Role of Digital Health and Artificial Intelligence in Inflammatory Bowel Disease: A Scoping Review
Abstract
1. Introduction
1.1. What Is Inflammatory Bowel Disease?
Pathogenesis
1.2. Current Standard of Care for Inflammatory Bowel Disease
1.3. The Roles of Digital Health and Artificial Intelligence in the Care of Inflammatory Bowel Disease
1.3.1. Digital Health
1.3.2. Artificial Intelligence
2. Methods
2.1. Scoping Review
2.2. Research Question
2.3. Identifying Articles in Published Literature
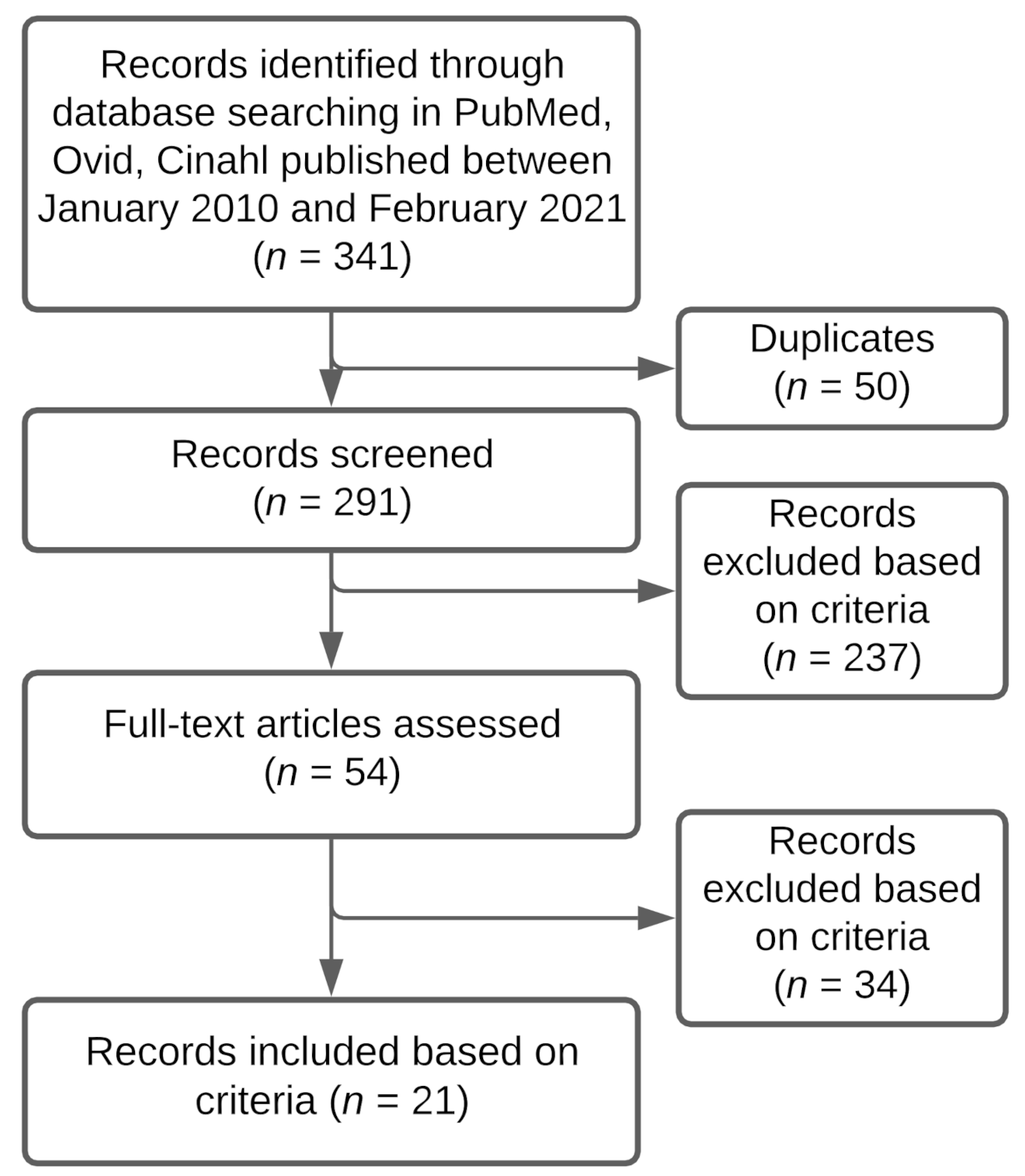
2.4. Article Selection
2.5. Data Charting
2.6. Collation and Summary
3. Results
3.1. Overview
3.2. Diagnosis
AI in Diagnosis
3.3. Treatment
3.3.1. AI in Treatment
3.3.2. DH in Treatment
3.4. Monitoring
3.4.1. AI in Monitoring
3.4.2. DH in Monitoring
3.5. Prognosis
3.5.1. AI and Prognosis
3.5.2. DH and Prognosis
4. Discussion
4.1. Summary of Evidence
4.2. Limitations of Current AI and DH
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- Inflammatory bowel disease/
- IBD/
- Crohn’s/
- Crohn’s disease/
- CD/
- Ulcerative colitis/
- UC/
- or/ 1-7
- Remote monitoring/
- Remote management/
- Digital health/
- mhealth/
- Mobile health applications/
- Mobile apps/
- self-management/
- telehealth/
- telemedicine/
- ehealth/
- Digital medicine/
- Electronic health/
- or/ 9-20
- 8 and 21
- Artificial intelligence/
- AI/
- Artificial intelligence in health care/
- or/ 23-25
- 8 and 26
References
- Engels, M.; Cross, R.K. Long Exercise in Patients with Inflammatory Bowel Diseases: Current Perspectives. Clin. Exp. Gastroenterol. 2017, 11, 1. [Google Scholar] [CrossRef]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.R.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.-F.; Gasche, C.; Geboes, K.; et al. Toward an Integrated Clinical, Molecular and Serological Classification of Inflammatory Bowel Disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5A–36A. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.-F. The Montreal Classification of Inflammatory Bowel Disease: Controversies, Consensus, and Implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Bruining, D.H.; Loftus, E.V., Jr.; Thia, K.T.; Schroeder, K.W.; Tremaine, W.J.; Faubion, W.A.; Kane, S.V.; Pardi, D.S.; de Groen, P.C.; et al. Validation of the Ulcerative Colitis Colonoscopic Index of Severity and Its Correlation with Disease Activity Measures. Clin. Gastroenterol. Hepatol. 2013, 11, 49–54.e1. [Google Scholar] [CrossRef]
- Pariente, B.; Cosnes, J.; Danese, S.; Sandborn, W.J.; Lewin, M.; Fletcher, J.G.; Chowers, Y.; D’Haens, G.; Feagan, B.G.; Hibi, T.; et al. Development of the Crohn’s Disease Digestive Damage Score, the Lémann Score. Inflamm. Bowel Dis. 2011, 17, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, J.E.; Cho, M.-L. Immunological Pathogenesis of Inflammatory Bowel Disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A Microbial Signature for Crohn’s Disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The Role of Diet in the Aetiopathogenesis of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized Diet Is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef]
- Mirkov, M.U.; Verstockt, B.; Cleynen, I. Genetics of Inflammatory Bowel Disease: Beyond NOD2. Lancet Gastroenterol. Hepatol. 2017, 2, 224–234. [Google Scholar] [CrossRef]
- Peters, L.A.; Perrigoue, J.; Mortha, A.; Iuga, A.; Song, W.-M.; Neiman, E.M.; Llewellyn, S.R.; Di Narzo, A.; Kidd, B.A.; Telesco, S.E.; et al. A Functional Genomics Predictive Network Model Identifies Regulators of Inflammatory Bowel Disease. Nat. Genet. 2017, 49, 1437–1449. [Google Scholar] [CrossRef]
- Ye, B.D.; McGovern, D.P.B. Genetic Variation in IBD: Progress, Clues to Pathogenesis and Possible Clinical Utility. Expert Rev. Clin. Immunol. 2016, 12, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association Analyses Identify 38 Susceptibility Loci for Inflammatory Bowel Disease and Highlight Shared Genetic Risk across Populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Kuchroo, V.K.; Ohashi, P.S.; Balfour Sartor, R.; Vinuesa, C.G. Dysregulation of Immune Homeostasis in Autoimmune Diseases. Nat. Med. 2012, 18, 42–47. [Google Scholar] [CrossRef]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut Microbiota in Ulcerative Colitis: Insights on Pathogenesis and Treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Seyed Tabib, N.S.; Madgwick, M.; Sudhakar, P.; Verstockt, B.; Korcsmaros, T.; Vermeire, S. Big Data in IBD: Big Progress for Clinical Practice. Gut 2020, 69, 1520–1532. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-Microbe Interactions Have Shaped the Genetic Architecture of Inflammatory Bowel Disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kanai, T. The Gut Microbiota and Inflammatory Bowel Disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Mitsuyama, K.; Niwa, M.; Takedatsu, H.; Yamasaki, H.; Kuwaki, K.; Yoshioka, S.; Yamauchi, R.; Fukunaga, S.; Torimura, T. Antibody Markers in the Diagnosis of Inflammatory Bowel Disease. World J. Gastroenterol. 2016, 22, 1304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Purohit, T.; Razonable, R.; Loftus, E.V., Jr. Opportunistic Infections due to Inflammatory Bowel Disease Therapy. Inflamm. Bowel Dis. 2014, 20, 196–212. [Google Scholar] [CrossRef]
- Schultsz, C.; Den Berg FM, V.; Ten Kate, F.W.; Tytgat, G.N.; Dankert, J. The Intestinal Mucus Layer from Patients with Inflammatory Bowel Disease Harbors High Numbers of Bacteria Compared with Controls. Gastroenterology 1999, 117. [Google Scholar] [CrossRef]
- Hollander, D. Crohn’s Disease—A Permeability Disorder of the Tight Junction? Gut 1988, 29, 1621. [Google Scholar] [CrossRef]
- Brazil, J.C.; Louis, N.A.; Parkos, C.A. The Role of Polymorphonuclear Leukocyte Trafficking in the Perpetuation of Inflammation during Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 1556–1565. [Google Scholar] [CrossRef]
- Uo, M.; Hisamatsu, T.; Miyoshi, J.; Kaito, D.; Yoneno, K.; Kitazume, M.T.; Mori, M.; Sugita, A.; Koganei, K.; Matsuoka, K.; et al. Mucosal CXCR4+ IgG Plasma Cells Contribute to the Pathogenesis of Human Ulcerative Colitis through FcγR-Mediated CD14 Macrophage Activation. Gut 2013, 62, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Das, K.M. Isolation and Characterization of a Colonic Autoantigen Specifically Recognized by Colon Tissue-Bound Immunoglobulin G from Idiopathic Ulcerative Colitis. J. Clin. Investig. 1985, 76, 311–318. [Google Scholar] [CrossRef]
- Das, K.M.; Dasgupta, A.; Mandal, A.; Geng, X. Autoimmunity to Cytoskeletal Protein Tropomyosin. A Clue to the Pathogenetic Mechanism for Ulcerative Colitis. J. Immunol. 1993, 150, 2487–2493. [Google Scholar]
- Targan, S.R.; Hanauer, S.B.; van Deventer, S.J.; Mayer, L.; Present, D.H.; Braakman, T.; DeWoody, K.L.; Schaible, T.F.; Rutgeerts, P.J. A Short-Term Study of Chimeric Monoclonal Antibody cA2 to Tumor Necrosis Factor α for Crohn’s Disease. Crohn’s Disease cA2 Study Group. N. Engl. J. Med. 1997, 337, 1029–1036. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Wehkamp, J.; Götz, M.; Herrlinger, K.; Steurer, W.; Stange, E.F. Inflammatory Bowel Disease. Dtsch. Arztebl. Int. 2016, 113, 72–82. [Google Scholar] [CrossRef]
- Chang, S.; Malter, L.; Hudesman, D. Disease Monitoring in Inflammatory Bowel Disease. World J. Gastroenterol. 2015, 21, 11246–11259. [Google Scholar] [CrossRef]
- Willén, R.; Agnarsdóttir, M.; Hultén, L. Prophylactic Surgery for Patients with Longstanding Ulcerative Colitis. Which Option? Histopathological and Clinical Implications. Ups. J. Med. Sci. 2007, 112, 49–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mattar, M.C.; Lough, D.; Pishvaian, M.J.; Charabaty, A. Current Management of Inflammatory Bowel Disease and Colorectal Cancer. Gastrointest. Cancer Res. 2011, 4, 53–61. [Google Scholar] [PubMed]
- Sica, G.S.; Biancone, L. Surgery for Inflammatory Bowel Disease in the Era of Laparoscopy. World J. Gastroenterol. 2013, 19, 2445–2448. [Google Scholar] [CrossRef]
- Patel, K.V.; Darakhshan, A.A.; Griffin, N.; Williams, A.B.; Sanderson, J.D.; Irving, P.M. Patient Optimization for Surgery Relating to Crohn’s Disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.; Doherty, G.A. Why Do We Need to Improve Monitoring of Patients with Inflammatory Bowel Disease (IBD) on Biologic Treatment? Expert Opin. Biol. Ther. 2019, 19, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W.; Skonieczna-Żydecka, K.; Dabos, K.J.; Łoniewski, I.; Koulaouzidis, A. Emerging Concepts in Non-Invasive Monitoring of Crohn’s Disease. Therap. Adv. Gastroenterol. 2018, 11, 1756284818769076. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Lee, J.C.; Biasci, D.; Roberts, R.; Gearry, R.B.; Mansfield, J.C.; Ahmad, T.; Prescott, N.J.; Satsangi, J.; Wilson, D.C.; Jostins, L.; et al. Genome-Wide Association Study Identifies Distinct Genetic Contributions to Prognosis and Susceptibility in Crohn’s Disease. Nat. Genet. 2017, 49, 262–268. [Google Scholar] [CrossRef]
- Rocchi, A.; Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Feagan, B.; Panaccione, R.; Glasgow, K.W.; Fernandes, A.; Ghosh, S. Inflammatory Bowel Disease: A Canadian Burden of Illness Review. Can. J. Gastroenterol. 2012, 26, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Mehta, F. Report: Economic Implications of Inflammatory Bowel Disease and Its Management. Am. J. Manag. Care 2016, 22, s51–s60. [Google Scholar]
- Del Hoyo, J.; Nos, P.; Faubel, R.; Muñoz, D.; Domínguez, D.; Bastida, G.; Valdivieso, B.; Correcher, M.; Aguas, M. A Web-Based Telemanagement System for Improving Disease Activity and Quality of Life in Patients with Complex Inflammatory Bowel Disease: Pilot Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e11602. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.J.; van der Meulen-de Jong, A.E.; Romberg-Camps, M.J.; Becx, M.C.; Maljaars, J.P.; Cilissen, M.; van Bodegraven, A.A.; Mahmmod, N.; Markus, T.; Hameeteman, W.M.; et al. Telemedicine for Management of Inflammatory Bowel Disease (myIBDcoach): A Pragmatic, Multicentre, Randomised Controlled Trial. Lancet 2017, 390, 959–968. [Google Scholar] [CrossRef]
- Eloi, C.; Foulon, G.; Bridoux-Henno, L.; Breton, E.; Pelatan, C.; Chaillou, E.; Grimal, I.; Darviot, E.; Carré, E.; Gastineau, S.; et al. Inflammatory Bowel Diseases and School Absenteeism. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 541–546. [Google Scholar] [CrossRef]
- Restall, G.J.; Simms, A.M.; Walker, J.R.; Graff, L.A.; Sexton, K.A.; Rogala, L.; Miller, N.; Haviva, C.; Targownik, L.E.; Bernstein, C.N. Understanding Work Experiences of People with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1688–1697. [Google Scholar] [CrossRef]
- Longobardi, T.; Jacobs, P.; Bernstein, C.N. Work Losses Related to Inflammatory Bowel Disease in the United States: Results from the National Health Interview Survey. Am. J. Gastroenterol. 2003, 98, 1064–1072. [Google Scholar] [CrossRef]
- Telford, J.J.; Rosenfeld, G.; Thakkar, S.; Bansback, N. Patients’ Experiences and Priorities for Accessing Gastroenterology Care. J. Can. Assoc. Gastroenterol. 2021, 4, 3–9. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Kuenzig, M.E.; Bernstein, C.N.; Nguyen, G.C.; Guttmann, A.; Jones, J.L.; Potter, B.K.; Targownik, L.E.; Catley, C.A.; Nugent, Z.J.; et al. Rural and Urban Disparities in the Care of Canadian Patients with Inflammatory Bowel Disease: A Population-Based Study. Clin. Epidemiol. 2018, 10, 1613–1626. [Google Scholar] [CrossRef]
- Atreja, A.; Otobo, E.; Ramireddy, K.; Deorocki, A. Remote Patient Monitoring in IBD: Current State and Future Directions. Curr. Gastroenterol. Rep. 2018, 20, 6. [Google Scholar] [CrossRef]
- Pelaccia, T.; Forestier, G.; Wemmert, C. Deconstructing the Diagnostic Reasoning of Human versus Artificial Intelligence. CMAJ 2019, 191, E1332–E1335. [Google Scholar] [CrossRef] [PubMed]
- Atreja, A.; Otobo, E.; Szigethy, E.; Kohli, A.; Shroff, H.; Chang, H.; Rogers, J.; Ullman, T.; Cohen, B.; Itzkowitz, S.; et al. P057 Improved Quality of Care and Quality of Life For Ibd Patients Using Mobile Based Remote Monitoring Platform: A Randomized Control Trial. Inflamm. Bowel Dis. 2018, 24, S21–S22. [Google Scholar] [CrossRef]
- Kelso, M.; Feagins, L.A. Can Smartphones Help Deliver Smarter Care for Patients with Inflammatory Bowel Disease? Inflamm. Bowel Dis. 2018, 24, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.; Holzwanger, E.A.; Levy, A.N. Emerging Use of Artificial Intelligence in Inflammatory Bowel Disease. World J. Gastroenterol. 2020, 26, 6923–6928. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, W.; Bradfield, J.; Li, J.; Cardinale, C.; Frackelton, E.; Kim, C.; Mentch, F.; Van Steen, K.; Visscher, P.M.; et al. Large Sample Size, Wide Variant Spectrum, and Advanced Machine-Learning Technique Boost Risk Prediction for Inflammatory Bowel Disease. Am. J. Hum. Genet. 2013, 92, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Isakov, O.; Dotan, I.; Ben-Shachar, S. Machine Learning-Based Gene Prioritization Identifies Novel Candidate Risk Genes for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Iadanza, E.; Fabbri, R.; Bašić-ČiČak, D.; Amedei, A.; Telalovic, J.H. Gut Microbiota and Artificial Intelligence Approaches: A Scoping Review. Health Technol. 2020, 10, 1343–1358. [Google Scholar] [CrossRef]
- Mossotto, E.; Ashton, J.J.; Coelho, T.; Beattie, R.M.; MacArthur, B.D.; Ennis, S. Classification of Paediatric Inflammatory Bowel Disease Using Machine Learning. Sci. Rep. 2017, 7, 2427. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, G.; Lin, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Serum Biomarkers for Inflammatory Bowel Disease. Front. Med. 2020, 7, 123. [Google Scholar] [CrossRef]
- Tsilimigras, M.C.B.; Fodor, A.A. Compositional Data Analysis of the Microbiome: Fundamentals, Tools, and Challenges. Ann. Epidemiol. 2016, 26, 330–335. [Google Scholar] [CrossRef]
- Gubatan, J.; Levitte, S.; Patel, A.; Balabanis, T.; Wei, M.T.; Sinha, S.R. Artificial Intelligence Applications in Inflammatory Bowel Disease: Emerging Technologies and Future Directions. World J. Gastroenterol. 2021, 27, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Pasolli, E.; Truong, D.T.; Malik, F.; Waldron, L.; Segata, N. Machine Learning Meta-Analysis of Large Metagenomic Datasets: Tools and Biological Insights. PLoS Comput. Biol. 2016, 12, e1004977. [Google Scholar] [CrossRef]
- Argollo, M.; Kotze, P.G.; Kakkadasam, P.; D’Haens, G. Optimizing Biologic Therapy in IBD: How Essential Is Therapeutic Drug Monitoring? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 702–710. [Google Scholar] [CrossRef]
- Cohen, R.D. The Pharmacoeconomics of Biologic Therapy for IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 103–109. [Google Scholar] [CrossRef]
- Doherty, M.K.; Ding, T.; Koumpouras, C.; Telesco, S.E.; Monast, C.; Das, A.; Brodmerkel, C.; Schloss, P.D. Fecal Microbiota Signatures Are Associated with Response to Ustekinumab Therapy among Crohn’s Disease Patients. mBio 2018, 9. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Zhou, N.; Fu, D.; Lian, G.; Yi, J.; Peng, Y.; Liu, X. A Random Forest Model Predicts Responses to Infliximab in Crohn’s Disease Based on Clinical and Serological Parameters. Scand. J. Gastroenterol. 2021, 56, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Sucharew, H. Methods for Research Evidence Synthesis: The Scoping Review Approach. Available online: https://cdn.mdedge.com/files/s3fs-public/issues/articles/jhm014070416.pdf (accessed on 4 June 2021).
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for Reporting Qualitative Research. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Berger, V.W.; Alperson, S.Y. A General Framework for the Evaluation of Clinical Trial Quality. Rev. Recent Clin. Trials 2009, 4, 79–88. [Google Scholar] [CrossRef]
- Elkjaer, M.; Shuhaibar, M.; Burisch, J.; Bailey, Y.; Scherfig, H.; Laugesen, B.; Avnstrøm, S.; Langholz, E.; O’Morain, C.; Lynge, E.; et al. E-Health Empowers Patients with Ulcerative Colitis: A Randomised Controlled Trial of the Web-Guided “Constant-Care” Approach. Gut 2010, 59, 1652–1661. [Google Scholar] [CrossRef]
- Pedersen, N.; Thielsen, P.; Martinsen, L.; Bennedsen, M.; Haaber, A.; Langholz, E.; Végh, Z.; Duricova, D.; Jess, T.; Bell, S.; et al. eHealth: Individualization of Mesalazine Treatment Through a Self-Managed Web-Based Solution in Mild-to-Moderate Ulcerative Colitis. Inflamm. Bowel Dis. 2014, 20, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; van Langenberg, D.R.; Little, R.D.; Sparrow, M.P.; De Cruz, P.; Ward, M.G. A Virtual Clinic Increases Anti-TNF Dose Intensification Success via a Treat-to-Target Approach Compared with Standard Outpatient Care in Crohn’s Disease. Aliment. Pharmacol. Ther. 2020, 51, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Marín-Jiménez, I.; Nos, P.; Domènech, E.; Riestra, S.; Gisbert, J.P.; Calvet, X.; Cortés, X.; Iglesias, E.; Huguet, J.M.; Taxonera, C.; et al. Diagnostic Performance of the Simple Clinical Colitis Activity Index Self-Administered Online at Home by Patients with Ulcerative Colitis: CRONICA-UC Study. Am. J. Gastroenterol. 2016, 111, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Thompson, K.D.; Peterson, T.; Huneven, S.; Carmichael, J.; Glazer, F.J.; Darling, K.; Siegel, C.A. Delivering High Value Inflammatory Bowel Disease Care Through Telemedicine Visits. Inflamm. Bowel Dis. 2017, 23, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Marshall, J.K.; Nguyen, G.C.; Atreja, A.; Narula, N. Impact of Digital Health Monitoring in the Management of Inflammatory Bowel Disease. J. Med. Syst. 2021, 45, 23. [Google Scholar] [CrossRef]
- Cross, R.K.; Langenberg, P.; Regueiro, M.; Schwartz, D.A.; Tracy, J.K.; Collins, J.F.; Katz, J.; Ghazi, L.; Patil, S.A.; Quezada, S.M.; et al. A Randomized Controlled Trial of TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD). Am. J. Gastroenterol. 2019, 114, 472–482. [Google Scholar] [CrossRef]
- Quinn, C.C.; Chard, S.; Roth, E.G.; Kevin Eckert, J.; Russman, K.M.; Cross, R.K. The Telemedicine for Patients With Inflammatory Bowel Disease (TELE-IBD) Clinical Trial: Qualitative Assessment of Participants’ Perceptions. J. Med. Internet Res. 2019, 21, e14165. [Google Scholar] [CrossRef]
- Östlund, I.; Werner, M.; Karling, P. Self-Monitoring with Home Based Fecal Calprotectin Is Associated with Increased Medical Treatment. A Randomized Controlled Trial on Patients with Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2021, 56, 38–45. [Google Scholar] [CrossRef] [PubMed]
- McCombie, A.; Walmsley, R.; Barclay, M.; Ho, C.; Langlotz, T.; Regenbrecht, H.; Gray, A.; Visesio, N.; Inns, S.; Schultz, M. A Noninferiority Randomized Clinical Trial of the Use of the Smartphone-Based Health Applications IBDsmart and IBDoc in the Care of Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2020, 26, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Kim, S.K.; Jang, B.I.; Kim, K.O.; Kim, E.Y.; Lee, Y.J.; Lee, H.S.; Kwak, S.G. Crohn’s and Colitis Association in Daegu-Gyeongbuk (CCAiD) Disease Activity Patterns Recorded Using a Mobile Monitoring System Are Associated with Clinical Outcomes of Patients with Crohn’s Disease. Dig. Dis. Sci. 2018, 63, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-S.; Kim, D. Inferring Crohn’s Disease Association from Exome Sequences by Integrating Biological Knowledge. BMC Med. Genom. 2016, 9, 35. [Google Scholar] [CrossRef][Green Version]
- Tong, Y.; Lu, K.; Yang, Y.; Li, J.; Lin, Y.; Wu, D.; Yang, A.; Li, Y.; Yu, S.; Qian, J. Can Natural Language Processing Help Differentiate Inflammatory Intestinal Diseases in China? Models Applying Random Forest and Convolutional Neural Network Approaches. BMC Med. Inform. Decis. Mak. 2020, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Matalka, I.I.; Al-Omari, F.A.; Salama, R.M.; Mohtaseb, A.H. A Novel Approach for Quantitative Assessment of Mucosal Damage in Inflammatory Bowel Disease. Diagn. Pathol. 2013, 8, 156. [Google Scholar] [CrossRef]
- Takenaka, K.; Ohtsuka, K.; Fujii, T.; Negi, M.; Suzuki, K.; Shimizu, H.; Oshima, S.; Akiyama, S.; Motobayashi, M.; Nagahori, M.; et al. Development and Validation of a Deep Neural Network for Accurate Evaluation of Endoscopic Images from Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 2150–2157. [Google Scholar] [CrossRef]
- Waljee, A.K.; Joyce, J.C.; Wang, S.; Saxena, A.; Hart, M.; Zhu, J.; Higgins, P.D.R. Algorithms Outperform Metabolite Tests in Predicting Response of Patients with Inflammatory Bowel Disease to Thiopurines. Clin. Gastroenterol. Hepatol. 2010, 8, 143–150. [Google Scholar] [CrossRef]
- Waljee, A.K.; Liu, B.; Sauder, K.; Zhu, J.; Govani, S.M.; Stidham, R.W.; Higgins, P.D.R. Predicting Corticosteroid-Free Endoscopic Remission with Vedolizumab in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2018, 47, 763–772. [Google Scholar] [CrossRef]
- Maeda, Y.; Kudo, S.-E.; Mori, Y.; Misawa, M.; Ogata, N.; Sasanuma, S.; Wakamura, K.; Oda, M.; Mori, K.; Ohtsuka, K. Fully Automated Diagnostic System with Artificial Intelligence Using Endocytoscopy to Identify the Presence of Histologic Inflammation Associated with Ulcerative Colitis (with Video). Gastrointest. Endosc. 2019, 89, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Waljee, A.K.; Lipson, R.; Wiitala, W.L.; Zhang, Y.; Liu, B.; Zhu, J.; Wallace, B.; Govani, S.M.; Stidham, R.W.; Hayward, R.; et al. Predicting Hospitalization and Outpatient Corticosteroid Use in Inflammatory Bowel Disease Patients Using Machine Learning. Inflamm. Bowel Dis. 2017, 24, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tontini, G.E.; Vecchi, M.; Pastorelli, L.; Neurath, M.F.; Neumann, H. Differential Diagnosis in Inflammatory Bowel Disease Colitis: State of the Art and Future Perspectives. World J. Gastroenterol. 2015, 21, 21–46. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S. Treatment of Inflammatory Bowel Disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F.; Siegmund, B. Personalizing Treatment in IBD: Hype or Reality in 2020? Can We Predict Response to Anti-TNF? Front. Med. 2020, 7, 517. [Google Scholar] [CrossRef]
- Domènech, E. Inflammatory Bowel Disease: Current Therapeutic Options. Digestion 2006, 73 (Suppl. 1), 67–76. [Google Scholar] [CrossRef]
- Waljee, A.K.; Sauder, K.; Patel, A.; Segar, S.; Liu, B.; Zhang, Y.; Zhu, J.; Stidham, R.W.; Balis, U.; Higgins, P.D.R. Machine Learning Algorithms for Objective Remission and Clinical Outcomes with Thiopurines. J. Crohn’s Colitis 2017, 11, 801–810. [Google Scholar] [CrossRef] [PubMed]
- de Boer, N.K.H.; van Bodegraven, A.A.; Jharap, B.; de Graaf, P.; Mulder, C.J.J. Drug Insight: Pharmacology and Toxicity of Thiopurine Therapy in Patients with IBD. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 686–694. [Google Scholar] [CrossRef]
- Cohen-Mekelburg, S.; Wallace, B.I.; Van, T.; Wiitala, W.L.; Govani, S.M.; Burns, J.; Lipson, R.; Yun, H.; Hou, J.; Lewis, J.D.; et al. Association of Anti-Tumor Necrosis Factor Therapy with Mortality Among Veterans With Inflammatory Bowel Disease. JAMA Netw. Open 2021, 4, e210313. [Google Scholar] [CrossRef]
- Jawad, I.; Watson, S.; Haddad, P.M.; Talbot, P.S.; McAllister-Williams, R.H. Medication Nonadherence in Bipolar Disorder: A Narrative Review. Ther. Adv. Psychopharmacol. 2018, 8, 349–363. [Google Scholar] [CrossRef]
- Karve, S.; Cleves, M.A.; Helm, M.; Hudson, T.J.; West, D.S.; Martin, B.C. Good and Poor Adherence: Optimal Cut-Point for Adherence Measures Using Administrative Claims Data. Curr. Med. Res. Opin. 2009, 25, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Hekler, E.B.; Andersson, G.; Collins, L.M.; Doherty, A.; Hollis, C.; Rivera, D.E.; West, R.; Wyatt, J.C. Evaluating Digital Health Interventions: Key Questions and Approaches. Am. J. Prev. Med. 2016, 51, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef] [PubMed]
- McQueenie, R.; Ellis, D.A.; McConnachie, A.; Wilson, P.; Williamson, A.E. Morbidity, Mortality and Missed Appointments in Healthcare: A National Retrospective Data Linkage Study. BMC Med. 2019, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Denson, L.A.; Curran, M.; McGovern, D.P.B.; Koltun, W.A.; Duerr, R.H.; Kim, S.C.; Sartor, R.B.; Sylvester, F.A.; Abraham, C.; de Zoeten, E.F.; et al. Challenges in IBD Research: Precision Medicine. Inflamm. Bowel Dis. 2019, 25, S31–S39. [Google Scholar] [CrossRef]
- George, L.A.; Cross, R.K. Remote Monitoring and Telemedicine in IBD: Are We There Yet? Curr. Gastroenterol. Rep. 2020, 22, 12. [Google Scholar] [CrossRef]
- Yin, A.L.; Hachuel, D.; Pollak, J.P.; Scherl, E.J.; Estrin, D. Digital Health Apps in the Clinical Care of Inflammatory Bowel Disease: Scoping Review. J. Med. Internet Res. 2019, 21, e14630. [Google Scholar] [CrossRef]
- Amann, J.; Blasimme, A.; Vayena, E.; Frey, D.; Madai, V.I. Explainability for Artificial Intelligence in Healthcare: A Multidisciplinary Perspective. BMC Med. Inform. Decis. Mak. 2020, 20, 310. [Google Scholar] [CrossRef]
- Nakase, H.; Hirano, T.; Wagatsuma, K.; Ichimiya, T.; Yamakawa, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Kazama, T.; Yoshii, S.; et al. Artificial Intelligence-Assisted Endoscopy Changes the Definition of Mucosal Healing in Ulcerative Colitis. Dig. Endosc. 2020, 33, 903–911. [Google Scholar] [CrossRef]
- Altman, D.G.; Lyman, G.H. Methodological Challenges in the Evaluation of Prognostic Factors in Breast Cancer. Breast Cancer Res. Treat. 1998, 52, 289–303. [Google Scholar] [CrossRef]
- Riley, R.D.; Abrams, K.R.; Sutton, A.J.; Lambert, P.C.; Jones, D.R.; Heney, D.; Burchill, S.A. Reporting of Prognostic Markers: Current Problems and Development of Guidelines for Evidence-Based Practice in the Future. Br. J. Cancer 2003, 88, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; Côté, P.; Steenstra, I.A.; Bombardier, C. QUIPS-LBP Working Group Identifying Phases of Investigation Helps Planning, Appraising, and Applying the Results of Explanatory Prognosis Studies. J. Clin. Epidemiol. 2008, 61, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.D.; Gray, K.; Knowles, S.R.; De Cruz, P. EHealth Technologies in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2016, 10, 1103–1121. [Google Scholar] [CrossRef] [PubMed]
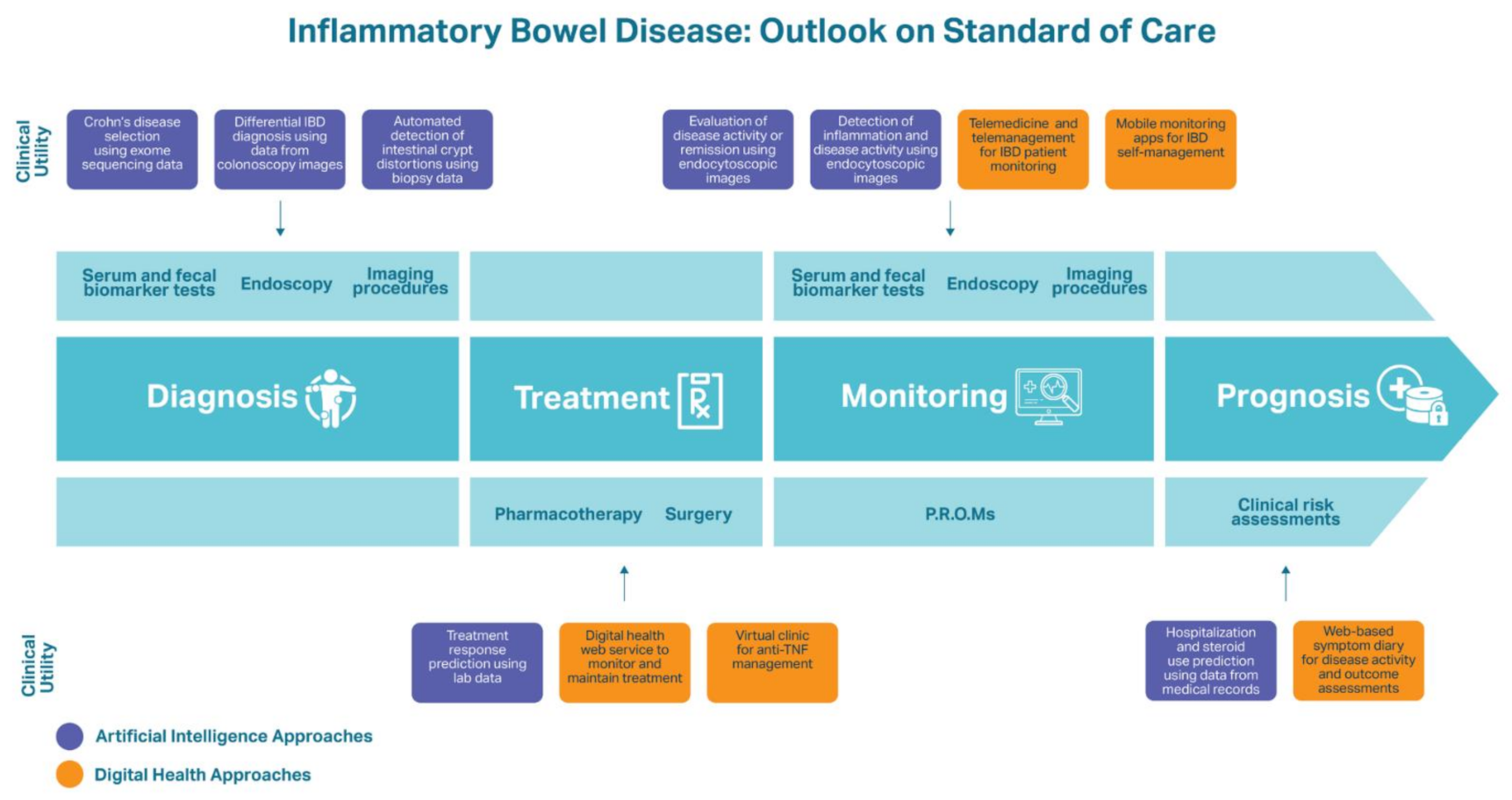
| Digital Health | ||
|---|---|---|
| Diagnosis | ||
| Treatment | Treatment Adherence and Maintenance “Constant-care” web service Significantly improved adherence to 5-aminosalicylate treatment, knowledge of IBD, and QoL compared to patients receiving standard care [75]. Helped UC patients optimize their maintenance treatment using mesalazine and improve treatment adherence, disease activity, and QoL [76]. | |
| Treatment Management Virtual clinic for anti-TNF therapy management Significantly shortened time until treatment success, provided suitable dose intensification, improved disease control, and improved treatment de-escalation compared to standard CD care [77]. | ||
| Monitoring | Telemedicine and Telemanagement Approaches | Mobile Applications |
| myIBDcoach Significantly reduced the number of outpatient visits compared to IBD patients using standard care while maintaining QoC and disease monitoring (p < 0.0001) [49]. TECCU Reduced outpatient clinic visits among IBD patients. TECCU users experienced improvements in disease activity and 81% of these patients were in clinical remission by the end of the study, compared to 71.4% of patients receiving standard care [48]. CRONICA The self-administered SCCAI via the CRONICA web platform was a trustworthy self-assessment tool for UC patients to monitor their. Online SCCAI scores were 85% in agreement with physician’s assessments of remission or UC disease activity [78]. IBD telemedicine clinic Appointments were evaluated to assess the quality of care provided at a low cost in comparison to standard care. Telemedicine patients saved a mean of $62 in travel costs and at least half a day of time without negative impacts on quality of care [79]. | HealthPROMISE Led to a significant reduction in hospitalizations and emergency room visits within one year among IBD patients compared to those who received standard care [80]. TELE-IBD TELE-IBD groups experienced a decline in IBD-related hospitalizations, with a significant decrease when receiving TELE-IBD messages weekly compared to standard care. TELE-IBD educational messages did not significantly improve disease activity and QoL in comparison to standard care, potentially due to the patients having more severe CD and UC [81]. Interviews with patients using TELE-IBD revealed that they considered the service a beneficial supplement to traditional follow-ups and a useful component in IBD self-management to stay educated on IBD, monitor their symptoms, and connect with their physician [82]. IBD-Home 29% of patients were compliant to the IBD-Home application and FC test kit after one year. Patients who were compliant experienced a rise in medical treatment, providing the value to remote disease monitoring [83]. Self-monitoring applications (IBDsmart and IBDoc) Led to significantly fewer outpatient appointments than standard care patients (mean of 0.6 vs. 1.7) without affecting health outcomes or HRQoL (p < 0.001) [84]. | |
| Prognosis | IBD-Related Predictions Web-based symptom diary for CD Patient-reported IBD-related symptoms were associated with significant increases in hospitalizations, unscheduled visits, and bowel resection surgeries among CD patients with more severe disease [85]. | |
| Artificial Intelligence | ||
| Diagnosis | IBD Detection Tri-matrix factorization model used a combination of exome sequencing data and biological knowledge to differentiate healthy individuals from CD patients (AUC = 0.816) [86]. RF model differentially diagnosed CD and UC using descriptions of colonoscopy images (AUC = 0.936) [87]. AI system built using a probabilistic neural network assessed intestinal crypt architecture distortion and mucosal damage from patient biopsies and diagnosed IBD with 98.31% precision and recall [88]. Deep neural network for evaluation of UC predicted endoscopic remission with 90.1% accuracy and histologic remission with 92.9% accuracy using endoscopic images and biopsies from UC patients [89]. | |
| Treatment | Treatment Response Predictions RF algorithms predicted clinical responders and non-responders (AuROC = 0.856) and non-adherence to thiopurine therapy (AuROC = 0.813). Can be used to personalize thiopurine dosages [90]. RF model predicted corticosteroid-free endoscopic remission at 52 weeks of vedolizumab treatment using data acquired during week 6 of therapy (AuROC = 0.73) [91]. | |
| Monitoring | Inflammation and Disease Activity Monitoring Deep neural network for evaluation of UC predicted endoscopic remission with 90.1% accuracy and histologic remission with 92.9% accuracy using endoscopic images and biopsies from UC patients [89]. Proprietary ML algorithm was 91% accurate at detecting histologic inflammation from endocytoscopic images and therefore assessing disease activity and risk of clinical exacerbation [92]. | |
| Prognosis | IBD Assessment and Predictions Proprietary ML algorithm was 91% accurate at detecting histologic inflammation from endocytoscopic images and therefore assessing disease activity and risk of clinical exacerbation [92]. RF model constructed from medical records of IBD patients predicted IBD-related hospitalizations and outpatient steroid use (AuROC = 0.85) [93]. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majidova, K.; Handfield, J.; Kafi, K.; Martin, R.D.; Kubinski, R. Role of Digital Health and Artificial Intelligence in Inflammatory Bowel Disease: A Scoping Review. Genes 2021, 12, 1465. https://doi.org/10.3390/genes12101465
Majidova K, Handfield J, Kafi K, Martin RD, Kubinski R. Role of Digital Health and Artificial Intelligence in Inflammatory Bowel Disease: A Scoping Review. Genes. 2021; 12(10):1465. https://doi.org/10.3390/genes12101465
Chicago/Turabian StyleMajidova, Kamila, Julia Handfield, Kamran Kafi, Ryan D. Martin, and Ryszard Kubinski. 2021. "Role of Digital Health and Artificial Intelligence in Inflammatory Bowel Disease: A Scoping Review" Genes 12, no. 10: 1465. https://doi.org/10.3390/genes12101465
APA StyleMajidova, K., Handfield, J., Kafi, K., Martin, R. D., & Kubinski, R. (2021). Role of Digital Health and Artificial Intelligence in Inflammatory Bowel Disease: A Scoping Review. Genes, 12(10), 1465. https://doi.org/10.3390/genes12101465









