The Evolutionary Relevance of Social Learning and Transmission in Non-Social Arthropods with a Focus on Oviposition-Related Behaviors
Abstract
1. Introduction
2. Genetics, Epigenetics and Social Inheritance in the Context of Oviposition Site Selection
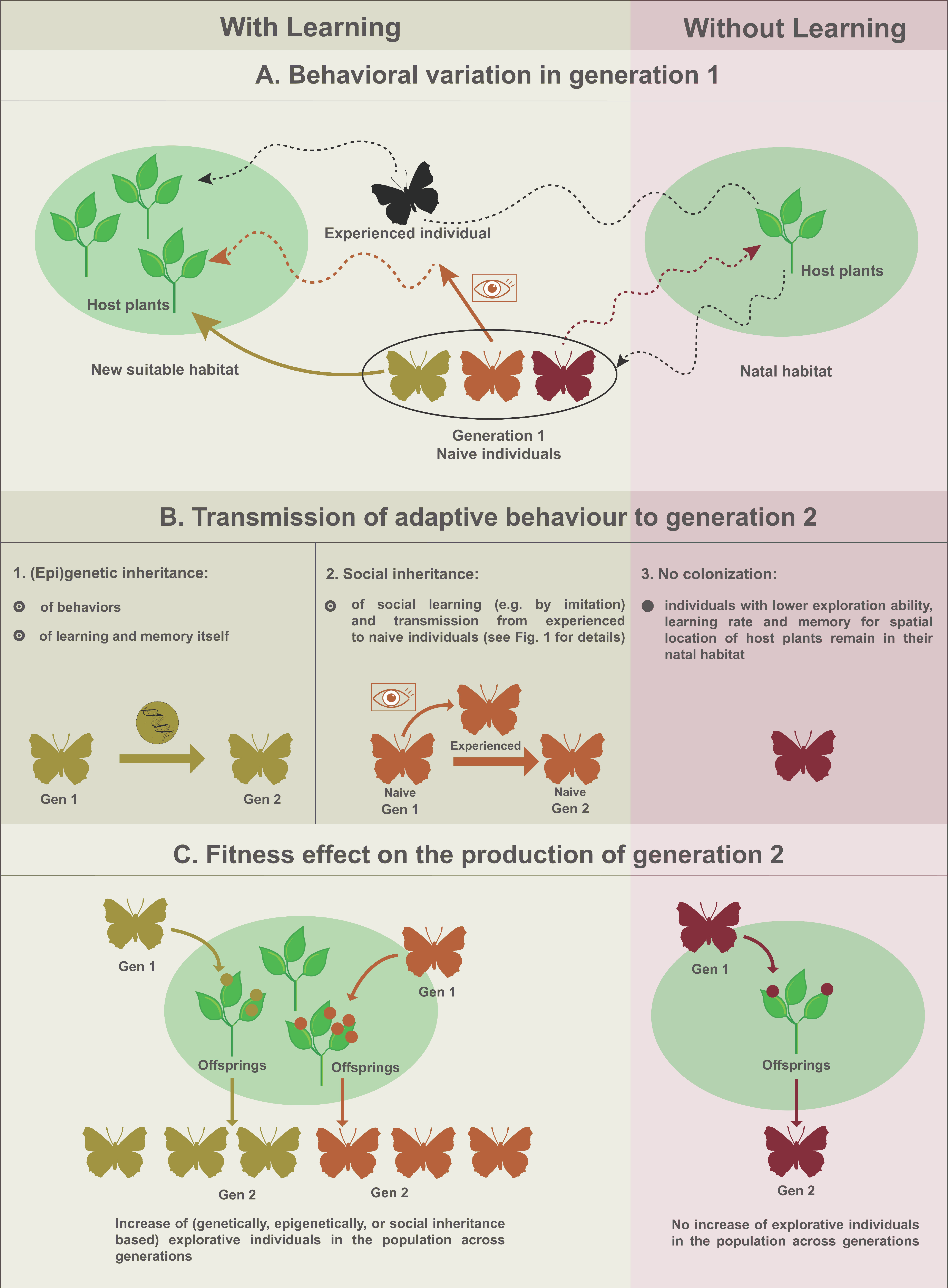
3. Relevance of Social Inheritance in Non-Social Arthropods
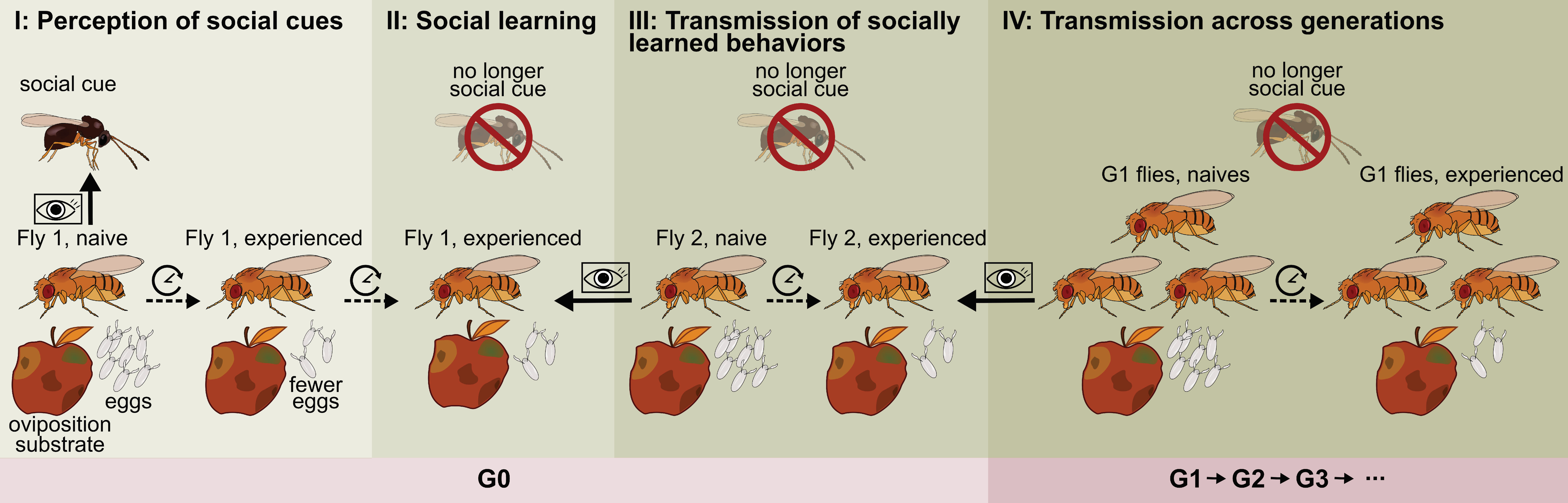
4. Social Learning of Oviposition-Related Behavior from Con- and Hetero-Specifics
| Species | Order | Social Cue | Behavior | Learning from con- (c) or Hetero- (h) Specifics | Step Towards Social Inheritance | Fitness Tested | Reference |
|---|---|---|---|---|---|---|---|
| D. melanogaster | D | Experienced females with preferred oviposition site | Site selection | c | 1, 2, 3 | y | [25] |
| D. melanogaster | D | Parasitoid presence (i.e., threat to offspring survival) | Clutch size | c | 1, 2 | y | [21] |
| Drosophila spp. | D | Parasitoid presence (i.e., threat to offspring survival) | Clutch size | c + h | 1, 2, 3 | y | [22] |
| D. melanogaster | D | Mated females | Site selection | c | 1, 2 | y | [97] |
| Leptopilina boulardi | H | Host insect | Site selection | h | 1, 2 | n | [98] |
| Necremnus tutae | H | Host insect and plant species | Host species preference | h | 1, 2 | n | [99] |
| Osmia sp.* | H | Nest site parasitism | Site selection | h | 1, 2 | n | [95] |
| Trichogramma evanescens | H | Host adult and eggs | Phoresy to oviposition substrate | h | 1, 2 | n | [96] |
| Anisopteromalus calandrae | H | Host insect | Host preference + host-finding + parasitism rates | h | 1, 2 | y | [100] |
| Phratora vulgatissima | C | Adult females | Distance between clutches | c | 1, 2 | y | [101] |
| Tetranychus urticae, T. kanzawai | T | Predator | Site selection (leaf surface vs web) | h | 1, 2 | n | [102] |
5. The Adaptive Value of Social Learning
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whiten, A.; Caldwell, C.A.; Mesoudi, A. Cultural diffusion in humans and other animals. Curr. Opin. Psychol. 2016, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Hinde, R. Opening of milk bottles by birds. Br. Birds 1950, 42, 347–357. [Google Scholar] [CrossRef]
- Heyes, C.M.; Street, G. Social learning in animals: Categories and mechanisms. Biol. Rev. 1994, 69, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Morand-Ferron, J. Why learn? The adaptive value of associative learning in wild populations. Curr. Opin. Behav. Sci. 2017, 16, 73–79. [Google Scholar] [CrossRef]
- Morand-Ferron, J.; Cole, E.F.; Quinn, J.L. Studying the evolutionary ecology of cognition in the wild: A review of practical and conceptual challenges. Biol. Rev. 2016, 91, 367–389. [Google Scholar] [CrossRef]
- Baldwin, J. A new factor in evolution. Am. Nat. 1896, 30, 441–451. [Google Scholar] [CrossRef]
- Whiten, A. A second inheritance system: The extension of biology through culture. Interface Focus 2017, 7, 20160142. [Google Scholar] [CrossRef]
- Whiten, A. Culture extends the scope of evolutionary biology in the great apes. Proc. Natl. Acad. Sci. USA 2017, 114, 7790–7797. [Google Scholar] [CrossRef]
- Hoppitt, W.; Laland, K. Social Learning: An Introduction to Mechanisms, Methods, and Models; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Slater, P.J.B. The cultural transmission of bird song. Trends Ecol. Evol. 1986, 1, 94–97. [Google Scholar] [CrossRef]
- Deecke, V.B.; Ford, J.K.B.; Spong, P. Dialect change in resident killer whales: Implications for vocal learning and cultural transmission. Anim. Behav. 2000, 60, 629–638. [Google Scholar] [CrossRef]
- Garland, E.C.; Goldizen, A.W.; Rekdahl, M.L.; Constantine, R.; Garrigue, C.; Hauser, N.D.; Poole, M.M.; Robbins, J.; Noad, M.J. Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 2011, 21, 687–691. [Google Scholar] [CrossRef]
- Cantor, M.; Whitehead, H. The interplay between social networks and culture: Theoretically and among whales and dolphins. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120340. [Google Scholar] [CrossRef]
- Lamon, N.; Neumann, C.; Gruber, T.; Zuberbühler, K. Kin-based cultural transmission of tool use in wild chimpanzees. Sci. Adv. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Alem, S.; Perry, C.J.; Zhu, X.; Loukola, O.J.; Ingraham, T.; Søvik, E.; Chittka, L. Associative Mechanisms Allow for Social Learning and Cultural Transmission of String Pulling in an Insect. PLoS Biol. 2016, 14, e1002564. [Google Scholar] [CrossRef]
- Nieberding, C.M.; van Alphen, J.J. Culture in bumblebees. Peer Community Evol. Biol. 2017, 2–4. [Google Scholar] [CrossRef]
- Grüter, C.; Leadbeater, E. Insights from insects about adaptive social information use. Trends Ecol. Evol. 2014, 29, 177–184. [Google Scholar] [CrossRef]
- Avarguès-Weber, A.; Lihoreau, M.; Isabel, G.; Giurfa, M. Information transfer beyond the waggle dance: Observational learning in bees and flies. Front. Ecol. Evol. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Worden, B.D.; Papaj, D.R. Flower choice copying in bumblebees. Biol. Lett. 2005, 1, 504–507. [Google Scholar] [CrossRef]
- Jones, P.L.; Agrawal, A.A. Learning in Insect Pollinators and Herbivores. Annu. Rev. Entomol. 2017, 62, 53–71. [Google Scholar] [CrossRef]
- Kacsoh, B.Z.; Bozler, J.; Ramaswami, M.; Bosco, G. Social communication of predator-induced changes in Drosophila behavior and germline physiology. Elife 2015, 4, 1–36. [Google Scholar] [CrossRef]
- Kacsoh, B.; Bozler, J.; Bosco, G. Drosophila species learn dialects through communal living. PLoS Genet. 2018, 14, e1007430. [Google Scholar] [CrossRef] [PubMed]
- Danchin, É.; Nobel, S.; Pocheville, A.; Dagaeff, A.-C.; Demay, L.; Alphand, M.; Ranty-Roby, S.; van Renssen, L.; Monier, M.; Gazagne, E.; et al. Cultural flies: Conformist social learning in fruitflies predicts long-lasting mate-choice traditions. Science 2019, 366, 1–7. [Google Scholar] [CrossRef]
- Durisko, Z.; Dukas, R. Attraction to and learning from social cues in fruitfly larvae. Proc. R. Soc. B Biol. Sci. 2013, 280, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Battesti, M.; Moreno, C.; Joly, D.; Mery, F. Spread of social information and dynamics of social transmission within Drosophila groups. Curr. Biol. 2012, 22, 309–313. [Google Scholar] [CrossRef]
- Coolen, I.; Dangles, O.; Casas, J. Social learning in noncolonial insects? Curr. Biol. 2005, 15, 1931–1935. [Google Scholar] [CrossRef]
- Davies, A.D.; Lewis, Z.; Dougherty, L.R. A meta-analysis of factors influencing the strength of mate-choice copying in animals. Behav. Ecol. 2020, 31, 1279–1290. [Google Scholar] [CrossRef]
- Mery, F.; Varela, S.A.M.; Danchin, É.; Blanchet, S.; Parejo, D.; Coolen, I.; Wagner, R.H. Public versus personal information for mate copying in an invertebrate. Curr. Biol. 2009, 19, 730–734. [Google Scholar] [CrossRef]
- Belkina, E.G.; Shiglik, A.; Sopilko, N.G.; Lysenkov, S.N.; Markov, A.V. Mate choice copying in Drosophila is probably less robust than previously suggested. Anim. Behav. 2021, 176, 175–183. [Google Scholar] [CrossRef]
- Dion, E.; Monteiro, A.; Nieberding, C.M. The role of learning on insect and spider sexual behaviors, sexual trait evolution, and speciation. Front. Ecol. Evol. 2019, 6, 225. [Google Scholar] [CrossRef]
- Dukas, R. Evolutionary biology of insect learning. Annu. Rev. Entomol. 2008, 53, 145–160. [Google Scholar] [CrossRef]
- Wright, G.A.; Schiestl, F.P. The evolution of floral scent: The influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 2009, 23, 841–851. [Google Scholar] [CrossRef]
- Webster, S.J.; Fiorito, G. Socially guided behaviour in non-insect invertebrates. Anim. Cogn. 2001, 4, 69–79. [Google Scholar] [CrossRef]
- Hoedjes, K.M.; Kruidhof, H.M.; Huigens, M.E.; Dicke, M.; Vet, L.E.M.; Smid, H.M. Natural variation in learning rate and memory dynamics in parasitoid wasps: Opportunities for converging ecology and neuroscience. Proc. R. Soc. B Biol. Sci. 2011, 278, 889–897. [Google Scholar] [CrossRef]
- Jones, P.L.; Agrawal, A.A. Beyond preference and performance: Host plant selection by monarch butterflies, Danaus plexippus. Oikos 2019, 128, 1092–1102. [Google Scholar] [CrossRef]
- Traynier, R.M.M. Associative learning in the ovipositional behaviour of the cabbage butterfly, Pieris rapae. Physiol. Entomol. 1984, 9, 465–472. [Google Scholar] [CrossRef]
- Papaj, D.R. Interpopulation differences in host preference and the evolution of learning in the butterfly, Battus philenor. Evolution 1986, 40, 518–530. [Google Scholar] [CrossRef]
- Traynier, R.M.M. Visual learning in assays of sinigrin solution as an oviposition releaser for the cabbage butterfly, Pieris rapae. Entomol. Exp. Appl. 1986, 40, 25–33. [Google Scholar] [CrossRef]
- Visser, M.E.; van Alphen, J.J.; Hemerik, L. Adaptive superparasitism and patch time allocation in solitary parasitoids: An ESS model. J. Anim. Ecol. 1992, 61, 93–101. [Google Scholar] [CrossRef]
- Vet, L.E.M.; De Jong, A.G.; Franchi, E.; Papaj, D.R. The effect of complete versus incomplete information on odour discrimination in a parasitic wasp. Anim. Behav. 1998, 55, 1271–1279. [Google Scholar] [CrossRef]
- Mery, F.; Kawecki, T.J. Experimental evolution of learning ability in fruit flies. Proc. Natl. Acad. Sci. USA 2002, 99, 14274–14279. [Google Scholar] [CrossRef]
- Liu, S.S.; Li, Y.H.; Liu, Y.Q.; Zalucki, M.P. Experience-induced preference for oviposition repellents derived from a non-host plant by a specialist herbivore. Ecol. Lett. 2005, 8, 722–729. [Google Scholar] [CrossRef]
- Braem, S.; Turlure, C.; Nieberding, C.; van Dyck, H. Oviposition site selection and learning in a butterfly under niche expansion: An experimental test. Anim. Behav. (In press). 2021. [CrossRef]
- Kawecki, T.J. Evolutionary ecology of learning: Insights from fruit flies. Popul. Ecol. 2010, 52, 15–25. [Google Scholar] [CrossRef]
- Feldman, M.W.; Laland, K.N. Gene-culture coevolutionary theory. Trends Ecol. Evol. 1996, 11, 453–457. [Google Scholar] [CrossRef]
- Danchin, É.; Wagner, R.H. Inclusive heritability: Combining genetic and non-genetic information to study animal behavior and culture. Oikos 2010, 119, 210–218. [Google Scholar] [CrossRef]
- Mesoudi, A.; Chang, L.; Dall, S.R.X.; Thornton, A. The evolution of individual and cultural variation in social learning. Trends Ecol. Evol. 2016, 31, 215–225. [Google Scholar] [CrossRef]
- Danchin, E.; Pocheville, A.; Rey, O.; Pujol, B.; Blanchet, S. Epigenetically facilitated mutational assimilation: Epigenetics as a hub within the inclusive evolutionary synthesis. Biol. Rev. 2019, 94, 259–282. [Google Scholar] [CrossRef]
- Fitzpatrick, M.J.; Ben-Shahar, Y.; Smid, H.M.; Vet, L.E.M.; Robinson, G.E.; Sokolowski, M.B. Candidate genes for behavioural ecology. Trends Ecol. Evol. 2005, 20, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Reaume, C.J.; Sokolowski, M.B. Conservation of gene function in behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2100–2110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bengston, S.E.; Dahan, R.A.; Donaldson, Z.; Phelps, S.M.; Van Oers, K.; Sih, A.; Bell, A.M. Genomic tools for behavioural ecologists to understand repeatable individual differences in behaviour. Nat. Ecol. Evol. 2018, 2, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, R.; Höglund, A.; Fogelholm, J.; Abbey-Lee, R.; Johnsson, M.; Dingemanse, N.J.; Wright, D. Intra-individual behavioural variability: A trait under genetic control. Int. J. Mol. Sci. 2020, 21, 8069. [Google Scholar] [CrossRef]
- Bubac, C.M.; Miller, J.M.; Coltman, D.W. The genetic basis of animal behavioural diversity in natural populations. Mol. Ecol. 2020, 29, 1957–1971. [Google Scholar] [CrossRef]
- Williams-Simon, P.A.; Posey, C.; Mitchell, S.; Ng’oma, E.; Mrkvicka, J.A.; Zars, T.; King, E.G. Multiple genetic loci affect place learning and memory performance in Drosophila melanogaster. Genes Brain Behav. 2019, 18, 1–16. [Google Scholar] [CrossRef]
- Mery, F. Natural variation in learning and memory. Curr. Opin. Neurobiol. 2013, 23, 52–56. [Google Scholar] [CrossRef]
- Hughes, E.; Shymansky, T.; Swinton, E.; Lukowiak, K.S.; Swinton, C.; Sunada, H.; Protheroe, A.; Phillips, I.; Lukowiak, K. Strain-specific differences of the effects of stress on memory in Lymnaea. J. Exp. Biol. 2017, 220, 891–899. [Google Scholar] [CrossRef]
- Giunti, G.; Canale, A.; Messing, R.H.; Donati, E.; Stefanini, C.; Michaud, J.P.; Benelli, G. Parasitoid learning: Current knowledge and implications for biological control. Biol. Control 2015, 90, 208–219. [Google Scholar] [CrossRef]
- Liefting, M.; Verwoerd, L.; Dekker, M.L.; Hoedjes, K.M.; Ellers, J. Strain differences rather than species differences contribute to variation in associative learning ability in Nasonia. Anim. Behav. 2020, 168, 25–31. [Google Scholar] [CrossRef]
- Osborne, K.A.; Robichon, A.; Burgess, E.; Butland, S.; Shaw, R.A.; Coulthard, A.; Pereira, H.S.; Greenspan, R.J.; Sokolowski, M.B. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science 1997, 277, 834–836. [Google Scholar] [CrossRef]
- Sokolowski, M.B. Drosophila: Genetics meets behaviour. Nat. Rev. Genet. 2001, 2, 879–890. [Google Scholar] [CrossRef]
- Wahlberg, N.; Wheat, C.W.; Peña, C. Timing and patterns in the taxonomic diversification of Lepidoptera (butterflies and moths). PLoS ONE 2013, 8, e80875. [Google Scholar] [CrossRef]
- Wheat, C.W. Dispersal genetics: Emerging insights from fruitflies, butterflies, and beyond. In Dispersal Ecology and Evolution; Clobert, J., Baguette, M., Benton, T., Bullock, J., Eds.; Oxford University Press: Oxford, UK, 2012; p. 498. [Google Scholar]
- Fitzpatrick, M.J.; Sokolowski, M.B. In search of food: Exploring the evolutionary link between cGMP-dependent protein kinase (PKG) and behaviour. Integr. Comp. Biol. 2004, 44, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, A.G.; Seroussi, U.; Claycomb, J.M. Next-Gen Learning: The C. elegans Approach. Cell 2019, 177, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Ledón-Rettig, C.C.; Richards, C.L.; Martin, L.B. Epigenetics for behavioral ecologists. Behav. Ecol. 2013, 24, 311–324. [Google Scholar] [CrossRef]
- Dias, B.G.; Ressler, K.J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014, 17, 89–96. [Google Scholar] [CrossRef]
- Gowri, V.; Dion, E.; Viswanath, A.; Piel, F.M.; Monteiro, A. Transgenerational inheritance of learned preferences for novel host plant odors in Bicyclus anynana butterflies. Evolution 2019, 73, 2401–2414. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Bargmann, C.I. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005, 438, 179–184. [Google Scholar] [CrossRef]
- Moore, R.S.; Kaletsky, R.; Murphy, C.T. Piwi/PRG-1 Argonaute and TGF-β mediate transgenerational learned pathogenic avoidance. Cell 2019, 177, 1827–1841.e12. [Google Scholar] [CrossRef]
- Posner, R.; Toker, I.A.; Antonova, O.; Star, E.; Anava, S.; Azmon, E.; Hendricks, M.; Bracha, S.; Gingold, H.; Rechavi, O. Neuronal small RNAs control behavior transgenerationally. Cell 2019, 177, 1814–1826.e15. [Google Scholar] [CrossRef]
- Rösvik, A.; Lhomme, P.; Khallaf, M.A.; Anderson, P. Plant-induced transgenerational plasticity affecting performance but not preference in a polyphagous moth. Front. Ecol. Evol. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Barrett, L.P.; Stanton, L.A.; Benson-Amram, S. The cognition of ‘nuisance’ species. Anim. Behav. 2019, 147, 167–177. [Google Scholar] [CrossRef]
- Rausher, M.D. Search image for leaf shape in a butterfly. Science 1978, 200, 1071–1073. [Google Scholar] [CrossRef]
- Williams, K.S.; Gilbert, L.E. Insects as selective agents on plant vegetative morphology: Egg mimicry reduces egg laying by butterflies. Science 1981, 212, 467–469. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506. [Google Scholar] [CrossRef]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef]
- Ellner, S.; Hairston, N.G., Jr. Role of overlapping generations in maintaining genetic variation in a fluctuating environment. Am. Nat. 1994, 143, 403–417. [Google Scholar] [CrossRef]
- Choe, J.; Crespi, B. The Evolution of Social Behavior in Insects and Arachnids; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Costa, J. The Other Insect Societies; Harvard University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Costa, J. Social evolution in “other” insects and arachnids. In Encyclopedia of Animal Behavior; Breed, M., Moore, J., Eds.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Costa, J.T. The other insect societies: Overview and new directions. Curr. Opin. Insect Sci. 2018, 28, 40–49. [Google Scholar] [CrossRef]
- Aluja, M.; Díaz-Fleischer, F. Foraging behavior of Anastrepha ludens, A. obliqua, and A. serpentina in response to feces extracts containing host marking pheromone. J. Chem. Ecol. 2006, 32, 367–389. [Google Scholar] [CrossRef]
- Decker, A.; D’elia, B.; Kuhl, A.; Rosen, S.; Disney, A.; Dial, C.; Linietsky, M.; Taylor-Lilquist, J.; Taylor-Lilquist, B.; Kim, E.; et al. Acoustic stimulus influences ovipositioning in Drosophila melanogaster. Bull. Insectol. 2020, 73, 103–109. [Google Scholar]
- Corbet, S.A. Mandibular gland secretion of larvae of the flour moth, Anagasta kuehniella, contains an epideictic pheromone and elicits oviposition movements in a hymenopteran parasite. Nature 1971, 232, 481–484. [Google Scholar] [CrossRef]
- Otake, R.; Dobata, S. Copy if dissatisfied, innovate if not: Contrasting egg-laying decision making in an insect. Anim. Cogn. 2018, 21, 805–812. [Google Scholar] [CrossRef]
- Malek, H.L.; Long, T.A.F. On the use of private versus social information in oviposition site choice decisions by Drosophila melanogaster females. Behav. Ecol. 2020, 31, 739–749. [Google Scholar] [CrossRef]
- Battesti, M.; Moreno, C.; Joly, D.; Mery, F. Biased social transmission in Drosophila oviposition choice. Behav. Ecol. Sociobiol. 2015, 69, 83–87. [Google Scholar] [CrossRef]
- Battesti, M.; Pasquaretta, C.; Moreno, C.; Teseo, S.; Joly, D.; Klensch, E.; Petit, O.; Sueur, C.; Mery, F. Ecology of information: Social transmission dynamics within groups of non-social insects. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142480. [Google Scholar] [CrossRef]
- Elsensohn, J.E.; Aly, M.F.K.; Schal, C.; Burrack, H.J. Social signals mediate oviposition site selection in Drosophila suzukii. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Rodriguez-Saona, C.; Meyer, W.L. Recognition of foreign oviposition-marking pheromone in a multi-trophic context. Naturwissenschaften 2009, 96, 585–592. [Google Scholar] [CrossRef]
- Pasqualone, A.A.; Davis, J.M. The use of conspecific phenotypic states as information during reproductive decisions. Anim. Behav. 2011, 82, 281–284. [Google Scholar] [CrossRef]
- Yadav, P.; Desireddy, S.; Kasinathan, S.; Bessière, J.M.; Borges, R.M. History matters: Oviposition resource acceptance in an exploiter of a nursery pollination mutualism. J. Chem. Ecol. 2018, 44, 18–28. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioural and Evolutionary Ecology; Princeton University Press: West Sussex, NJ, USA, 1994. [Google Scholar]
- Loukola, O.J.; Gatto, E.; Híjar-Islas, A.C.; Chittka, L. Selective interspecific information use in the nest choice of solitary bees. Anim. Biol. 2020, 70, 215–225. [Google Scholar] [CrossRef]
- Huigens, M.E.; Pashalidou, F.G.; Qian, M.H.; Bukovinszky, T.; Smid, H.M.; Van Loon, J.J.A.; Dicke, M.; Fatouros, N.E. Hitch-hiking parasitic wasp learns to exploit butterfly antiaphrodisiac. Proc. Natl. Acad. Sci. USA 2009, 106, 820–825. [Google Scholar] [CrossRef]
- Sarin, S.; Dukas, R. Social learning about egg-laying substrates in fruitflies. Proc. R. Soc. B Biol. Sci. 2009, 276, 4323–4328. [Google Scholar] [CrossRef] [PubMed]
- Couty, A.; Kaiser, L.; Huet, D.; Pham-Delegue, M.H. The attractiveness of different odour sources from the fruit-host complex on Leptopilina boulardi, a larval parasitoid of frugivorous Drosophila spp. Physiol. Entomol. 1999, 24, 76–82. [Google Scholar] [CrossRef]
- Bodino, N.; Ferracini, C.; Tavella, L. Is host selection influenced by natal and adult experience in the parasitoid Necremnus tutae (Hymenoptera: Eulophidae)? Anim. Behav. 2016, 112, 221–228. [Google Scholar] [CrossRef]
- Ghimire, M.N.; Phillips, T.W. Effects of prior experience on host selection and host utilization by two populations of Anisopteromalus calandrae (Hymenoptera: Pteromalidae). Environ. Entomol. 2008, 37, 1300–1306. [Google Scholar] [CrossRef][Green Version]
- Stephan, J.G.; Stenberg, J.A.; Björkman, C. How far away is the next basket of eggs? Spatial memory and perceived cues shape aggregation patterns in a leaf beetle. Ecology 2015, 96, 908–914. [Google Scholar] [CrossRef]
- Murase, A.; Fujita, K.; Yano, S. Behavioural flexibility in spider mites: Oviposition site shifts based on past and present stimuli from conspecifics and predators. R. Soc. Open Sci. 2017, 4, 170328. [Google Scholar] [CrossRef]
- Kujtan, L.; Dukas, R. Learning magnifies individual variation in heterospecific mating propensity. Anim. Behav. 2009, 78, 549–554. [Google Scholar] [CrossRef]
- Mair, M.M.; Seifert, N.; Ruther, J. Previous interspecific courtship impairs female receptivity to conspecifics in the parasitoid wasp Nasonia longicornis but not in N. vitripennis. Insects 2018, 9, 112. [Google Scholar] [CrossRef]
- Hostachy, C.; Couzi, P.; Portemer, G.; Hanafi-Portier, M.; Murmu, M.; Deisig, N.; Dacher, M. Exposure to conspecific and heterospecific sex-pheromones modulates gustatory habituation in the moth Agrotis ipsilon. Front. Physiol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Romano, D.; Benelli, G.; Stefanini, C. Opposite valence social information provided by bio-robotic demonstrators shapes selection processes in the green bottle fly. J. R. Soc. Interface 2021, 18, 20210056. [Google Scholar] [CrossRef]
- Verzijden, M.N.; ten Cate, C.; Servedio, M.R.; Kozak, G.M.; Boughman, J.W.; Svensson, E. The impact of learning on sexual selection and speciation. Trends Ecol. Evol. 2012, 27, 511–519. [Google Scholar] [CrossRef]
- Vosteen, I.; van den Meiracker, N.; Poelman, E.H. Getting confused: Learning reduces parasitoid foraging efficiency in some environments with non-host-infested plants. Oecologia 2019, 189, 919–930. [Google Scholar] [CrossRef]
- Magrath, R.D.; Haff, T.M.; Fallow, P.M.; Radford, A.N. Eavesdropping on heterospecific alarm calls: From mechanisms to consequences. Biol. Rev. 2015, 90, 560–586. [Google Scholar] [CrossRef]
- Muramatsu, D. Sand-bubbler crabs distinguish fiddler crab signals to predict intruders. Behav. Ecol. Sociobiol. 2021, 75, 1–11. [Google Scholar] [CrossRef]
- Rieucau, G.; Giraldeau, L.A. Exploring the costs and benefits of social information use: An appraisal of current experimental evidence. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 949–957. [Google Scholar] [CrossRef]
- Nieberding, C.M.; Van Dyck, H.; Chittka, L. Adaptive learning in non-social insects: From theory to field work, and back. Curr. Opin. Insect Sci. 2018, 27, 75–81. [Google Scholar] [CrossRef]
- Costa, T.M.; Hebets, E.A.; Melo, D.; Willemart, R.H. Costly learning: Preference for familiar food persists despite negative impact on survival. Biol. Lett. 2016, 12, 20160256. [Google Scholar] [CrossRef]
- Botero, C.A.; Weissing, F.J.; Wright, J.; Rubenstein, D.R. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl. Acad. Sci. USA 2015, 112, 184–189. [Google Scholar] [CrossRef]
- Dechaume-Moncharmont, F.X.; Dornhaus, A.; Houston, A.I.; McNamara, J.M.; Collins, E.J.; Franks, N.R. The hidden cost of information in collective foraging. Proc. R. Soc. B Biol. Sci. 2005, 272, 1689–1695. [Google Scholar] [CrossRef]
- Greggor, A.L.; Trimmer, P.C.; Barrett, B.J.; Sih, A. Challenges of Learning to Escape Evolutionary Traps. Front. Ecol. Evol. 2019, 7, 408. [Google Scholar] [CrossRef]
- Fleury, F.; Gibert, P.; Ris, N.; Allemand, R. Ecology and life history evolution of frugivorous Drosophila parasitoids. Adv. Parasitol. 2009, 70, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, T.; De Roode, J.C.; Kacsoh, B.Z.; Schlenke, T.A. Defence strategies against a parasitoid wasp in Drosophila: Fight or flight? Biol. Lett. 2012, 8, 230–233. [Google Scholar] [CrossRef] [PubMed]
- van Lenteren, J.C.; Bakker, K. Behavioural aspects of the functional responses of a parasite (Pseudocoila bochei) to its host (Drosophila melanogaster). Netherlands J. Zool. 1978, 28, 213–233. [Google Scholar] [CrossRef]
- Vet, L.E.; Papaj, D. Effects of experience on parasitoid movement in odour plumes. Physiol. Entomol. 1992, 17, 90–96. [Google Scholar] [CrossRef]
- Wertheim, B.; Vet, L.E.M.; Dicke, M. Increased risk of parasitism as ecological costs of using aggregation pheromones: Laboratory and field study of Drosophila-Leptopilina interaction. Oikos 2003, 100, 269–282. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieberding, C.M.; Marcantonio, M.; Voda, R.; Enriquez, T.; Visser, B. The Evolutionary Relevance of Social Learning and Transmission in Non-Social Arthropods with a Focus on Oviposition-Related Behaviors. Genes 2021, 12, 1466. https://doi.org/10.3390/genes12101466
Nieberding CM, Marcantonio M, Voda R, Enriquez T, Visser B. The Evolutionary Relevance of Social Learning and Transmission in Non-Social Arthropods with a Focus on Oviposition-Related Behaviors. Genes. 2021; 12(10):1466. https://doi.org/10.3390/genes12101466
Chicago/Turabian StyleNieberding, Caroline M., Matteo Marcantonio, Raluca Voda, Thomas Enriquez, and Bertanne Visser. 2021. "The Evolutionary Relevance of Social Learning and Transmission in Non-Social Arthropods with a Focus on Oviposition-Related Behaviors" Genes 12, no. 10: 1466. https://doi.org/10.3390/genes12101466
APA StyleNieberding, C. M., Marcantonio, M., Voda, R., Enriquez, T., & Visser, B. (2021). The Evolutionary Relevance of Social Learning and Transmission in Non-Social Arthropods with a Focus on Oviposition-Related Behaviors. Genes, 12(10), 1466. https://doi.org/10.3390/genes12101466






