Comparative Transcriptome Profiling of Ovary Tissue between Black Muscovy Duck and White Muscovy Duck with High- and Low-Egg Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Sample Collection
2.2. Library Construction and Sequencing
2.3. Differential Expression Analysis
2.4. GO and KEGG Analysis
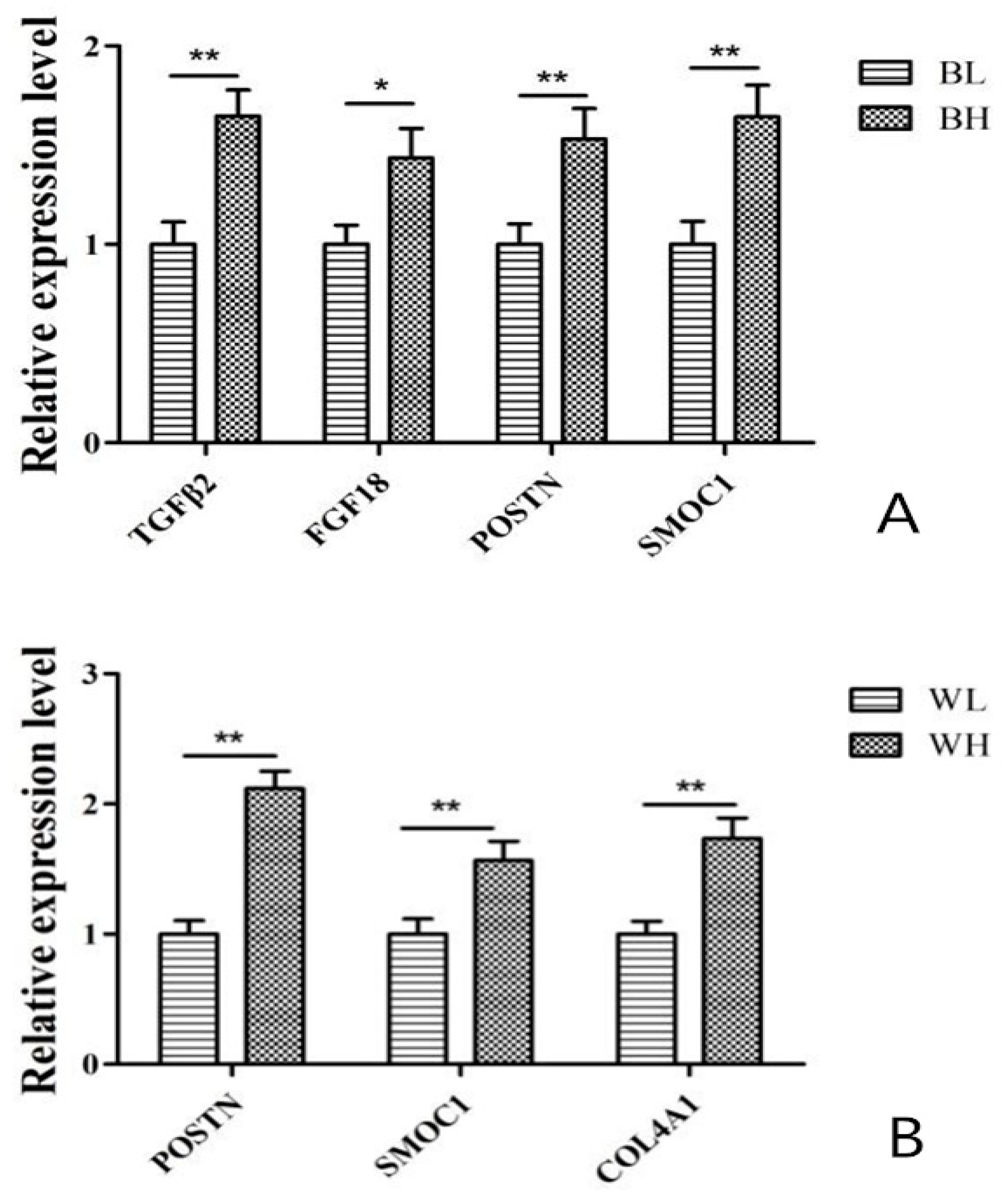
2.5. Verification of RNA-seq by qPCR
2.6. Statistical Analysis
3. Results
3.1. High-Throughput Sequencing and Read Mapping
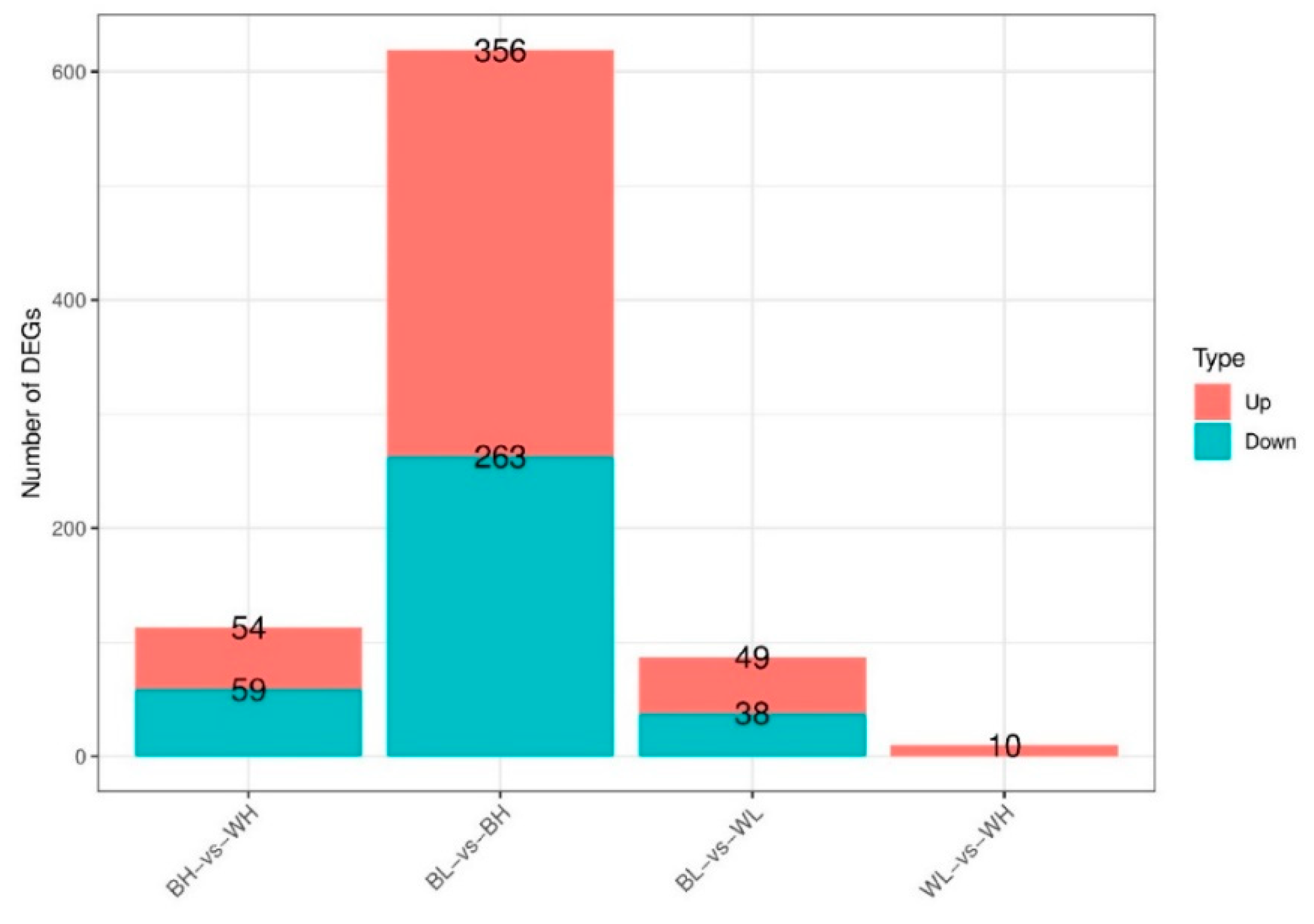
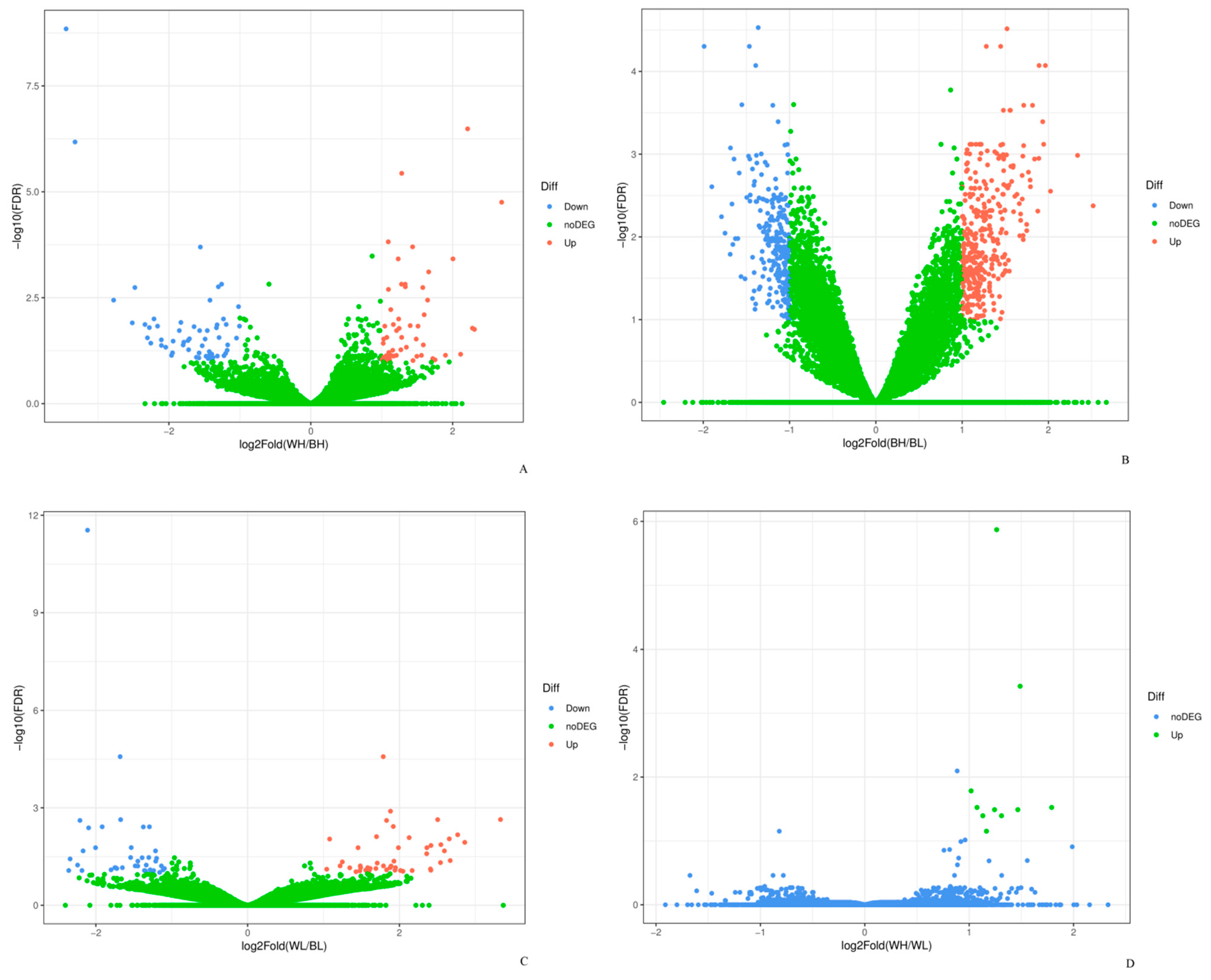
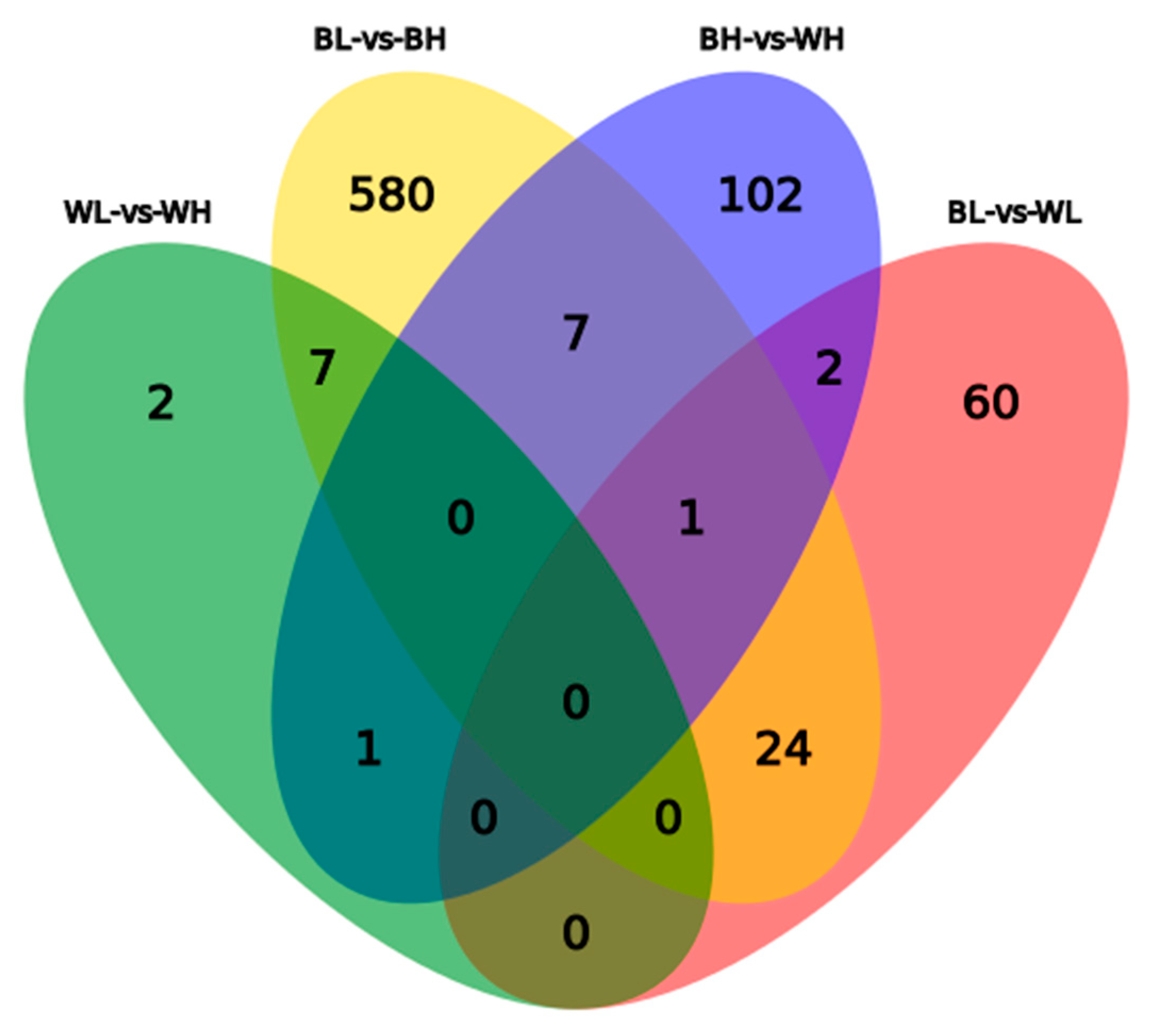
3.2. Differentially Expressed Genes
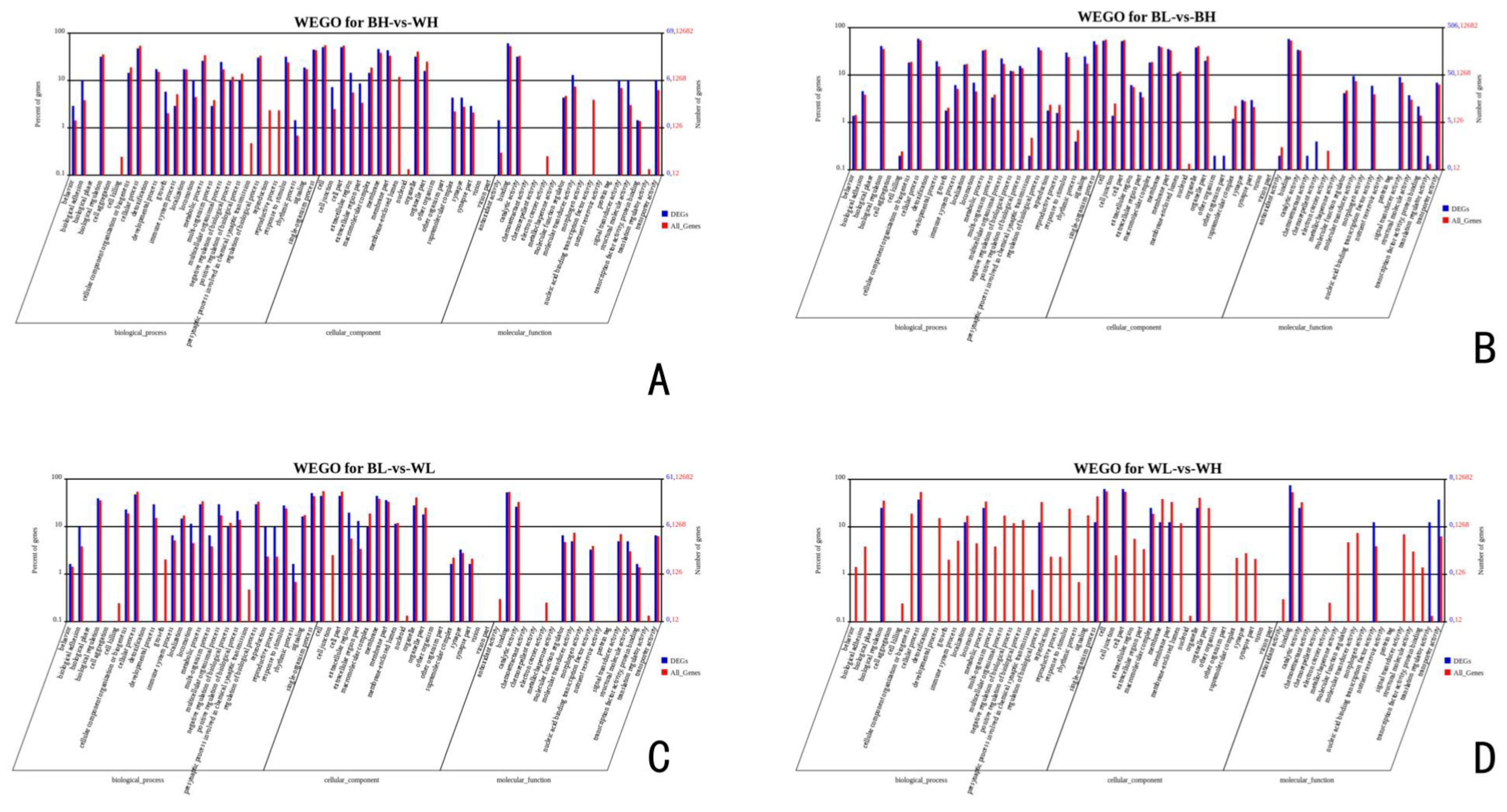
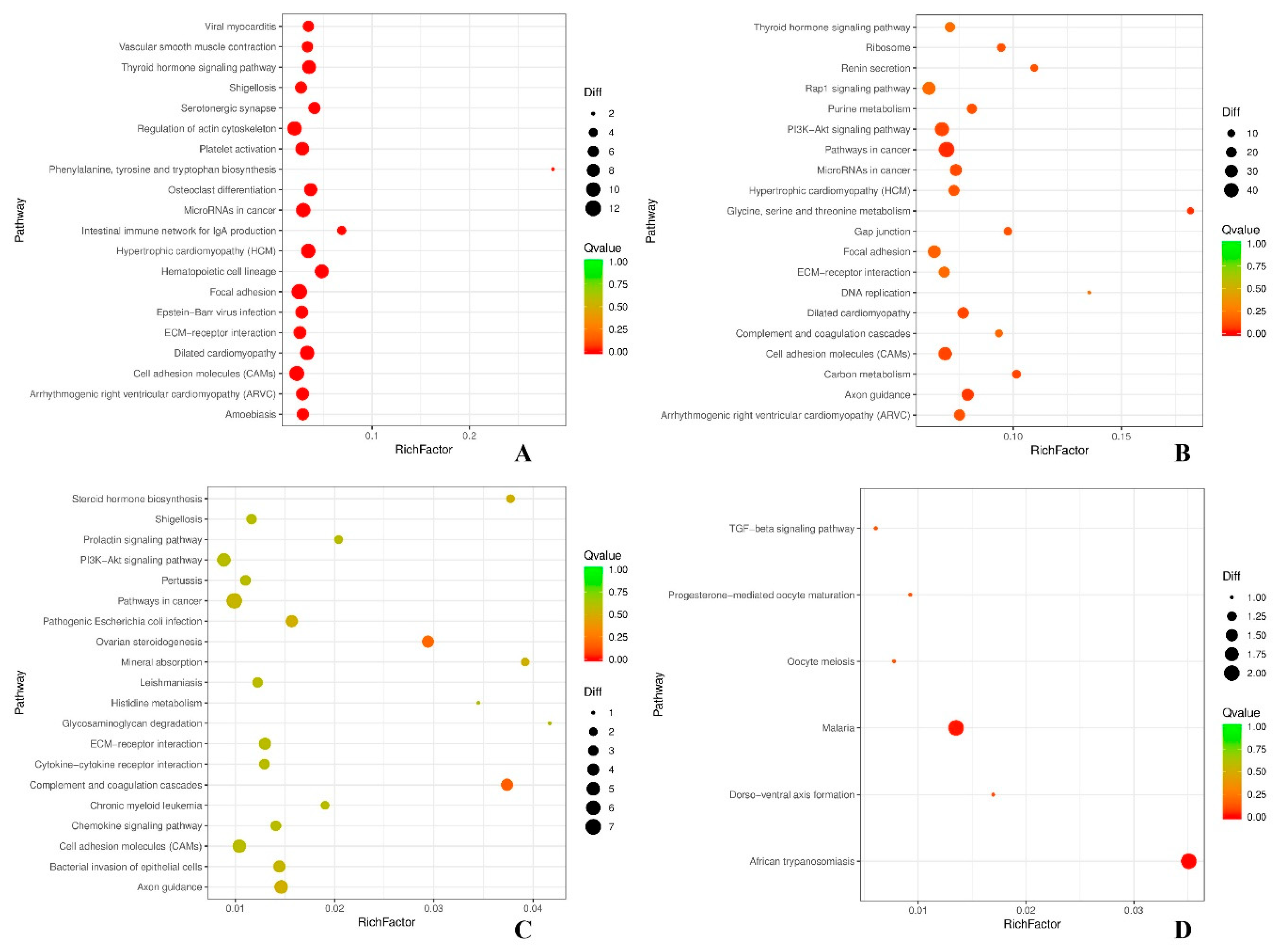
3.3. Gene Ontology and KEGG Analysis
4. Discussion
4.1. Analysis of DEGs
4.2. Analysis of GO and KEGG
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Z.M.; Miao, Z.W.; Chen, H.P.; Xin, Q.W.; Li, L.; Lin, R.L.; Huang, Q.L.; Zheng, N.Z. Ovarian transcriptomic analysis of shanma ducks at peak and late stages of egg production. Asian-Australas J. Anim. Sci. 2017, 30, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Yao, Y.; Cao, Z.F.; Gu, T.T.; Xu, Q.; Chen, G. Histological characteristics of follicles and reproductive hormone secretion during ovarian follicle development in laying geese. Poult. Sci. 2019, 98, 6063–6070. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K.; Shea, L.D. The Role of the Extracellular Matrix in Ovarian Follicle Development. Reprod. Sci. 2007, 14, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Zheng, W.; Liu, K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol. Metab. 2010, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, C.-Q. Effects of Daidzein on Messenger Ribonucleic Acid Expression of Gonadotropin Receptors in Chicken Ovarian Follicles. Poult. Sci. 2008, 87, 541–545. [Google Scholar] [CrossRef]
- Cao, W.N.; Dong, X.Y. Research progress of mechanism of mTOR pathway involves follicular development. J. Reprod. Med. 2016, 5, 469–472. [Google Scholar]
- Onagbesan, O.; Bruggeman, V.; Decuypere, E. Intra-ovarian growth factors regulating ovarian function in avian species: A review. Anim. Reprod. Sci. 2009, 111, 121–140. [Google Scholar] [CrossRef]
- Zhang, C.; Shimada, K.; Saito, N.; Kansaku, N. Expression of messenger ribonucleic acids of luteinizing hormone and folli-cle-stimulating hormone receptors in granulosa and theca layers of chicken preovulatory follicles. Gen. Comp. Endocrinol. 1997, 105, 402–409. [Google Scholar] [CrossRef]
- Wu, X.; Wan, X.P.; Lan, J.; Yan, M.; Lian, S.; Rijal, M.; Huang, Z.; Li, A. Cloning, expression and polymorphism at the 5′-flanking region of the GnRH gene and their association with laying traits in Muscovy duck (Cairina moschata). Br. Poult. Sci. 2015, 56, 531–542. [Google Scholar] [CrossRef]
- Johnson, J.L.; Solovieva, E.V.; Bridgham, J.T. Relationship between steroidogenic acute regulatory protein expression and proges-terone production in hen granulosa cells during follicle development. Biol. Reprod. 2002, 67, 1313–1320. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Liu, Y.; Wang, Y.; Zhao, X.; Zhu, Q. Genetic effect of the prolactin receptor gene on egg production traits in chickens. Genet. Mol. Res. 2012, 11, 4307–4315. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lou, Y.; Zhao, A. Transcriptome analysis of follicles reveals the importance of autophagy and hormones in regulating broodiness of Zhedong white goose. Sci. Rep. 2016, 6, 36877. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Zhou, B.; Xu, F.; Chen, R.; Shen, C.; Liang, T.; Li, Y.; Schinckel, A.P. Genome-wide differential mRNA expression profiles in follicles of two breeds and at two stages of estrus cycle of gilts. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hatzirodos, N.; Hummitzsch, K.; Irving-Rodgers, H.F.; Breen, J.; Perry, V.E.A.; Anderson, R.A.; Rodgers, R.J. Transcript abundance of stromal and thecal cell related genes during bovine ovarian development. PLoS ONE 2019, 14, e0213575. [Google Scholar] [CrossRef]
- Talebi, R.; Ahmadi, A.; Afraz, F.; Sarry, J.; Plisson-Petit, F.; Genet, C.; Fabre, S. Transcriptome analysis of ovine granulosa cells reveals differences between small antral follicles collected during the follicular and luteal phases. Theriogenology 2018, 108, 103–117. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.; Han, K.; Zhang, X.; Zhang, G.; Dai, G.; Wang, J.; Xie, K. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. Anim. Reprod. Sci. 2019, 208, 106114. [Google Scholar] [CrossRef]
- Tao, Z.; Song, W.; Zhu, C.; Xu, W.; Liu, H.; Zhang, S.; HuiFang, L. Comparative transcriptomic analysis of high and low egg-producing duck ovaries. Poult. Sci. 2017, 96, 4378–4388. [Google Scholar] [CrossRef]
- Luan, X.; Liu, D.; Cao, Z.; Luo, L.; Liu, M.; Gao, M.; Zhang, X. Transcriptome Profiling Identifies Differentially Expressed Genes in Huoyan Goose Ovaries between the Laying Period and Ceased Period. PLoS ONE 2014, 9, e113211. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, X.; Lu, L.; Duan, X.; Qin, H.; Du, X.; Li, G.; Tao, Z.; Zhong, S.; Wang, G.L. Transcriptomic analysis of different stages of pigeon ovaries by RNA-sequencing. Mol. Reprod. Dev. 2016, 83, 640–648. [Google Scholar] [CrossRef]
- Wu, X.; Pan, X.L.; Cao, S.M.; Xu, F.Q.; Lan, L.M.; Zhang, Y.Y.; Lian, S.Y.; Yan, M.J.; Li, A. iTRAQ-based quantitative proteomic analysis provides insights into strong broodiness in Muscovy duck (Cairina moschata) combined with metabolomics analysis. J. Proteomics 2019, 204, 103401. [Google Scholar] [CrossRef]
- Ye, Q.; Xu, J.; Gao, X.; Ouyang, H.; Luo, W.; Nie, Q. Associations of IGF2 and DRD2 polymorphisms with laying traits in Muscovy duck. PeerJ 2017, 5, e4083. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2009, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Cui, X.X.; Zhang, Y.J.; Yang, C.H.; Jiang, Y.L. Identification of miRNAs associated with sexual maturity in chicken ovary by Illumina small RNA deep sequencing. BMC Genom. 2013, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Rider, C.C.; Mulloy, B. Heparin, Heparan Sulphate and the TGF-βCytokine Superfamily. Molecules 2017, 22, 713. [Google Scholar] [CrossRef]
- Zhang, Y.; Yanli, Z.; FengZhe, L.; Yang, H.; Zhu, A.; Pang, J.; Han, L.; Zhang, T.; Yao, X.; Wang, F. Genome-wide analysis of DNA Methylation profiles on sheep ovaries associated with prolificacy using whole-genome Bisulfite sequencing. BMC Genom. 2017, 18, 1–17. [Google Scholar] [CrossRef]
- Jackowska, M.; Kempisty, B.; Woźna, M.; Piotrowska, H.; Antosik, P.; Zawierucha, P.; Bukowska, D.; Nowicki, M.; Jaśkowski, J.M.; Brüssow, K.-P. Differential expression of GDF9, TGFB1, TGFB2 and TGFB3 in porcine oocytes isolated from follicles of different size before and after culture in vitro. Acta Vet. Hung. 2013, 61, 99–115. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Saxena, V.K.; Sastry, K.V.H.; Nagarajan, K.; Jain, P.; Singh, R.; Anish, D.; Ravindra, P.V.; Saxena, M.; Ahmed, K.A. Cytokines and chemokines in postovulatory follicle regression of domestic chicken (Gallus gallus domesticus). Dev. Comp. Immunol. 2008, 32, 253–264. [Google Scholar] [CrossRef]
- Ma, M.H.; Furuta, H.; Hiyama, Y.; Kato, Y.; Fujihara, N. Prominent expression of transforming growth factor beta2 gene in the chicken embryonic gonad as revealed by suppressive subtraction cloning. Gen. Comp. Endocrinol. 2002, 125, 311–316. [Google Scholar]
- Gao, X.G.; Daugherty, R.L.; Tourtellotte, W.G. Regulation of low affinity neurotrophin receptor (NGFR) by early growth response (egr) transcriptional regulators. Mol. Cell Neurosci. 2007, 36, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Yang, J.C.; Shan, A.T.; Tian, J.J.; Zhang, Q. Progress on NGF and its receptor expression in reproductive organs of femals animals. Prog. Vet. Med. 2016, 37, 113–115. [Google Scholar]
- Jana, B.M.; Koszykowska, M.; Czarzasta, J. Expression of nerve growth factor and its receptors, TrkA and p75, in porcine ovaries. J. Reprod. Dev. 2011, 57, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Medan, M.S.; Weng, Q.; Jin, W.; Li, C.; Watanabe, G.; Taya, K. Immunolocalization of Nerve Growth Factor (NGF) and Its Receptors (TrkA and p75LNGFR) in the Reproductive Organs of Shiba Goats. J. Reprod. Dev. 2005, 51, 399–404. [Google Scholar] [CrossRef][Green Version]
- Ramji, D.P.; Foka, P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem. J. 2002, 365, 561–575. [Google Scholar] [CrossRef]
- Becker, C.; Riedmaier, I.; Reiter, M.; Tichopad, A.; Groot, M.J.; Stolker, L.; Pfaffl, M.W.; Nielen, M.; Meyer, H. Influence of anabolic combinations of an androgen plus an estrogen on biochemical pathways in bovine uterine endometrium and ovary. J. Steroid Biochem. Mol. Biol. 2011, 125, 192–201. [Google Scholar] [CrossRef]
- Groisman, I.; Ivshina, M.; Marin, V.; Kennedy, N.J.; Davis, R.J.; Richter, J.D. Control of cellular senescence by CPEB. Genes Dev. 2006, 20, 2701–2712. [Google Scholar] [CrossRef][Green Version]
- Wang, X.-P.; Cooper, N.G.F. Comparative in silico Analyses of Cpeb1–4 with Functional Predictions. Bioinform. Biol. Insights 2010, 4, 61–83. [Google Scholar] [CrossRef]
- Kurihara, Y.; Tokuriki, M.; Myojin, R.; Hori, T.; Kuroiwa, A.; Matsuda, Y.; Sakurai, T.; Kimura, M.; Hecht, N.B.; Uesugi, S. CPEB2, a novel putative translational regulator in mouse haploid germ cells. Biol. Reprod. 2003, 69, 261–268. [Google Scholar] [CrossRef]
- Prochazkova, B.; Komrskova, P.; Kubelka, M. CPEB2 Is Necessary for Proper Porcine Meiotic Maturation and Embryonic De-velopment. Int. J. Mol. Sci. 2018, 19, 3138. [Google Scholar] [CrossRef]
- Gillan, L.; Matei, D.; Fishman, D.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar] [PubMed]
- Matsuzawa, M.; Arai, C.; Nomura, Y.; Murata, T.; Yamakoshi, Y.; Oida, S.; Hanada, N.; Nakamura, Y. Periostin of human periodontal ligament fibroblasts promotes migration of human mesenchymal stem cell through the alphavbeta3 integrin/FAK/PI3K/Akt pathway. J. Periodontal Res. 2015, 50, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Kulus, M.; Sujka-Kordowska, P.; Konwerska, A.; Celichowski, P.; Kranc, W.; Kulus, J.; Piotrowska-Kempisty, H.; Antosik, P.; Bukowska, D.; Iżycki, D.; et al. New Molecular Markers Involved in Regulation of Ovarian Granulosa Cell Morphogenesis, Development and Differentiation during Short-Term Primary In Vitro Culture-Transcriptomic and Histochemical Study Based on Ovaries and Individual Separated Follicles. Int. J. Mol. Sci. 2019, 20, 3966. [Google Scholar] [CrossRef] [PubMed]
- Ożegowska, K.; Brązert, M.; Ciesiółka, S.; Nawrocki, M.J.; Kranc, W.; Celichowski, P.; Jankowski, M.; Bryja, A.; Jeseta, M.; Antosik, P.; et al. In VitroGenes Involved in the Pro-cesses of Cell Proliferation, Migration, Adhesion and Tissue Development as New Potential Markers of Porcine Granulosa Cellular Processes: A Microarray Approach. DNA Cell Biol. 2019, 38, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, J.; Han, B.; Wang, L.; Chen, Y.; Liu, M.; Huang, J. Proteomic profiling of follicle fluids after superstimulation in one-month-old lambs. Reprod. Domest. Anim. 2017, 53, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Pazin, D.E.; Albrecht, K.H. Developmental expression of Smoc1 and Smoc2 suggests potential roles in fetal gonad and repro-ductive tract differentiation. Dev. Dyn. 2009, 238, 2877–2890. [Google Scholar] [CrossRef]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor Specificity of the Fibroblast Growth Factor Family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef]
- Portela, V.M.; Dirandeh, E.; Guerrero-Netro, H.M.; Zamberlam, G.; Barreta, M.H.; Goetten, A.; Price, C. The Role of Fibroblast Growth Factor-18 in Follicular Atresia in Cattle1. Biol. Reprod. 2015, 92, 14. [Google Scholar] [CrossRef]
- Han, P.; Guerrero-Netro, H.; Estienne, A.; Price, C. Effects of fibroblast growth factors and the transcription factor, early growth response 1, on bovine theca cells. Mol. Cell. Endocrinol. 2018, 476, 96–102. [Google Scholar] [CrossRef]
- Da Silva, R.B.; Yang, M.Y.; Caixeta, E.S.; Castilho, A.C.; Amorim, R.L.; Price, C.A.; Fortune, J.E.; Buratini, J. Fibroblast growth factor 18 regulates steroidogenesis in fetal bovine ovarian tissue in vitro. Mol. Reprod. Dev. 2019, 86, 166–174. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, Q.T.; Sun, T.; Wang, F.; Liu, J.; Leach, R.; Johnson, A.; Puscheck, E.E.; Rappolee, D.A. FGF ligand family mRNA ex-pression profile for mouse preimplantation embryos, early gestation human placenta and mouse trophoblast stem cells. Mol. Reprod. Dev. 2006, 73, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Hatzirodos, N.; Hummitzsch, K.; Irving-Rodgers, H.F.; Harland, M.L.; Morris, S.; Rodgers, R.J. Transcriptome profiling of granulosa cells from bovine ovarian follicles during atresia. BMC Genom. 2014, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Worku, T.; Wang, K.; Ayers, D.; Wu, D.; Rehman, Z.U.; Zhou, H.; Yang, L. Regulatory roles of ephrinA5 and its novel signaling pathway in mouse primary granulosa cell apoptosis and proliferation. Cell Cycle 2018, 17, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 2010, 26, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.R.; Humphries, M.J.; Bass, M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007, 8, 957–969. [Google Scholar] [CrossRef]
- Bellin, R.M.; Capila, I.; Lincecum, J.; Park, P.W.; Reizes, O.; Bernfield, M.R. Unlocking the secrets of syndecans: Transgenic organisms as a potential key. Glycoconj. J. 2002, 19, 295–304. [Google Scholar] [CrossRef]
- Ishiguro, K.; Kojima, T.; Taguchi, O.; Saito, H.; Muramatsu, T.; Kadomatsu, K. Syndecan-4 expression is associated with follicular atresia in mouse ovary. Histochem. Cell Biol. 1999, 112, 25–33. [Google Scholar] [CrossRef]
- Lussier, J.G.; Diouf, M.N.; Lévesque, V.; Sirois, J.; Ndiaye, K. Gene expression profiling of upregulated mRNAs in granulosa cells of bovine ovulatory follicles following stimulation with hCG. Reprod. Biol. Endocrinol. 2017, 15, 88. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Rep. 2018, 19, 783–791. [Google Scholar] [CrossRef]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.; Casey, S.M.; Canty, M.J.; Crowe, M.A.; Martin, F.; Evans, A.C. Akt and Erk signal transduction pathways are early markers of differentiation in dominant and subordinate ovarian follicles in cattle. Reproduction 2007, 133, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.J.; Nagaraju, G.; Liu, Z.L.; Liu, K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mamma-lian ovary. Mol. Cell Endocrinol. 2012, 356, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Reverchon, M.; Cloix, L.; Froment, P.; Rame, C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 2012, 56, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Lin, S.; Zhu, M.; Li, C.; Chen, S.; Pu, L.; Lin, J.; Cao, L.; Zhang, Y. Acupuncture Reduces Apoptosis of Granulosa Cells in Rats with Premature Ovarian Failure Via Restoring the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 6311. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chen, B.L.; Zhu, Q.; Xu, Z.X.; Ning, C.Y.; Yin, H.D.; Wang, Y.; Zhao, X.L.; Fan, X.L.; Yang, M.Y.; et al. Transcriptome analysis reveals diferentially expressed genes associated with high rates of egg production in chicken hypothalamic-pituitary-ovarian axis. Sci. Rep. 2020, 10, 5976. [Google Scholar] [CrossRef]
- Etches, R.J.; Petitte, J.N. Reptilian and avian follicular hierarchies: Models for the study of ovarian development. J. Exp. Zoöl. 1990, 256, 112–122. [Google Scholar] [CrossRef]
- Miller, W.L.; Bose, H.S. Early steps in steroidogenesis: Intracellular cholesterol trafficking. J. Lipid Res. 2011, 52, 2111–2135. [Google Scholar] [CrossRef]
| Ingredient | Content (%) | Nutrient | Content (%) |
|---|---|---|---|
| Corn | 56.00 | Crude protein | 15.700 |
| Soybean meal | 23.80 | Calcium | 0.900 |
| Corn gluten meal | 10.00 | Total phosphorus | 0.680 |
| Limestone | 7.00 | Available phosphorus | 0.450 |
| CaHPO4 | 1.50 | Salt | 0.370 |
| Premix | 1.00 | Lysine | 0.760 |
| NaCl | 0.30 | Methionine | 0.387 |
| Lys·HCl | 0.30 | Methionine + Cystine | 0.654 |
| DL-Met | 0.10 | Isoleucine | 0.534 |
| Total | 100.00 | Threonine | 0.579 |
| Tryptophan | 0.194 | ||
| Crude fiber | 4.100 | ||
| Crude fat | 3.400 | ||
| Crude ash | 5.200 | ||
| Avian metabolizable energy | 2875 Mcal·kg−1 |
| Primer | Sequences(5′–3′) | GeneBank Accession Number | Product Length (bp) |
|---|---|---|---|
| POSTN | F:AACACGCTTGAAGTTGGC R:TCAATGAGGTGGATAACG | XM_005018343.4 | 107 |
| SMOC1 | F:CCGCTTCAGACCTGGCAATC R:CTCAAGAGACAGGCCCAGTTTCTAC | XM_013101462.3 | 149 |
| TGFβ2 | F:ATCTACAACAGCACCAGGGAC R:TAGCTTGGTGGGATGGCA | XM_027453142.1 | 155 |
| FGF18 | F: ATGTTTGTTGCCGAGGAG R:TGTTTCCCGCTTGTCCTG | XM_027468582.1 | 125 |
| COL4A1 | F:GGAGAAATGGGAGTTATGGG R:TTGGCCTTTGAGACTAACC | XM_027444310.1 | 213 |
| β-actin | F:TATGTCGCCCTGGATTTCG R:CTCAAGAGACAGGCCCAGTTTCTAC | XM_013101462.3 | 162 |
| Reads | Clean Reads | Mapped Reads | Unique Mapped Reads | Detected Genes |
|---|---|---|---|---|
| BH1 | 46,357,720 | 63.07% | 60.66% | 17,786 |
| BH2 | 46,947,348 | 63.87% | 61.37% | 17,822 |
| BH3 | 46,070,474 | 62.75% | 60.33% | 17,699 |
| BL1 | 47,534,374 | 67.40% | 64.36% | 16,847 |
| BL2 | 47,067,606 | 64.27% | 61.86% | 17,926 |
| BL3 | 45,252,048 | 63.94% | 60.66% | 16,276 |
| WH1 | 46,942,582 | 64.33% | 61.76% | 17,371 |
| WH2 | 44,667,726 | 61.96% | 59.67% | 17,553 |
| WH3 | 47,963,328 | 63.71% | 61.09% | 17,549 |
| WL1 | 46,411,962 | 65.58% | 62.91% | 17,553 |
| WL2 | 46,391,890 | 60.28% | 57.81% | 17,417 |
| WL3 | 44,344,070 | 64.77% | 61.92% | 17,286 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Song, Y.; Li, T.; Zhang, S.; Huang, L.; Zhang, S.; Cao, J.; Liu, X.; Zhang, J. Comparative Transcriptome Profiling of Ovary Tissue between Black Muscovy Duck and White Muscovy Duck with High- and Low-Egg Production. Genes 2021, 12, 57. https://doi.org/10.3390/genes12010057
Bao X, Song Y, Li T, Zhang S, Huang L, Zhang S, Cao J, Liu X, Zhang J. Comparative Transcriptome Profiling of Ovary Tissue between Black Muscovy Duck and White Muscovy Duck with High- and Low-Egg Production. Genes. 2021; 12(1):57. https://doi.org/10.3390/genes12010057
Chicago/Turabian StyleBao, Xiuyu, Yiping Song, Tao Li, Shanshan Zhang, Lihua Huang, Shuya Zhang, Junting Cao, Xiaolin Liu, and Jianqin Zhang. 2021. "Comparative Transcriptome Profiling of Ovary Tissue between Black Muscovy Duck and White Muscovy Duck with High- and Low-Egg Production" Genes 12, no. 1: 57. https://doi.org/10.3390/genes12010057
APA StyleBao, X., Song, Y., Li, T., Zhang, S., Huang, L., Zhang, S., Cao, J., Liu, X., & Zhang, J. (2021). Comparative Transcriptome Profiling of Ovary Tissue between Black Muscovy Duck and White Muscovy Duck with High- and Low-Egg Production. Genes, 12(1), 57. https://doi.org/10.3390/genes12010057







