmission was also observed [50]. The elucidation of the role of neuroplastin in the regulation and organization of the GABAergic system may contribute to a better understanding of the mechanisms underlying psychiatric disorders.
2.2.3. Neuroplastin Binding to TRAF6
The tumor necrosis factor receptor-associated factor 6 (TRAF6), is an intracellular adaptor protein with E3 ligase activity that is largely known for its function in the activation and tolerance of immune cells, cell differentiation, and cancer [83,84,85,86]. TRAF6 is also involved in the regulation of programmed cell death that normally occurs during early development of the mesencephalon and diencephalon [87]. Furthermore, TRAF6 is proposed to play a role in Alzheimer’s disease (AD) and neuroinflammation [86]. TRAF binding sites have been identified in neuroplastin [38,60] (see below, association of neuroplastin to cancer). In particular, we showed that the binding of TRAF6 to its binding motif (RKRPDEVPD) within the C-terminal domain of neuroplastin promotes spinogenesis [60]. Genetic inactivation of Nptn or TRAF6-RNA interference strongly reduced the protrusion density of young hippocampal neurons, which could be rescued by the over-expression of Np55 or Np65. In mature neurons, TRAF6 does not co-localize with neuroplastin and does not promote spinogenesis, thus limiting the function of neuroplastin-TRAF6 interactions to the early neuronal spinogenetic phase [60]. Synapse malformation and alterations in synaptic density occurring during early neuronal development have been associated with schizophrenia [88,89]. Furthermore, altered TRAF6 mRNA levels were detected in hippocampus and striatum of SZ patients [90]. Therefore, it is tempting to speculate about a potential involvement of neuroplastin-TRAF6 interaction in the origin of schizophrenia (see below, associations of neuroplastin to schizophrenia).
2.2.4. Neuroplastin Binding to Plasma Membrane Ca2+ ATPases (PMCA)
Recently, the expression of plasma membrane Ca2+ ATPases (PMCAs) was found to critically depend on neuroplastin in the mouse brain [35,50]. PMCAs are encoded by four distinct genes and expressed in numerous isoforms originating from alternative splicing [91]. PMCAs are essential for the extrusion of cytoplasmic Ca2+ to the extracellular side [92]. The loss of neuroplastin does not affect the transcription of PMCA genes [35], but in the absence of neuroplastin, the levels of PMCA proteins are reduced resulting in less Ca2+ extrusion and elevated intracellular Ca2+ levels with prolonged decay time to reach resting Ca2+ levels after stimulation [35,55,56]. Neuroplastin interacts directly with PMCAs forming functional complexes [35,55,56,57]. Gong et al. 2018 showed that the transmembrane domain of neuroplastin is responsible for binding to PMCAs. Cryogenic electron microscopy analysis of the neuroplastin–PMCA complex showed that the transmembrane domain of Np interacts with the 10th transmembrane domain and the 8th–9th transmembrane linker of PMCA, resulting in a conformational change required for the activity of PMCA and exposing the cytosolic Ca2+ binding site [57].
The function of the pairing of neuroplastin–PMCA must be regarded with respect to regulation of Ca2+ homeostasis, Ca2+ signal transduction, and synaptic activity, which are dysfunctional in neuropsychiatric diseases like ASD and SZ [93] and neurodegenerative diseases such as AD [94,95]. PMCA activity was found to be altered in AD human brain and was reduced by amyloid-β (Aβ) [96]. Furthermore, an interplay between PMCA with Aβ and tau protein was proposed [97]. Interestingly, decreased PMCA activity by Aβ or tau can be rescued by fostering the activity of the pump using endogenous regulatory proteins [98] or a synthetic phenothiazine [99]. In addition, numerous PMCA mutations are associated with human diseases and impairments, like deafness and ataxia (for review see: [100]). Interestingly, the genetically driven ablation of Nptn results in decreased PMCA levels and deafness in mice ([43]; see below, Section 3.5.1 Deafness).
3. Neuroplastin in Neurological Diseases
A particular syndrome has not yet been attributed to neuroplastin malfunction. However, the observed functions in mouse models and the expression, structure, and interaction partners of neuroplastin, indicate that the impairment of neuroplastin function in humans may result in deleterious consequences for the nervous system. Here, we will review the evidence for the contribution of neuroplastin to neurological pathologies.
3.1. Schizophrenia (SZ) and Autism Spectrum Disorder (ASD)
SZ and ASD manifest as distinct neurodevelopmental diseases. ASD frequently presents in childhood, whereas SZ manifests later in young adults. For both disorders, a heritable genetic contribution was observed, but explicit monogenetic causes have not been identified. Furthermore, a significant association between ASD and SZ was detected [101]. Strikingly, many gene loci related to synaptic function were identified as contributing to both SZ and ASD, suggesting that pathological malfunctions of synapses or synaptopathies may be causal (for review see: [102]). In addition, these two diseases frequently co-occur with attention deficit hyperactivity (ADHD) and bipolar disorder (BD); this is likely resulting from a developmental synaptopathy [103,104]. Brain images from ASD children have shown increased brain size and weight [105] affecting axons and synaptic density [106], which indicate an acceleration of brain development and more synaptic connections. A lack of adolescent synaptic pruning was observed in ASD patients [107], which may account for the dysfunction of brain circuits in ASD [108].
Unlike ASD, which shows an increase in brain growth in all brain regions except occipital grey matter [109], loss of grey matter in SZ was observed [110]. Excessive synaptic pruning in prefrontal cortical synapses was found in SZ neuropathology [111], which indicated reduced synapses and further impairments of the brain circuitry and cognitive functions [112,113].
3.1.1. Neuroplastin Relation to Schizophrenia
In rat models displaying schizophrenia-like symptoms after injection of the two different psychostimulants methamphetamine (MAP) or phencyclidine (PCP), neuroplastin was significantly up-regulated [114]. MAP is a dopamine transporter inhibitor that causes a positive symptom, clinically similar to paranoid schizophrenia in an acute phase [115]. PCP is an NMDA receptor antagonist, which induces both negative and positive schizophrenia-like symptomatology [116]. Subsequent genetic studies of patients with schizophrenia identified three single-nucleotide polymorphisms (SNPs) in the 5′-upstream region of NPTN that were strongly correlated to schizophrenia [44].
Pre-pulse inhibition (PPI) of the startle response is often considered as a characteristic in the diagnosis of schizophrenia [117]. In Nptn-deficient mice, PPI is severely impaired [50], although this could simply result from the profound hearing deficit of these mice [43], rather than processing deficits. Nevertheless, the significant reduction in paired-pulse facilitation in the auditory cortex of Nptn-deficient mice suggests altered cortical synaptic transmission [43]. In addition, the PPI deficit in heterozygous Nptn-deficient mice [50] points to potential central alterations as contributors to the phenotype. As detailed above, the neuroplastin interaction partners AMPA receptor subunit GluA1 and TRAF6 have also been associated with schizophrenia.
3.1.2. Autism Spectrum Disorder (ASD)
Some patients suffering from the heterogeneous 15q24 microdeletion syndrome display ASD and attention deficit hyperactivity disorder (ADHD), in addition to various other deficits [118,119]. NPTN is located at cytogenetic band 15q24.1 and it is deleted or duplicated in some 15q24 microdeletion syndrome patients [118,119]. Furthermore, PMCA2 was identified by GWAS studies to be associated with ASD [120]. A study which included 717 children associated the cortical morphology, such as cortical thickness and surface area, with autistic traits [121]. Interestingly, a single-nucleotide polymorphism in NPTN was found to be associated with cortical thickness [49]. Furthermore, the paths to ASDs may involve unbalanced excitatory–inhibitory synaptic transmission and abnormal synaptogenesis [122]. Several studies have observed an imbalance of excitatory-inhibitory transmission and altered synaptogenesis in different Nptn-deficient mice [40,51,59,60,123]. In addition, neuroplastin-deficient mice displayed altered social interactions avoiding unfamiliar mice [50]. In conclusion, genetic association studies suggest a link of neuroplastin to autism spectrum disorder, but the role of NPTN in ASD still needs to be specifically addressed. It remains to be seen whether a direct malfunction or loss of neuroplastin, rather than an indirect effect, e.g., via PMCAs, contributes to ASD.
3.2. Depression and Anxiety Disorder
Depression and anxiety are the most common mental disorders in society today, and both frequently co-occur in patients [124,125]. Etiological factors related to depression and anxiety disorder could be linked to childhood trauma, environmental adversity, as well as stressful life events [126]. Furthermore, several genes have been associated with depression and anxiety, among them 5-HTT, NPSR1, and RGS2 [126]. Genetic inactivation of Nptn results in elevated corticosterone levels and increased depressive-like behavior, but reduced anxiety-related behaviors in mice [50]. Mice that lacked only Np65 displayed the opposite phenotype, with reduced depressive-like behavior and increased anxiety [59]. In addition, the neuroplastin binding partners GluA1 and GABAA receptor are associated with anxiety disorder and depression (see Section 2.2.1 and Section 2.2.2).
3.3. Alzheimer’s (AD) Disease
An alteration of neuroplastin expression in AD patients was reported recently [58]. In the early phase of confirmed AD neuropathology, neuroplastin was significantly upregulated in the hippocampus (dentate gyrus, CA2/3 region, and subiculum) without changes in neuron number or tissue volume. Interestingly, patients experiencing a longer duration of AD disease (5–7 years) showed a decreased expression level of neuroplastin compared to patients with a short duration AD (≤4 years), which may indicate a role of neuroplastin in the early phase of AD. The analysis of neuropathological amyloid plaques and neurofibrillary tangles (NFT) showed a negative correlation between neuroplastin expression level and the number of amyloid plaques in the CA1 area and a weak negative correlation between neuroplastin and NFT in CA1, CA2/3, and subiculum. In human brain, both NPTN and PMCAs exhibit similar expression patterns at the transcriptomic level [127]. In comparison to the aging brain, the expression and activity of PMCA in AD patients were reduced, and the AD hallmarks tau and Aβ showed a negative impact on PMCA activity, which may indicate an altered Ca2+ homeostasis in the AD brain [96,128,129]. Alternatively, Ca2+ dys-homeostasis could promote the accumulation of Aβ and phosphorylated tau protein, which result in the neuropathy and brain function deficits in AD patients [128,129,130]. Aβ is produced by the β- and γ-secretase cleavages of the amyloid precursor protein (APP) [131]. The principal β-secretase for generation of Aβ in vivo is the β-site APP cleaving enzyme 1 (BACE1) [131,132]. The use of BACE1-specific inhibitors has been proposed as a potential intervention in AD [133]; however, this approach must be regarded carefully, as BACE1 cleaves numerous substrates. Interestingly, both neuroplastin and basigin were identified as potential BACE1 substrates [134,135]. Although further studies on the cleavage of Np by BACE1 were not conducted, an attractive hypothesis is that increased neuroplastin cleavage by BACE1 could result in the cognitive deficits observed in AD (Figure 3).
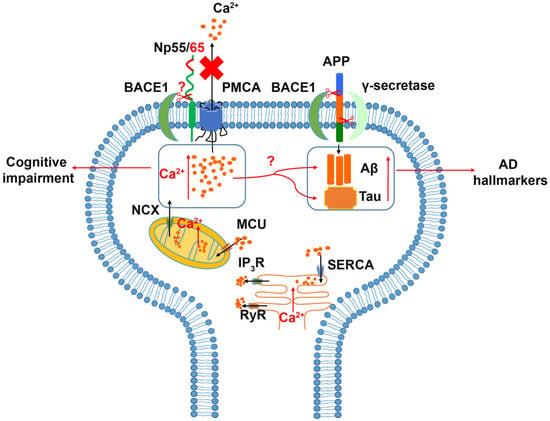
Figure 3.
Relation of Neuroplastin to Alzheimer’s disease. The Amyloid Precursor Protein (APP) is cleaved aberrantly by β-secretase (BACE1) and γ-secretase resulting in Aβ. Aβ and tau are considered hallmarks of Alzheimer’s disease. Increased intracellular Ca2+ concentrations are associated with cognitive impairment and increases in Aβ and tau. Intracellular Ca2+ can be deposited into or released from mitochondria and ER as intracellular calcium stores. Energy driven extrusion of Ca2+ is mediated by PMCAs. In the absence of neuroplastin, PMCA levels are reduced and intracellular Ca2+ is increased. The hypothetical cleavage of neuroplastin by BACE1 may result in loss of PMCAs and elevated Ca2+ levels.
3.4. Cognition, Antero- and Retrograde Amnesia
A large-scale genetic association study in adolescents associated NPTN with cortical thickness and intellectual ability [49], suggesting a role for NPTN in cognition and learning and memory. Furthermore, NPTN variants were recently related to creativity [136].
In recent years, we have addressed the role of neuroplastin in learning and memory using several mouse mutants. When neuroplastin was missing from only glutamatergic neurons, achieved using an Emx1-promoter driven Cre-recombinase, associative learning was slightly improved. However, the continuity of task execution was affected, suggesting altered striatum-dependent decision making [35]. The complete loss of neuroplastin expression resulted in a complex phenotype, which included the inability to learn associative tasks [50]. The comparison of Nptn-ablation in glutamatergic versus all neurons suggests a particular role of neuroplastin, expressed by gabaergic interneurons for associative learning. When neuroplastin expression was specifically ablated in all types of neurons after a normal development, again, the anterograde memory was not formed for associative tasks [50]. Furthermore, when the associative tasks were first acquired perfectly before neuroplastin ablation, the induced loss of neuroplastin resulted in specific retrograde amnesia for these associative memories but not for spatial memories [50]. Interestingly, the β-blocker propranolol has retrograde amnestic side effects. Therefore, propranolol is applied as off-label use for the treatment of intrusive thoughts associated with post-traumatic stress disorder (PTSD) (for a review, see: [137]). Propranolol also acts as an inhibitor of the PMCAs [138], and thus its amnestic effects may be related to PMCA inhibition. In vivo, the absence of neuroplastin leads to dramatically reduced PMCA levels [35,50], suggesting that the neuroplastin-PMCA assembly may be critical for associative learning and memory. If this hypothesis can be substantiated, it may provide an opportunity to address PTSD and other psychiatric conditions involving intrusive thoughts.
3.5. Other Diseases Related to Neuroplastin
3.5.1. Deafness
Deafness resulting from the loss of neuroplastin function has been studied using Nptn-deficient and neuroplastin mutant mice [43,51,52]. It was proposed that Np55 expression by outer hair cells is required for cochlear amplification [52], and Carrott et al. [51] proposed Np65-driven synaptogenesis by inner hair cells as necessary for hearing. Recently, we showed that neuroplastin expression is essential for hearing during the development of the hearing system, and also for the maintenance of hearing capabilities in adults throughout their life [43]. Neuroplastin is required for PMCA2 targeting and Ca2+ extrusion in cochlear hair cells [43]. Interestingly, PMCA2 loss of function mutations result in deafness in mice and human [139], verifying that the interaction of neuroplastin with PMCA is decisive for Ca2+ extrusion.
3.5.2. Cancer
The first evidence linking NPTN to cancer came from a bioinformatic analysis showing that NPTN was one of 166 genes with an altered expression in colorectal adenomatous polyps [140]. In a study screening for potential tumor antigens from breast cancer patients, neuroplastin was identified and showed strongly increased immunoreactivity in invasive carcinoma tissues [141]. Moreover, over-expression of neuroplastin in a breast-cancer-derived cell line strongly increased tumor growth and angiogenesis, as well as the production of vascular endothelia growth factor (VEGF), suggesting an angiogenic mechanism regulated by VEGF in the aberrant neuroplastin-expressing tumors [141]. Furthermore, the role of neuroplastin and its interaction with S100A8/A9, resulting in the activation of a cascade for lung cancer disseminative progression and aggressive development, has been proposed [142,143].
3.5.3. Various Diseases
Not surprisingly, the widespread expression of neuroplastin in nearly all organs may result in the discovery of further pathological conditions influenced by neuroplastin, e.g., within the immune system [55] or in heart disease [144].
4. Future Research Directions
The multiple binding partners place neuroplastin centrally in the interwoven processes of (a), synapse formation and synaptic plasticity, and (b), intracellular calcium signaling. While developmental dysfunctions of neuroplastin-mediated synaptic processes may be more related to neuropsychiatric diseases, neurodegenerative diseases may instead involve neuroplastin-related alterations in synaptic calcium extrusion. However, both aspects must not be mutually exclusive. Therefore, the association of neuroplastin, PMCAs, and AMPA receptors in synaptic assemblies will be a topic of further investigation. Furthermore, the potential cleavage of neuroplastin by BACE1 or other proteases, and its successive cognitive impairment and neurodegeneration, should be addressed. It is likely that neuroplastin variants will be identified as causal for specific human disease syndromes, possibly not only affecting the nervous system. Future research will address by which mechanisms neuroplastin influences learning and memory. Of particular interest is the loss of associative memories after neuroplastin ablation. The possibility to elicit retrograde amnesia in a controlled manner in a mouse model allows for the study of underlying mechanisms, and can increase the understanding of the molecular and circuit processes of memory. This retrograde amnesia model opens experimental means of developing treatment approaches for posttraumatic stress disorder, traumatic experiences, and intrusive thoughts. In particular, the analysis of neuronal subsets and the role of neuroplastin expression in gabaergic interneurons may reveal decisive circuits for associative memories.
5. Conclusions and Perspectives
The study of neuroplastin in recent decades has elucidated a complex and interwoven network of binding partners. Neuroplastin is evolving as an important molecule, with essential functions in the nervous system for optimal synapse formation, synaptic plasticity, and learning and memory. Accordingly, the functions of neuroplastin can now be related to neuropathological conditions. Expression in most organs implies a critical function of neuroplastin, which may be affected in other diseases, such as cancer or heart disease. In particular, the recent identification of neuroplastin as a decisive component of plasma membrane Ca2+ ATPase complexes has opened new perspectives for the mechanistic understanding of learning and memory processes. The unique animal model for induction of retrograde amnesia may help to understand mechanistically memory loss, and might provide useful insights to develop further strategies for the treatment of PTSD.
Author Contributions
All authors contributed to conceptualization, writing, and visualization of this review article. All authors have read and agreed to the published version of the manuscript.
Funding
We are very grateful to Jonathan Lindquist, Otto-von-Guericke University, Magdeburg for critically proofreading the manuscript. This work was funded by German Federal Ministry of Education and Research (BMBF grant CONICYT to Eckart D. Gundelfinger, Karl-Heinz Smalla, Constanze Seidenbecher, and D.M.) and by the China Scholarship Council (to X.L. CSC No. 201506290028 and Y.L. CSC No. 202008080376). R.H.-M. thanks the Center for Behavioral and Brain Sciences (LSA-fellowship) and the DAAD 57514679. The APC was funded by Leibniz Institute for Neurobiology (LIN).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
N/A.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abate, K.A.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sloane, P.D.; Zimmerman, S.; Suchindran, C.; Reed, P.; Wang, L.; Boustani, M.; Sudha, S. The public health impact of Alzheimer’s disease, 2000–2050: Potential implication of treatment advances. Annu. Rev. Public Health 2002, 23, 213–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, H.Y.; Teoh, S.L.; Wu, D.B.; Kotirum, S.; Chiou, C.F.; Chaiyakunapruk, N. Global economic burden of schizophrenia: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 357–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogge, N.; Janssen, J. The Economic Costs of Autism Spectrum Disorder: A Literature Review. J. Autism Dev. Disord. 2019, 49, 2873–2900. [Google Scholar] [CrossRef]
- Baresic, A.; Nash, A.J.; Dahoun, T.; Howes, O.; Lenhard, B. Understanding the genetics of neuropsychiatric disorders: The poten-tial role of genomic regulatory blocks. Mol. Psychiatry 2020, 25, 6–18. [Google Scholar] [CrossRef]
- Sakurai, K.; Migita, O.; Toru, M.; Arinami, T. An association between a missense polymorphism in the close homologue of L1 (CHL1, CALL) gene and schizophrenia. Mol. Psychiatry 2002, 7, 412–415. [Google Scholar] [CrossRef] [Green Version]
- Munafo, M.R.; Attwood, A.S.; Flint, J. Neuregulin 1 genotype and schizophrenia. Schizophr. Bull. 2008, 34, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, H.G.; Bogerts, B. Neuregulin-1 alpha, the underestimated molecule: Emerging new roles in normal brain function and the pathophysiology of schizophrenia? Genome 2013, 56, 703–704. [Google Scholar] [CrossRef]
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.R.; Farh, K.; Holmans, P.A.; Lee, P.; Bulik-Sullivan, B.; Collier, D.A.; Huang, H.; et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar]
- Dennison, C.A.; Legge, S.E.; Pardinas, A.F.; Walters, J.T.R. Genome-wide association studies in schizophrenia: Recent advances, challenges and future perspective. Schizophr. Res. 2020, 217, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Quach, H.; Betancur, C.; Rastam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Oliveira, G.; Coutinho, A.; Yang, C.; Feng, J.; Katz, C.; Sram, J.; Bockholt, A.; Jones, I.R.; Craddock, N.; et al. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol. Psychiatry 2005, 10, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Sudhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Yao, X.; March, M.E.; Meng, X.; Li, J.; Wei, Z.; Sleiman, P.M.A.; Hakonarson, H.; Xia, Q.; Li, J. Target Genes of Autism Risk Loci in Brain Frontal Cortex. Front. Genet. 2019, 10, 707. [Google Scholar] [CrossRef] [Green Version]
- Anney, R.J.L.; Ripke, S.; Anttila, V.; J Grove, J.; Holmans, P.; Huang, H.; Klei, L.; Lee, P.H.; Medland, S.E.; Neale, B.; et al. Me-ta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol. Autism 2017, 8, 21. [Google Scholar]
- Hung, A.Y.; Futai, K.; Sala, C.; Valtschanoff, J.G.; Ryu, J.; Woodworth, M.A.; Kidd, F.L.; Sung, C.C.; Miyakawa, T.; Bear, M.F.; et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J. Neurosci. 2008, 28, 1697–1708. [Google Scholar] [CrossRef] [Green Version]
- Peca, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; McCoy, P.A.; Rodriguiz, R.M.; Pan, Y.; Je, H.S.; Roberts, A.C.; Kim, C.J.; Berrios, J.; Colvin, J.S.; Bousquet-Moore, D.; et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum. Mol. Genet. 2011, 20, 3093–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Won, H.; Lee, H.R.; Gee, H.Y.; Mah, W.; Kim, J.I.; Lee, J.; Ha, S.; Chung, C.; Jung, E.S.; Cho, Y.S.; et al. Autistic-like social behav-iour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012, 486, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Christian, K.M.; Song, H.; Ming, G.L. Synaptic dysfunction in complex psychiatric disorders: From genetics to mecha-nisms. Genome Med. 2018, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Penzes, P.; Buonanno, A.; Passafaro, M.; Sala, C.; Sweet, R.A. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J. Neurochem. 2013, 126, 165–182. [Google Scholar] [CrossRef]
- Lopatina, O.L.; Malinovskaya, N.A.; Komleva, Y.K.; Gorina, Y.V.; Shuvaev, A.N.; Olovyannikova, R.Y.; Belozor, O.S.; Belova, O.A.; Higashida, H.; Salmina, A.B. Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Rev. Neurosci. 2019, 30, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Hadjichrysanthou, C.; Evans, S.; Wong, M.M. Why do so many clinical trials of therapies for Alzheimer’s disease fail? Lancet 2017, 390, 2327–2329. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef]
- Paik, J. Olanzapine/Samidorphan: First Approval. Drugs 2021, 81, 1431–1436. [Google Scholar] [CrossRef]
- LeClerc, S.; Easley, D. Pharmacological therapies for autism spectrum disorder: A review. Pharm. Ther. 2015, 40, 389–397. [Google Scholar]
- Baribeau, D.; Anagnostou, E. Novel treatments for autism spectrum disorder based on genomics and systems biology. Pharmacol. Ther. 2021, 107939. [Google Scholar] [CrossRef]
- Doherty, J.L.; Owen, M.J. Genomic insights into the overlap between psychiatric disorders: Implications for research and clinical practice. Genome Med. 2014, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Beesley, P.W.; Herrera-Molina, R.; Smalla, K.H.; Seidenbecher, C. The Neuroplastin adhesion molecules: Key regulators of neu-ronal plasticity and synaptic function. J. Neurochem. 2014, 131, 268–283. [Google Scholar] [CrossRef]
- Hill, I.E.; Selkirk, C.P.; Hawkes, R.B.; Beesley, P.W. Characterization of novel glycoprotein components of synaptic membranes and postsynaptic densities, gp65 and gp55, with a monoclonal antibody. Brain Res. 1988, 461, 27–43. [Google Scholar] [CrossRef]
- Herrera-Molina, R.; Mlinac-Jerkovic, K.; Ilic, K.; Stober, F.; Vemula, S.K.; Sandoval, M.; Milosevic, N.J.; Simic, G.; Smalla, K.H.; Goldschmidt, J.; et al. Neuroplastin deletion in glutamatergic neurons impairs selective brain functions and calcium regula-tion: Implication for cognitive deterioration. Sci. Rep. 2017, 7, 7273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langnaese, K.; Beesley, P.W.; Gundelfinger, E.D. Synaptic membrane glycoproteins gp65 and gp55 are new members of the im-munoglobulin superfamily. J. Biol. Chem. 1997, 272, 821–827. [Google Scholar] [CrossRef] [Green Version]
- Langnaese, K.; Mummery, R.; Gundelfinger, E.D.; Beesley, P.W. Immunoglobulin superfamily members gp65 and gp55: Tissue distribution of glycoforms. FEBS Lett. 1998, 429, 284–288. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, M.; Yamamoto, M.; Miyai, M.; Maeda, T.; Hiruma, J.; Murata, H.; Kinoshita, R.; Winarsa Ruma, I.M.; Putranto, E.W.; Inoue, Y.; et al. Identification of an S100A8 Receptor Neuroplastin-beta and its Heterodimer Formation with EMMPRIN. J. Invest. Derm. 2016, 136, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Smalla, K.H.; Matthies, H.; Langnase, K.; Shabir, S.; Bockers, T.M.; Wyneken, U.; Staak, S.; Krug, M.; Beesley, P.W.; Gundelfinger, E.D. The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal CA1 synapses. Proc. Natl. Acad. Sci. USA 2000, 97, 4327–4332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-Molina, R.; Sarto-Jackson, I.; Montenegro-Venegas, C.; Heine, M.; Smalla, K.H.; Seidenbecher, C.I.; Beesley, P.W.; Gun-delfinger, E.D.; Montag, D. Structure of excitatory synapses and GABAA receptor localization at inhibitory synapses are regulated by neuroplastin-65. J. Biol. Chem. 2014, 289, 8973–8988. [Google Scholar] [CrossRef] [Green Version]
- Kreutz, M.R.; Langnaese, K.; Dieterich, D.C.; Seidenbecher, C.I.; Zuschratter, W.; Beesley, P.W.; Gundelfinger, E.D. Distribution of transcript and protein isoforms of the synaptic glycoprotein neuroplastin in rat retina. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1907–1914. [Google Scholar] [PubMed]
- Bernstein, H.G.; Smalla, K.H.; Bogerts, B.; Gordon-Weeks, P.R.; Beesley, P.W.; Gundelfinger, E.D.; Kreutz, M.R. The immuno-localization of the synaptic glycoprotein neuroplastin differs substantially between the human and the rodent brain. Brain Res. 2007, 1134, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Brunk, M.G.K.; Yuanxiang, P.; Curran, A.W.; Zhang, E.; Stober, F.; Goldschmidt, J.; Gundelfinger, E.D.; Vollmer, M.; Hap-pel, M.F.K.; et al. Neuroplastin expression is essential for hearing and hair cell PMCA expression. Brain Struct. Funct. 2021, 226, 1533–1551. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Fujikura-Ouchi, Y.; Kuramasu, A.; Shimoda, K.; Akiyama, K.; Matsuoka, H.; Ito, C. Association study of putative pro-moter polymorphisms in the neuroplastin gene and schizophrenia. Neurosci. Lett. 2007, 411, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, S.; Kiryushko, D.; Larsen, M.H.; Kastrup, J.S.; Gajhede, M.; Sandi, C.; Berezin, V.; Bock, E.; Soroka, V. Neuroplastin-55 binds to and signals through the fibroblast growth factor receptor. FASEB J. 2010, 24, 1139–1150. [Google Scholar] [CrossRef]
- Owczarek, S.; Soroka, V.; Kiryushko, D.; Larsen, M.H.; Yuan, Q.; Sandi, C.; Berezin, V.; Bock, E. Neuroplastin-65 and a mimetic peptide derived from its homophilic binding site modulate neuritogenesis and neuronal plasticity. J. Neurochem. 2011, 117, 984–994. [Google Scholar] [CrossRef]
- Sarto-Jackson, I.; Milenkovic, I.; Smalla, K.H.; Gundelfinger, E.D.; Kaehne, T.; Herrera-Molina, R.; Thomas, S.; Kiebler, M.A.; Sieghart, W. The cell adhesion molecule neuroplastin-65 is a novel interaction partner of gamma-aminobutyric acid type A receptors. J. Biol. Chem. 2012, 287, 14201–14214. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.C.; Kraus, M.; Marzban, H.; Sarna, J.R.; Wang, Y.; Hawkes, R.; Halestrap, A.P.; Beesley, P.W. The neuroplastin adhesion molecules are accessory proteins that chaperone the monocarboxylate transporter MCT2 to the neuronal cell surface. PLoS ONE 2013, 8, e78654. [Google Scholar]
- Desrivieres, S.; Lourdusamy, A.; Tao, C.; Toro, R.; Jia, T.; Loth, E.; Medina, L.M.; Kepa, A.; Fernandes, A.; Ruggeri, B.; et al. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Mol. Psychiatry 2015, 20, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Herrera-Molina, R.; Sabanov, V.; Ahmed, T.; Iscru, E.; Stober, F.; Richter, K.; Fischer, K.D.; Angenstein, F.; Gold-schmidt, J.; et al. Genetically Induced Retrograde Amnesia of Associative Memories After Neuroplastin Ablation. Biol. Psy-chiatry 2017, 81, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Carrott, L.; Bowl, M.R.; Aguilar, C.; Johnson, S.L.; Chessum, L.; West, M.; Morse, S.; Dorning, J.; Smart, E.; Hardisty-Hughes, R.; et al. Absence of Neuroplastin-65 Affects Synaptogenesis in Mouse Inner Hair Cells and Causes Profound Hearing Loss. J. Neurosci. 2016, 36, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.Z.; Grillet, N.; Dewey, J.B.; Trouillet, A.; Krey, J.F.; Barr-Gillespie, P.G.; Oghalai, J.S.; Muller, U. Neuroplastin Isoform Np55 Is Expressed in the Stereocilia of Outer Hair Cells and Required for Normal Outer Hair Cell Function. J. Neurosci. 2016, 36, 9201–9216. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, J.; Imanishi, E.; Nagata, S. Xkr8 phospholipid scrambling complex in apoptotic phosphatidylserine exposure. Proc. Natl. Acad. Sci. USA 2016, 113, 9509–9514. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhan, Q.; Zhang, H.; Liu, X.; Huang, L.; Li, H.; Yuan, Q. Increased Susceptibility to Ischemic Brain Injury in Neuroplastin 65-Deficient Mice Likely via Glutamate Excitotoxicity. Front. Cell Neurosci. 2017, 11, 110. [Google Scholar] [CrossRef]
- Korthals, M.; Langnaese, K.; Smalla, K.H.; Kahne, T.; Herrera-Molina, R.; Handschuh, J.; Lehmann, A.C.; Mamula, D.; Naumann, M.; Seidenbecher, C.; et al. A complex of Neuroplastin and Plasma Membrane Ca(2+) ATPase controls T cell activation. Sci. Rep. 2017, 7, 8358. [Google Scholar] [CrossRef]
- Schmidt, N.; Kollewe, A.; Constantin, C.E.; Henrich, S.; Ritzau-Jost, A.; Bildl, W.; Saalbach, A.; Hallermann, S.; Kulik, A.; Fakler, B.; et al. Neuroplastin and Basigin Are Essential Auxiliary Subunits of Plasma Membrane Ca(2+)-ATPases and Key Regula-tors of Ca(2+) Clearance. Neuron 2017, 96, 827–838 e829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, D.; Chi, X.; Ren, K.; Huang, G.; Zhou, G.; Yan, N.; Lei, J.; Zhou, Q. Structure of the human plasma membrane Ca(2+)-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat. Commun. 2018, 9, 3623. [Google Scholar] [CrossRef] [PubMed]
- Ilic, K.; Mlinac-Jerkovic, K.; Jovanov-Milosevic, N.; Simic, G.; Habek, N.; Bogdanovic, N.; Kalanj-Bognar, S. Hippocampal expres-sion of cell-adhesion glycoprotein neuroplastin is altered in Alzheimer’s disease. J. Cell. Mol. Med. 2019, 23, 1602–1607. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Gao, X.; Liu, L.; Amuti, S.; Wu, D.; Jiang, F.; Huang, L.; Wang, G.; Zeng, J.; et al. Neuroplastin 65 modulates anxiety- and depression-like behavior likely through adult hippocampal neurogenesis and central 5-HT activity. FEBS J. 2019, 286, 3401–3415. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.K.; Malci, A.; Junge, L.; Lehmann, A.C.; Rama, R.; Hradsky, J.; Matute, R.A.; Weber, A.; Prigge, M.; Naumann, M.; et al. The Interaction of TRAF6 With Neuroplastin Promotes Spinogenesis During Early Neuronal Development. Front. Cell Dev. Biol. 2020, 8, 579513. [Google Scholar] [CrossRef]
- Yagi, T.; Asada, R.; Kanekura, K.; Eesmaa, A.; Lindahl, M.; Saarma, M.; Urano, F. Neuroplastin Modulates Anti-inflammatory Effects of MANF. iScience 2020, 23, 101810. [Google Scholar] [CrossRef]
- Jiang, C.H.; Wei, M.; Zhang, C.; Shi, Y.S. The amino-terminal domain of GluA1 mediates LTP maintenance via interaction with neuroplastin-65. Proc.Natl. Acad. Sci. USA 2021, 118, e2019194118. [Google Scholar] [CrossRef] [PubMed]
- Balog, M.; Blazetic, S.; Ivic, V.; Labak, I.; Krajnik, B.; Marin, R.; Canerina-Amaro, A.; de Pablo, D.P.; Bardak, A.; Gaspar, R.; et al. Disarranged neuroplastin environment upon aging and chronic stress recovery in female Sprague Dawley rats. Eur. J. Neu-rosci. 2021, 1–17. [Google Scholar] [CrossRef]
- Empson, R.M.; Buckby, L.E.; Kraus, M.; Bates, K.J.; Crompton, M.R.; Gundelfinger, E.D.; Beesley, P.W. The cell adhesion molecule neuroplastin-65 inhibits hippocampal long-term potentiation via a mitogen-activated protein kinase p38-dependent reduc-tion in surface expression of GluR1-containing glutamate receptors. J. Neurochem. 2006, 99, 850–860. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Y.T. GluA1-homomeric AMPA receptor in synaptic plasticity and neurological diseases. Neuropharmacology 2021, 197, 108708. [Google Scholar] [CrossRef]
- Gugustea, R.; Jia, Z. Genetic manipulations of AMPA glutamate receptors in hippocampal synaptic plasticity. Neuropharmacology 2021, 194, 108630. [Google Scholar] [CrossRef]
- Zamanillo, D.; Sprengel, R.; Hvalby, O.; Jensen, V.; Burnashev, N.; Rozov, A.; Kaiser, K.M.; Koster, H.J.; Borchardt, T.; Worley, P.; et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 1999, 284, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, D.J.; Sprengel, R.; Seeburg, P.H.; Bannerman, D.M. Deletion of the GluA1 AMPA receptor subunit alters the expression of short-term memory. Learn. Mem. 2011, 18, 128–131. [Google Scholar] [CrossRef] [Green Version]
- Wiedholz, L.M.; Owens, W.A.; Horton, R.E.; Feyder, M.; Karlsson, R.M.; Hefner, K.; Sprengel, R.; Celikel, T.; Daws, L.C.; Holmes, A. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol. Psychiatry 2008, 13, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Barkus, C.; Feyder, M.; Graybeal, C.; Wright, T.; Wiedholz, L.; Izquierdo, A.; Kiselycznyk, C.; Schmitt, W.; Sanderson, D.J.; Rawlins, J.N.; et al. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symp-toms of schizophrenia and schizoaffective disorder? Neuropharmacology 2012, 62, 1263–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minano-Molina, A.J.; Espana, J.; Martin, E.; Barneda-Zahonero, B.; Fado, R.; Sole, M.; Trullas, R.; Saura, C.A.; Rodriguez-Alvarez, J. Soluble oligomers of amyloid-beta peptide disrupt membrane trafficking of al-pha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor contributing to early synapse dysfunction. J. Biol. Chem. 2011, 286, 27311–27321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriguchi, S. Pharmacological study on Alzheimer’s drugs targeting calcium/calmodulin-dependent protein kinase II. J. Pharm. Sci. 2011, 117, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman-Albasini, L.; Diaz-Veliz, G.; Olave, F.A.; Aguayo, F.I.; Garcia-Rojo, G.; Corrales, W.A.; Silva, J.P.; Avalos, A.M.; Rojas, P.S.; Aliaga, E.; et al. Antidepressant-relevant behavioral and synaptic molecular effects of long-term fasudil treatment in chron-ically stressed male rats. Neurobiol. Stress 2020, 13, 100234. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Yao, D.; Li, S.; Li, J.; Si, Y.; Zhang, H.; Zhu, Z.; Song, D.; Li, H. N-cadherin regulates GluA1-mediated depressive-like behavior in adolescent female rat offspring following prenatal stress. Neuroendocrinology 2021. [Google Scholar] [CrossRef] [PubMed]
- Leal, R.B.; Lopes, M.W.; Formolo, D.A.; de Carvalho, C.R.; Hoeller, A.A.; Latini, A.; Sousa, D.S.; Wolf, P.; Prediger, R.D.; Bortolot-to, Z.A.; et al. Amygdala levels of the GluA1 subunit of glutamate receptors and its phosphorylation state at serine 845 in the anterior hippocampus are biomarkers of ictal fear but not anxiety. Mol. Psychiatry 2020, 25, 655–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.; Wang, J.; Zhang, X.; Duan, Y.; Xu, Y.; Lv, J.; Wang, D.; Zhang, H.; Richter-Levin, G.; Klavir, O.; et al. alphaCaMKII in the lateral amygdala mediates PTSD-Like behaviors and NMDAR-Dependent LTD. Neurobiol. Stress 2021, 15, 100359. [Google Scholar] [CrossRef]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABAA receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Castellano, D.; Shepard, R.D.; Lu, W. Looking for Novelty in an “Old” Receptor: Recent Advances Toward Our Understanding of GABAARs and Their Implications in Receptor Pharmacology. Front. Neurosci. 2020, 14, 616298. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, G.; Cho, R.Y.; Lewis, D.A. Alterations in cortical network oscillations and parvalbumin neurons in schizo-phrenia. Biol. Psychiatry 2015, 77, 1031–1040. [Google Scholar] [CrossRef] [Green Version]
- Marin, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef]
- Mori, T.; Mori, K.; Fujii, E.; Toda, Y.; Miyazaki, M.; Harada, M.; Hashimoto, T.; Kagami, S. Evaluation of the GABAergic nervous system in autistic brain: (123)I-iomazenil SPECT study. Brain Dev. 2012, 34, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; van Gerven, J.; Cohen, A.; Jacobs, G. Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety. Acta Pharm. Sin. 2019, 40, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signal-ing pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, P. TRAF molecules in cell signaling and in human diseases. J. Mol. Signal 2013, 8, 7. [Google Scholar] [CrossRef]
- Walsh, M.C.; Lee, J.; Choi, Y. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 2015, 266, 72–92. [Google Scholar] [CrossRef]
- Dou, Y.; Tian, X.; Zhang, J.; Wang, Z.; Chen, G. Roles of TRAF6 in Central Nervous System. Curr. Neuropharmacol. 2018, 16, 1306–1313. [Google Scholar] [CrossRef]
- Lomaga, M.A.; Yeh, W.C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999, 13, 1015–1024. [Google Scholar] [CrossRef] [Green Version]
- Boda, B.; Dubos, A.; Muller, D. Signaling mechanisms regulating synapse formation and function in mental retardation. Curr. Opin. Neurobiol. 2010, 20, 519–527. [Google Scholar] [CrossRef]
- Lima Caldeira, G.; Peca, J.; Carvalho, A.L. New insights on synaptic dysfunction in neuropsychiatric disorders. Curr. Opin. Neuro-biol. 2019, 57, 62–70. [Google Scholar] [CrossRef]
- Armstrong, A.P.; Tometsko, M.E.; Glaccum, M.; Sutherland, C.L.; Cosman, D.; Dougall, W.C. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J. Biol. Chem. 2002, 277, 44347–44356. [Google Scholar] [CrossRef] [Green Version]
- Krebs, J. The plethora of PMCA isoforms: Alternative splicing and differential expression. Biochim. Biophys. Acta 2015, 1853, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Lopreiato, R.; Giacomello, M.; Carafoli, E. The plasma membrane calcium pump: New ways to look at an old enzyme. J. Biol. Chem. 2014, 289, 10261–10268. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, L.; Papaleo, V.; Porcelli, V.; Scarcia, P.; Gaita, L.; Sacco, R.; Hager, J.; Rousseau, F.; Curatolo, P.; Manzi, B.; et al. Altered calcium homeostasis in autism-spectrum disorders: Evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol. Psychiatry 2010, 15, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Popugaeva, E.; Pchitskaya, E.; Bezprozvanny, I. Dysregulation of neuronal calcium homeostasis in Alzheimer’s disease—A ther-apeutic opportunity? Biochem. Biophys. Res. Commun. 2017, 483, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Chami, M.; Checler, F. Alterations of the Endoplasmic Reticulum (ER) Calcium Signaling Molecular Components in Alzheimer’s Disease. Cells 2020, 9, 2577. [Google Scholar] [CrossRef]
- Berrocal, M.; Marcos, D.; Sepulveda, M.R.; Perez, M.; Avila, J.; Mata, A.M. Altered Ca2+ dependence of synaptosomal plasma membrane Ca2+-ATPase in human brain affected by Alzheimer’s disease. FASEB J. 2009, 23, 1826–1834. [Google Scholar] [CrossRef]
- Mata, A.M. Functional interplay between plasma membrane Ca(2+)-ATPase, amyloid beta-peptide and tau. Neurosci. Lett. 2018, 663, 55–59. [Google Scholar] [CrossRef]
- Berrocal, M.; Saez, L.; Mata, A.M. Sorcin Activates the Brain PMCA and Blocks the Inhibitory Effects of Molecular Markers of Alzheimer’s Disease on the Pump Activity. Int. J. Mol. Sci. 2021, 22, 6055. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, M.; Caballero-Bermejo, M.; Gutierrez-Merino, C.; Mata, A.M. Methylene Blue Blocks and Reverses the Inhibitory Effect of Tau on PMCA Function. Int. J. Mol. Sci. 2019, 20, 3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brini, M.; Carafoli, E.; Cali, T. The plasma membrane calcium pumps: Focus on the role in (neuro)pathology. Biochem. Biophys. Res. Commun. 2017, 483, 1116–1124. [Google Scholar]
- Zheng, Z.; Zheng, P.; Zou, X. Association between schizophrenia and autism spectrum disorder: A systematic review and me-ta-analysis. Autism Res. 2018, 11, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.G. Synaptopathies: Diseases of the synaptome. Curr. Opin. Neurobiol. 2012, 22, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Brose, N.; O’Connor, V.; Skehel, P. Synaptopathy: Dysfunction of synaptic function? Biochem. Soc. Trans. 2010, 38, 443–444. [Google Scholar] [CrossRef]
- Eltokhi, A.; Janmaat, I.E.; Genedi, M.; Haarman, B.C.M.; Sommer, I.E.C. Dysregulation of synaptic pruning as a possible link between intestinal microbiota dysbiosis and neuropsychiatric disorders. J. Neurosci. Res. 2020, 98, 1335–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courchesne, E.; Carper, R.; Akshoomoff, N. Evidence of brain overgrowth in the first year of life in autism. JAMA 2003, 290, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, L.; Gozzi, M.; Lenroot, R.; Thurm, A.; Behseta, B.; Swedo, S.; Pierpaoli, C. Diffusion tensor imaging in young children with autism: Biological effects and potential confounds. Biol. Psychiatry 2012, 72, 1043–1051. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Gudsnuk, K.; Kuo, S.H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, M.K.; Allen, G.; Beckel-Mitchener, A.; Boulanger, L.M.; Carper, R.A.; Webb, S.J. Autism and abnormal development of brain connectivity. J. Neurosci. 2004, 24, 9228–9231. [Google Scholar] [CrossRef]
- Schumann, C.M.; Bloss, C.S.; Barnes, C.C.; Wideman, G.M.; Carper, R.A.; Akshoomoff, N.; Pierce, K.; Hagler, D.; Schork, N.; Lord, C.; et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 2010, 30, 4419–4427. [Google Scholar] [CrossRef]
- Gogtay, N. Cortical brain development in schizophrenia: Insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr. Bull. 2008, 34, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Keshavan, M.S.; Anderson, S.; Pettegrew, J.W. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J. Psychiatr. 1994, 28, 239–265. [Google Scholar] [CrossRef]
- Mallya, A.P.; Deutch, A.Y. (Micro)Glia as Effectors of Cortical Volume Loss in Schizophrenia. Schizophr. Bull. 2018, 44, 948–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019, 22, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Kubota, Y.; Kuramasu, A.; Watanabe, T.; Ito, C. Gene expression profiling in whole cerebral cortices of phencyclidine- or methamphetamine-treated rats. Mol. Brain Res. 2005, 140, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Chen, C.C.; Akiyama, K.; Otsuki, S. Acute exacerbation of paranoid psychotic state after long-term abstinence in pa-tients with previous methamphetamine psychosis. Biol. Psychiatry 1983, 18, 429–440. [Google Scholar]
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. J. Psychiatry 1991, 148, 1301–1308. [Google Scholar]
- Mena, A.; Ruiz-Salas, J.C.; Puentes, A.; Dorado, I.; Ruiz-Veguilla, M.; De la Casa, L.G. Reduced Prepulse Inhibition as a Biomarker of Schizophrenia. Front. Behav. Neurosci. 2016, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.; Spence, M.A.; Flodman, P. Nuclear and mitochondrial genome defects in autisms. Ann. N. Y. Acad. Sci. 2009, 1151, 102–132. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zarrei, M.; Dong, R.; Yang, X.; Zhao, D.; Scherer, S.W.; Gai, Z. Refining critical regions in 15q24 microdeletion syndrome pertaining to autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2020, 183, 217–226. [Google Scholar] [CrossRef]
- Carayol, J.; Sacco, R.; Tores, F.; Rousseau, F.; Lewin, P.; Hager, J.; Persico, A.M. Converging evidence for an association of ATP2B2 allelic variants with autism in male subjects. Biol. Psychiatry 2011, 70, 880–887. [Google Scholar] [CrossRef]
- Blanken, L.M.; Mous, S.E.; Ghassabian, A.; Muetzel, R.L.; Schoemaker, N.K.; El Marroun, H.; van der Lugt, A.; Jaddoe, V.W.; Hof-man, A.; Verhulst, F.C.; et al. Cortical morphology in 6- to 10-year old children with autistic traits: A population-based neuroimaging study. Am. J. Psychiatry 2015, 172, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Persico, A.M.; Bourgeron, T. Searching for ways out of the autism maze: Genetic, epigenetic and environmental clues. Trends Neurosci. 2006, 29, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Amuti, S.; Tang, Y.; Wu, S.; Liu, L.; Huang, L.; Zhang, H.; Li, H.; Jiang, F.; Wang, G.; Liu, X.; et al. Neuroplastin 65 mediates cogni-tive functions via excitatory/inhibitory synapse imbalance and ERK signal pathway. Neurobiol. Learn. Mem. 2016, 127, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, G.J.; McCarthy-Larzelere, M.E.; Williamson, D.A.; Mathews, A.; Manguno-Mire, G.M.; Bentz, B.G. Anxiety, depres-sion, and the content of worries. Depress. Anxiety 2001, 14, 247–250. [Google Scholar] [PubMed]
- Tiller, J.W. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31. [Google Scholar] [CrossRef]
- Gottschalk, M.G.; Domschke, K. Genetics of generalized anxiety disorder and related traits. Dialogues Clin. Neurosci. 2017, 19, 159–168. [Google Scholar]
- Ilic, K.; Mlinac-Jerkovic, K.; Sedmak, G.; Rosenzweig, I.; Kalanj-Bognar, S. Neuroplastin in human cognition: Review of literature and future perspectives. Transl. Psychiatry 2021, 11, 394. [Google Scholar] [CrossRef]
- Mark, R.J.; Hensley, K.; Butterfield, D.A.; Mattson, M.P. Amyloid beta-peptide impairs ion-motive ATPase activities: Evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J. Neurosci. 1995, 15, 6239–6249. [Google Scholar] [CrossRef]
- Berrocal, M.; Corbacho, I.; Vazquez-Hernandez, M.; Avila, J.; Sepulveda, M.R.; Mata, A.M. Inhibition of PMCA activity by tau as a function of aging and Alzheimer’s neuropathology. Biochim. Biophys. Acta 2015, 1852, 1465–1476. [Google Scholar] [CrossRef] [Green Version]
- Arancio, A.; Ilya, B.; Berger, T.; Bouteiller, J.M.; Carrillo, M.; Disterhoft, J.; Foskett, K.; Khachaturian, A.S.; LaFerla, F.; Landfield, P.W.; et al. Calcium Hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a com-prehensive theory of pathogenesis. Alzheimers Dement. 2017, 13, 178–182.e117. [Google Scholar]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharm. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Luo, Y.; Bolon, B.; Kahn, S.; Bennett, B.D.; Babu-Khan, S.; Denis, P.; Fan, W.; Kha, H.; Zhang, J.; Gong, Y.; et al. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001, 4, 231–232. [Google Scholar] [CrossRef]
- Das, B.; Yan, R. A Close Look at BACE1 Inhibitors for Alzheimer’s Disease Treatment. CNS Drugs 2019, 33, 251–263. [Google Scholar] [CrossRef]
- Johnson, J.L.; Chambers, E.; Jayasundera, K. Application of a Bioinformatics-Based Approach to Identify Novel Putative in vivo BACE1 Substrates. Biomed. Eng. Comput. Biol. 2013, 5, 1–15. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Piccininni, C.; Dibello, V.; Stallone, R.; Giannelli, G.; Bellomo, A.; Greco, A.; et al. BACE inhibitors in clinical development for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2018, 18, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Orwig, W.; Diez, I.; Bueicheku, E.; Vannini, P.; Beaty, R.; Sepulcre, J. Cortical Networks of Creative Ability Trace Gene Expression Profiles of Synaptic Plasticity in the Human Brain. Front. Hum. Neurosci 2021, 15, 694274. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Butcher, R. Propranolol for Post-Traumatic Stress Disorder: A Review of Clinical Effectiveness; CADTH Rapid Response Re-ports; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar]
- Roed, A.; Brodal, B. Inhibition of sarcolemma ATPases by some membrane-stabilizing drugs. Acta Pharm. Toxicol. 1981, 48, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzi, M.; Mammano, F. PMCA2 pump mutations and hereditary deafness. Neurosci. Lett. 2018, 663, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, C.; Cardoso, J.; Franken, P.; Molenaar, L.; Morreau, H.; Moslein, G.; Sampson, J.; Boer, J.M.; de Menezes, R.X.; Fodde, R. Cross-species comparison of human and mouse intestinal polyps reveals conserved mechanisms in adenomatous polyposis coli (APC)-driven tumorigenesis. Am. J. Pathol. 2008, 172, 1363–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Pinto, D.; Sparkowski, J.; Keough, M.P.; Phoenix, K.N.; Vumbaca, F.; Han, D.K.; Gundelfinger, E.D.; Beesley, P.; Claffey, K.P. Identification of novel tumor antigens with patient-derived immune-selected antibodies. Cancer Immunol. Im-munother. 2009, 58, 221–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumardika, I.W.; Chen, Y.; Tomonobu, N.; Kinoshita, R.; Ruma, I.M.W.; Sato, H.; Kondo, E.; Inoue, Y.; Yamauchi, A.; Murata, H.; et al. Neuroplastin-beta mediates S100A8/A9-induced lung cancer dissminative progression. Mol. Carcinog. 2019, 58, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Bajkowska, K.; Sumardika, I.W.; Tomonobu, N.; Chen, Y.; Yamamoto, K.I.; Kinoshita, R.; Murata, H.; Gede Yoni Komalasari, N.L.; Jiang, F.; Yamauchi, A.; et al. Neuroplastin beta-mediated upregulation of solute carrier family 22 member 18 antisense (SLC22A18AS) plays a crucial role in the epithelial-mesenchymal transition, leading to lung cancer cells’ enhanced motility. Biochem. Biophys. Rep. 2020, 22, 100768. [Google Scholar] [PubMed]
- Choy, M.K.; Javierre, B.M.; Williams, S.G.; Baross, S.L.; Liu, Y.; Wingett, S.W.; Akbarov, A.; Wallace, C.; Freire-Pritchett, P.; Rugg-Gunn, P.J.; et al. Promoter interactome of human embryonic stem cell-derived cardiomyocytes connects GWAS re-gions to cardiac gene networks. Nat. Commun. 2018, 9, 2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).