Prevalence and Spectrum of BRCA Germline Variants in Central Italian High Risk or Familial Breast/Ovarian Cancer Patients: A Monocentric Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
- -
- Knowledge of pathogenetic mutation in the family
- -
- Males affected by breast cancer
- -
- Women with breast and ovarian cancer
- -
- Women affected by breast cancer <36 years old
- -
- Women affected by triple negative breast cancer <60 years old
- -
- Women with bilateral breast cancer <50 years old
- -
- Women with breast cancer <50 years old AND first degree familiarity of:
- Breast cancer <50 years old
- No-mucinous, no-border line ovarian cancer (all ages)
- Bilateral breast cancer
- Male breast cancer
- Pancreatic cancer
- Prostate cancer
2.2. BRCA1/2 Analysis
2.3. Variant Classification
2.4. Data Collection and Statistical Analysis
2.5. Immunohistochemistry Analysis of Breast Tumor Samples
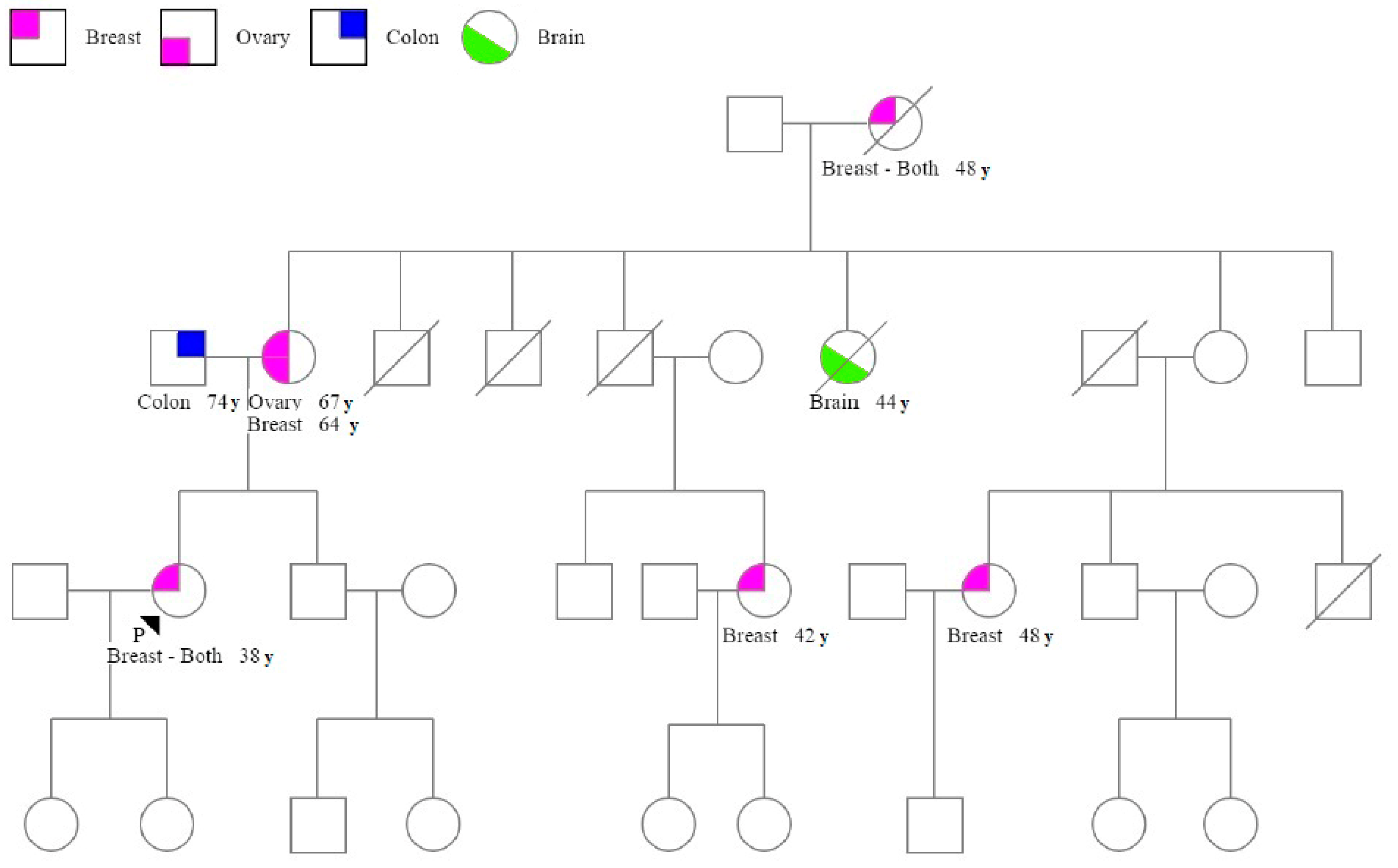
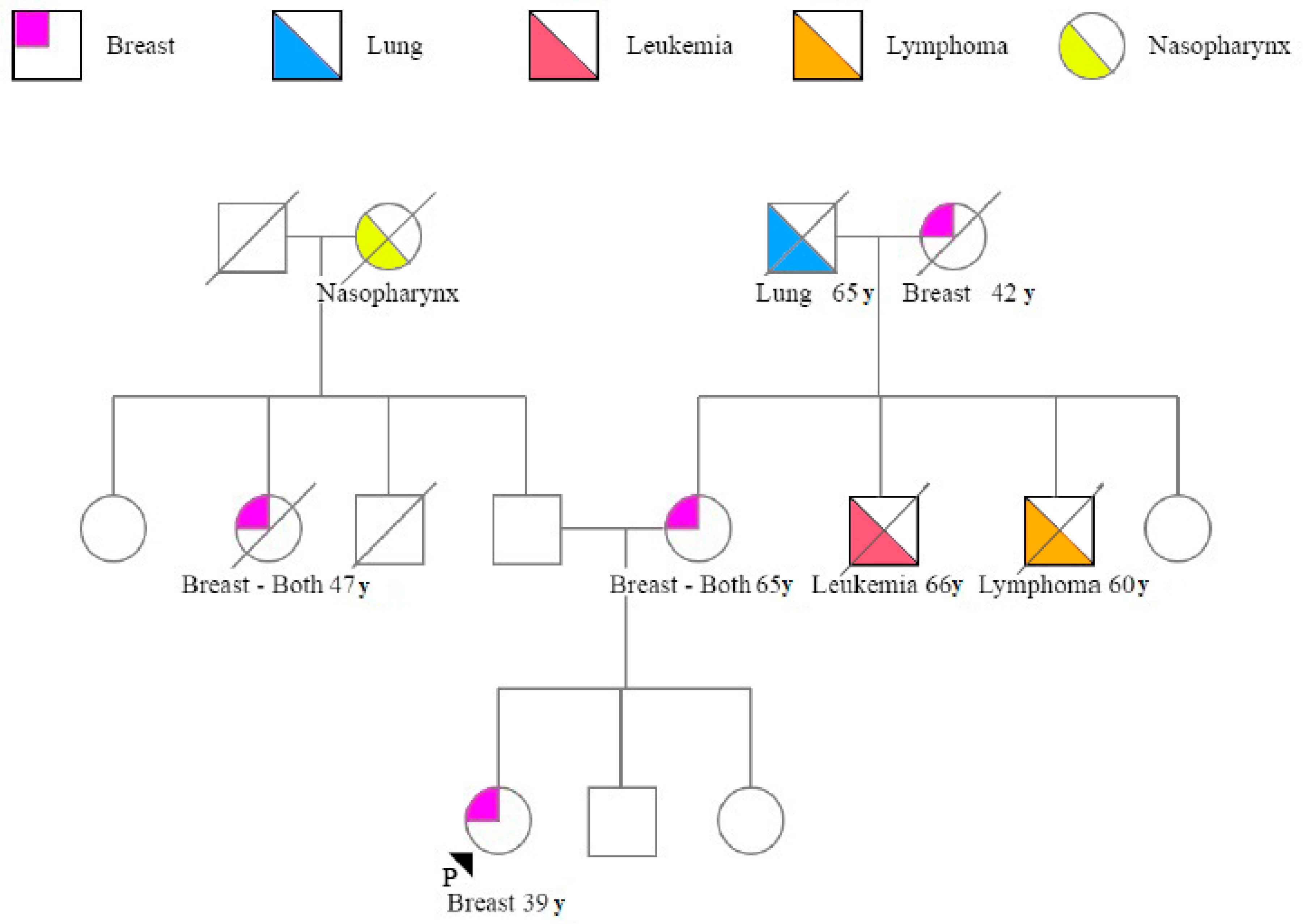
3. Results
3.1. Patient Characteristics
3.2. BRCA Variants and Patient Characteristics
3.3. Cohort Spectrum and Variant Detection Rate
3.4. Recurrent Pathogenic/Likely-Pathogenic BRCA1/2 Variants
3.5. Characteristics of Breast Cancer in BRCA Carrier Patients
3.6. Characteristics of Breast Cancer in Patients with VUS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferla, R.; Calo, V.; Cascio, S.; Rinaldi, G.; Badalamenti, G.; Carreca, I.; Surmacz, E.; Colucci, G.; Bazan, V.; Russo, A. Founder variants in BRCA1 and BRCA2 genes. Ann. Oncol. 2007, 18, vi93–vi98. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Haile, R.W.; Borg, Å.; Malone, K.E.; Concannon, P.; Thomas, D.C.; Langholz, B.; Bernstein, L.; Olsen, J.H.; Lynch, C.F.; et al. Variation of Breast Cancer Risk Among BRCA1/2 Carriers. JAMA 2008, 299, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, Q.; Ge, Z.; Yu, N.; Xu, S.; Fan, X. Common BRCA1 and BRCA2 variants in breast cancer families: A meta-analysis from systematic review. Mol. Biol. Rep. 2012, 39, 2109–2118. [Google Scholar] [CrossRef]
- Judkins, T.; Rosenthal, E.; Arnell, C.; Burbidge, L.A.; Geary, W.; Barrus, T.; Schoenberger, J.; Trost, J.; Wenstrup, R.J.; Roa, B.B. Clinical significance of large rearrangements in BRCA1 and BRCA2. Cancer 2012, 118, 5210–5216. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Variant Carriers. JAMA 2017, 317, 2402. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Wubbenhorst, B.; D’Andrea, K.; Garman, B.; Long, J.M.; Powers, J.; Rathbun, K.; Stopfer, J.E.; Zhu, J.; Bradbury, A.R.; et al. Prevalence of variants in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet. Med. 2015, 17, 630–638. [Google Scholar] [CrossRef]
- Schon, K.; Tischkowitz, M. Clinical implications of germline variants in breast cancer: TP53. Breast Cancer Res. Treat. 2018, 167, 417–423. [Google Scholar] [CrossRef]
- Pinto, C.; Mangone, L. Epidemiology of cancer in Italy: From real data to the need for cancer networks. Recenti Prog. Med. 2016, 107, 505–506. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 310–320. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. ESMO Guidelines Committee Prevention and screening in BRCA variant carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27, v103–v110. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Bella, M.A.; Capoluongo, E.; Carrera, P.; Clemente, C.; Colombo, N.; Cortesi, L.; De Rosa, G.; Fenizia, F.; Genuardi, M.; et al. Recommendations for the implementation of BRCA testing in the care and treatment pathways of ovarian cancer patients. Futur. Oncol. 2016, 12, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Underhill, C.; Toulmonde, M.; Bonnefoi, H. A review of PARP inhibitors: From bench to bedside. Ann. Oncol. 2011, 22, 268–279. [Google Scholar] [CrossRef]
- Linee Guida Neoplasie Della Mammella | AIOM. Available online: https://www.aiom.it/linee-guida-aiom-2018-neoplasie-della-mammella-11/ (accessed on 6 June 2019).
- Antoniou, A.C.; Easton, D.F. Models of genetic susceptibility to breast cancer. Oncogene 2006, 25, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- BRCAPRO | BayesMendel Lab. Available online: https://projects.iq.harvard.edu/bayesmendel/BRCApro (accessed on 6 June 2019).
- Parmigiani, G.; Berry, D.; Aguilar, O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am. J. Hum. Genet. 1998, 62, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, J.; Duffy, S.W.; Cuzick, J. A breast cancer prediction model incorporating familial and personal risk factors. Stat. Med. 2004, 23, 1111–1130. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Plon, S.E.; Eccles, D.M.; Easton, D.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.L.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V.; et al. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 29, 1282–1291. [Google Scholar] [CrossRef]
- Szabo, C.; Masiello, A.; Ryan, J.F.; Brody, L.C. The Breast Cancer Information Core: Database design, structure, and scope. Hum. Mutat. 2000, 16, 123–131. [Google Scholar] [CrossRef]
- Caputo, S.; Benboudjema, L.; Sinilnikova, O.; Rouleau, E.; Béroud, C.; Lidereau, R. French BRCA GGC Consortium Description and analysis of genetic variants in French hereditary breast and ovarian cancer families recorded in the UMD-BRCA1/BRCA2 databases. Nucleic Acids Res. 2012, 40, D992–D1002. [Google Scholar] [CrossRef]
- Fokkema, I.F.A.C.; Taschner, P.E.M.; Schaafsma, G.C.P.; Celli, J.; Laros, J.F.J.; den Dunnen, J.T. LOVD v.2.0: The next generation in gene variant databases. Hum. Mutat. 2011, 32, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef] [PubMed]
- den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.-F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E.M.; et al. On behalf of the Human Genome Variation Society (HGVS), the Human Variome Project (HVP), and the Human Genome Organisation (HUGO):HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 6, 564–569. [Google Scholar] [CrossRef]
- Bianconi, F.; Brunori, V.; Valigi, P.; La Rosa, F.; Stracci, F. Information technology as tools for cancer registry and regional cancer network integration. IEEE Trans. Syst. Man Cybern. Part A Syst. Hum. 2012, 42, 1410–1424. [Google Scholar] [CrossRef]
- Kim, Y.C.; Zhao, L.; Zhang, H.; Huang, Y.; Cui, J.; Xiao, F.; Downs, B.; Wang, S.M. Prevalence and spectrum of BRCA germline variants in mainland Chinese familial breast and ovarian cancer patients. Oncotarget 2016, 7, 9600–9612. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Tapia, T.; Perez-Moreno, E.; Gajardo-Meneses, P.; Ruiz, C.; Rios, M.; Missarelli, C.; Silva, M.; Cruz, A.; Matamala, L.; et al. BRCA1 and BRCA2 founder variants account for 78% of germline carriers among hereditary breast cancer families in Chile. Oncotarget 2017, 8, 74233–74243. [Google Scholar] [CrossRef]
- Sung, P.L.; Wen, K.C.; Chen, Y.J.; Chao, T.C.; Tsai, Y.F.; Tseng, L.M.; Qiu, J.T.T.; Chao, K.C.; Wu, H.H.; Chuang, C.M.; et al. The frequency of cancer predisposition gene variants in hereditary breast and ovarian cancer patients in Taiwan: From BRCA1/2 to multi-gene panels. PLoS ONE 2017, 12, e0185615. [Google Scholar] [CrossRef]
- Loizidou, M.A.; Hadjisavvas, A.; Pirpa, P.; Spanou, E.; Delikurt, T.; Tanteles, G.A.; Daniel, M.; Kountourakis, P.; Malas, S.; Ioannidis, G.; et al. BRCA1 and BRCA2 variant testing in Cyprus; a population based study. Clin. Genet. 2017, 91, 611–615. [Google Scholar] [CrossRef]
- Szabo, C.I.; King, M.C. Population genetics of BRCA1 and BRCA2. Am. J. Hum. Genet. 1997, 60, 1013–1020. [Google Scholar]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Metcalfe, K.; Lubinski, J.; Lynch, H.T.; Ghadirian, P.; Foulkes, W.D.; Kim-Sing, C.; Neuhausen, S.; Tung, N.; Rosen, B.; Gronwald, J.; et al. Family History of Cancer and Cancer Risks in Women with BRCA1 or BRCA2 Variants. J. Natl. Cancer Inst. 2010, 102, 1874–1878. [Google Scholar] [CrossRef]
- Stevens, K.N.; Vachon, C.M.; Couch, F.J. Genetic Susceptibility to Triple-Negative Breast Cancer. Cancer Res. 2013, 73, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; Gonzalez, L.D.; Vera-Badillo, F.E.; Tibau, A.; Goldstein, R.; Šeruga, B.; Srikanthan, A.; Pandiella, A.; Amir, E.; Ocana, A. Interaction between Hormonal Receptor Status, Age and Survival in Patients with BRCA1/2 Germline Variants: A Systematic Review and Meta-Regression. PLoS ONE 2016, 11, e0154789. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Zhang, P.; Fei, X.; Zong, Y.; Chen, X.; Huang, O.; He, J.-R.; Chen, W.; Li, Y.; et al. Prognostic and predictive value of Ki-67 in triple-negative breast cancer. Oncotarget 2016, 7, 31079–31087. [Google Scholar] [CrossRef]
- Roa, B.B.; Boyd, A.A.; Volcik, K.; Richards, C.S. Ashkenazi Jewish population frequencies for common variants in BRCA1 and BRCA2. Nat. Genet. 1996, 14, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Fackenthal, J.D.; Olopade, O.I. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat. Rev. Cancer 2007, 7, 937–948. [Google Scholar] [CrossRef]
- Azzollini, J.; Scuvera, G.; Bruno, E.; Pasanisi, P.; Zaffaroni, D.; Calvello, M.; Pasini, B.; Ripamonti, C.B.; Colombo, M.; Pensotti, V.; et al. Variant detection rates associated with specific selection criteria for BRCA1/2 testing in 1854 high-risk families: A monocentric Italian study. Eur. J. Intern. Med. 2016, 32, 65–71. [Google Scholar] [CrossRef]
- Finkelman, B.S.; Rubinstein, W.S.; Friedman, S.; Friebel, T.M.; Dubitsky, S.; Schonberger, N.S.; Shoretz, R.; Singer, C.F.; Blum, J.L.; Tung, N.; et al. Breast and ovarian cancer risk and risk reduction in Jewish BRCA1/2 variant carriers. J. Clin. Oncol. 2012, 30, 1321–1328. [Google Scholar] [CrossRef]
| BRCA1 | BRCA2 | BRCA1/2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogenic/Likely Pathogenic Variants | VUS | No Pathogenic Variants | Pathogenic/Likely Pathogenic Variants | VUS | No Pathogenic Variants | Pathogenic/Likely Pathogenic Variants | VUS | No Pathogenic Variants | |||
| *** Overall Central Italian individuals (N. %) | 363 (100.0) | 28 (7.7) | 9 (2.5) | 326 (89.8) | 23 (6.3) | 21 (5.8) | 319 (87.9) | 50 ** (13.8) | 28 (7.7) | 285 (78.5) | |
| Age at diagnosis, years | Median Range (Min-Max) | 47 (19–84) | 49 (22–69) | 54 (37–74) | 47 (19–84) | 48 (19–84) | 50 (27–72) | 47 (19–81) | 48 (19–84) | 51 (27–74) | 47 (19–81) |
| p-value * | 0.165 | 0.444 | 0.09 | ||||||||
| N (%) | |||||||||||
| Sex | |||||||||||
| Female | 351 (97.7) | 27 (96.4) | 8 (88.9) | 316 (96.9) | 22 (95.7) | 21 (100.0) | 308 (96.6) | 48 (96.0) | 27 (96.4) | 276 (96.8) | |
| Male | 12 (3.3) | 1 (3.6) | 1 (11.1) | 10 (3.1) | 1 (4.3) | 0 (0.0) | 11 (3.4) | 2 (4.0) | 1 (3.6) | 9 (3.2) | |
| p-value * | 0.411 | 0.665 | 0.961 | ||||||||
| Tumor Type | |||||||||||
| BC | |||||||||||
| First BC | 217 (59.9) | 15 (53.6) | 5 (55.6) | 197 (60.4) | 11 (47.8) | 13 (61.9) | 193 (60.5) | 25 (50.0) | 14 (57.1) | 176 (61.8) | |
| Second BC | 44 (12.1) | 4 (14.3) | 2 (22.2) | 38 (11.7) | 3 (13.0) | 7 (33.3) | 34 (10.7) | 7 (14.0) | 9 (32.2) | 28 (9.8) | |
| Third BC | 2 (0.5) | 0 (0.0) | 0 (0.0) | 2 (0.6) | 1 (4.4) | 0 (0.0) | 1 (0.3) | 1 (2.0) | 0 (0.0) | 1 (0.4) | |
| Other tumors | 16 (4.4) | 2 (7.1) | 1 (11.1) | 13 (4.0) | 2 (8.7) | 1 (4.8) | 13 (4) | 4 (8.0) | 2 (7.1) | 10 (3.5) | |
| No tumors | 84 (23.1) | 7 (25) | 1 (11.1) | 76 (23.3) | 6 (26.1) | 0 (0.0) | 78 (24.5) | 13 (26.0) | 1 (3.6) | 70 (24.5) | |
| p-value * | 0.898 | 0.006 | 0.006 | ||||||||
| OC | |||||||||||
| First OC | 3 (0.8) | 2 (7.1) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 3 (0.9) | 2 (4.0) | 0 (0.0) | 1 (0.3) | |
| Both BC and OC | 7 (1.9) | 5 (17.9) | 0 (0.0) | 2 (0.6) | 0 (0.0) | 1 (4.8) | 6 (1.9) | 5 (10.0) | 1 (3.6) | 1 (0.3) | |
| No | 269 (96.4) | 21 (75.0) | 9 (100.0) | 323 (99.1) | 23 (100.0) | 20 (95.2) | 310 (97.2) | 43 (86.0) | 27 (96.4) | 283 (99.3) | |
| p-value * | <0.001 | 0.779 | <0.001 | ||||||||
| Table 2 (A) List of BRCA1 and BRCA2 Pathogenic/Likely-Pathogenic Variants Detected on 50 Central Italian Individuals | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Gene | Exon/Intron | HGVS cDNA (BRCA1 NM_007294.3) (BRCA2 NM_000059.3) | HGVS Protein | Variant Type | IARC Classification | ClinVar | BRCA Share- BIC-LOVD | N. |
| 66,101 | BRCA1 | 2 | c.68_69delAG | p.(Glu23Valfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 2 |
| 315 | BRCA1 | 3 | c.116G > A | p.(Cys39Tyr) | Missense | Class-5 | Pathogenic | Pathogenic | 1 |
| 909 | BRCA1 | 5 | c.181T > G | p.(Cys61Gly) | Missense | Class-5 | Pathogenic | Pathogenic | 1 |
| 403 | BRCA1 | 11 | c.1999C > T | p.(Gln667Ter) | Nonsense | Class-5 | Pathogenic | Pathogenic | 1 |
| 833 | BRCA1 | 11 | c.3228_3229delAG | p.(Gly1077Alafs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| 265,287,471,524 | BRCA1 | 11 | c.2406_2409delGAGT | p.(Gln804Valfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 4 |
| 475,606,1341 | BRCA1 | 11 | c.3326-3329delAAAA | p.(Lys1109Serfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 3 |
| 223 | BRCA1 | 11 | c.3599_3600delAG | p.(Gln1200Argfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| 443 | BRCA1 | 12 | c.4117G > T | p.(Glu1373Ter) | Nonsense | Class-5 | Pathogenic | Pathogenic | 1 |
| 161 | BRCA1 | 16 | c.4964_4982del19 | p.(Ser1655Tyrfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| 270,300,358,1011 | BRCA1 | 17 | c.5062_5064delGTT | p.(Val1688del) | Inframe deletion | Class-5 | Pathogenic | Pathogenic | 4 |
| 50 | BRCA1 | 18 | c.5096G > A | p.(Arg1699Gln) | Missense | Class-5 | Pathogenic | Pathogenic | 1 |
| 47,150,746,938,943,609 | BRCA1 | 20 | c.5266dupC | p.(Gln1756Profs) | Frameshift insertion | Class-5 | Pathogenic | Pathogenic | 6 |
| 932 | BRCA1 | 23 | c.5445G > A | p.(Trp1815Ter) | Nonsense | Class-5 | Pathogenic | Pathogenic | 1 |
| 616 | BRCA2 | 2 | c.67 + 1G > A | - | Splicing | Class-5 | Pathogenic | Pathogenic | 1 |
| 606 | BRCA2 | 8 | c.632 − 2A > G | - | Splicing | Class-5 | Pathogenic | Pathogenic | 1 |
| 289 | BRCA2 | 8 | c.658_659delGT | p.(Val220Ilefs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| 426 | BRCA2 | 11 | c.3919delG | p.(Glu1307Lysfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| 352 | BRCA2 | 11 | c.4284dupT | p.(Gln1429Serfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| 959 | BRCA2 | 11 | c.5645C > A | p.(Ser1882Ter) | Nonsense | Class-5 | Pathogenic | Pathogenic | 1 |
| 865,946,1004 | BRCA2 | 11 | c.5722_5723delCT | p.(Leu1908Argfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 3 |
| 424 | BRCA2 | 11 | c.6039delA | p.(Val2014Tyrfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| 48,78,291,564,614,615 | BRCA2 | 11 | c.6313delA | p.(Ile2105Tyrfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 6 |
| 618 | BRCA2 | 17 | c.7828_7834delGTGGATC | p.(Val2610fs) | Frameshift deletion | Class-4 | - | - | 1 |
| 367 | BRCA2 | 17 | c.7852_7862delATTTGGGTTTA | p.(Ile2618fs) | Frameshift deletion | Class-4 | - | - | 1 |
| 260 | BRCA2 | 18 | c.8174G > A | p.(Trp2725Ter) | Nonsense | Class-5 | Pathogenic | Pathogenic | 1 |
| 393 | BRCA2 | 19 | c.8487 + 1G > A | - | Splicing | Class-5 | Pathogenic | UV/Pathogenic | 1 |
| 295 | BRCA2 | 20 | c.8537_8538delAG | p.(Glu2846Glyfs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| 640 | BRCA2 | 22 | c.8878C > T | p.(Gln2960Ter) | Nonsense | Class-5 | Pathogenic | Pathogenic | 1 |
| 571 | BRCA2 | 22 | c.8930delA | p.(Tyr2977Phefs) | Frameshift deletion | Class-5 | Pathogenic | Pathogenic | 1 |
| Table 2 (B) List of BRCA1 and BRCA2 Variant of Uncertain Significance (VUS) Variants Detected in 33 Central Italian Individuals | |||||||||
| Sample ID | Gene | Exon/Intron | HGVS cDNA (BRCA1 NM_007294.3) (BRCA2 NM_000059.3) | HGVS Protein | Variant Type | IARC Classification | Clin Var | BRCA Share-BIC-LOVD | N. |
| 879 | BRCA1 | 2 | c.-77delTGT (IVS0-77delTGT) | - | Intron | Class-3 | - | - | 1 |
| 733 | BRCA1 | 7 | c.335A > G | p.(Asn112Ser) | missense | Class-3 | - | VUS | 1 |
| 632 | BRCA1 | 11 | c.734A > T | p.(Asp245Val) | missense | Class-3 | VUS | VUS | 1 |
| 635 | BRCA1 | 11 | c.3711A > G | p.(Ile1237Met) | missense | Class-3 | VUS | VUS | 1 |
| 303 | BRCA1 | 12 | c.4132G > A | p.(Val1378Ile) | missense | Class-3 | VUS | VUS | 1 |
| 1013 | BRCA1 | 16 | c.4986 + 47A > G (IVS16+47A > G) | - | Intron | Class-3 | - | - | 1 |
| 635 | BRCA1 | 16 | c.4843G > A | p.(Ala1615Thr) | missense | Class-3 | VUS | VUS | 1 |
| 272,478 | BRCA1 | 20 | c.5277 + 60_5277 + 61insGTATTCCACTCC | - | Intron | Class-3 | VUS | Benign/VUS | 2 |
| 1012 | BRCA1 | 23 | c.5407-72delAAAA | - | Intron | Class-3 | - | - | 1 |
| 527 | BRCA2 | 2 | c.67 + 62T > G (IVS2+62T>G) | - | Intron | Class-3 | VUS | Benign/VUS | 1 |
| 553 | BRCA2 | 10 | c.1181A > C | p.(Glu394Ala) | missense | Class-3 | VUS | VUS | 1 |
| 886,930 | BRCA2 | 11 | c.4928T > C | p.(Val1643Ala) | missense | Class-3 | VUS | VUS | 2 |
| 633 | BRCA2 | 11 | c.4504C > A | p.(Gln1502Lys) | missense | Class-3 | - | - | 1 |
| 399,532,558,635,679 | BRCA2 | 11 | c.5972C > T | p.(Ala1991Val) | missense | Class-3 | VUS | VUS | 5 |
| 212,309 | BRCA2 | 11 | c.6131G > C | p.(Gly2044Ala) | missense | Class-3 | VUS | VUS | 2 |
| 423 | BRCA2 | 11 | c.6441C > G | p.His2147Gln) | missense | Class-3 | VUS | VUS | 1 |
| 259,296 | BRCA2 | 11 | c.6461A > C | p.(Tyr2154Ser) | missense | Class-3 | VUS | VUS | 2 |
| 752 | BRCA2 | 11 | c.6641C > T | p.(Thr2214Ile) | missense | Class-3 | VUS | VUS | 1 |
| 518 | BRCA2 | 15 | c.7505G > A | p.(Arg2502His) | missense | Class-3 | VUS | VUS | 1 |
| 367 | BRCA2 | 16 | c.7618-11delATTTT | - | Intron | Class-3 | - | - | 1 |
| 571 | BRCA2 | 25 | c.9275A > G | p.(Tyr3092Cys) | missense | Class-3 | VUS | VUS | 1 |
| 1012 | BRCA2 | 25 | c.9501 + 3A > T | - | Intron | Class-3 | VUS | VUS | 1 |
| 786 | BRCA2 | 26 | c.9648 + 84G > A | - | Intron | Class-3 | VUS | Likely Benign/VUS | 1 |
| 64 | BRCA2 | 27 | c.10024G > A | p.(Glu3342Lys) | missense | Class-3 | VUS | VUS | 1 |
| 1016 | BRCA2 | 27 | c.10095delinsGAATTATATCT | p.(Ser3366fs) | Frameshift deletion | Class-3 | VUS | Benign/VUS | 1 |
| BRCA1 | BRCA2 | BRCA1/2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variants | VUS | No Pathogenic Variants | Variants | VUS | No Pathogenic Variants | Variants | VUS | No Pathogenic Variants | |||
| *** Overall Central Italian individuals (N. %) | 263 (100) | 19 (7.2) | 7 (2.7) | 237 (90.1) | 15 (5.7) | 20 (7.6) | 228 (86.7) | 33 ** (12.6) | 25 (9.5) | 205 (77.9) | |
| Age at diagnosis, years | Median Range(Min-Max) | 46 (27–77) | 47 (31–63) | 47 (37–58) | 46 (27–77) | 44 (27–65) | 50 (34–68) | 46 (27–77) | 46 (27–65) | 48 (34–68) | 46 (27–77) |
| p-value * | 0.784 | 0.194 | 0.169 | ||||||||
| Histology | |||||||||||
| In situ carcinoma | 31 (11.8) | 0 (0.0) | 2 (28.6) | 29 (12.2) | 0 (0.0) | 6 (30.0) | 25 (11.0) | 0 (0.0) | 8 (32.0) | 23 (11.2) | |
| Invasive ductal carcinoma | 152 (57.8) | 13 (68.4) | 2 (28.6) | 137 (57.8) | 9 (60.0) | 8 (40.0) | 135 (59.2) | 21 (63.6) | 10 (40.0) | 121 (59.0) | |
| Invasive lobular carcinoma | 33 (12.6) | 3 (15.8) | 1 (14.2) | 29 (12.2) | 4 (26.7) | 1 (5.0) | 28 (12.3) | 7 (21.2) | 1 (4.0) | 25 (12.2) | |
| Other invasive hystotypes | 47 (17.8) | 3 (15.8) | 2 (28.6) | 42 (17.8) | 2 (13.3) | 5 (25.0) | 40 (17.5) | 5 (15.2) | 6 (24.0) | 36 (17.6) | |
| p-value * | 0.418 | 0.047 | 0.005 | ||||||||
| Grading | |||||||||||
| Well-differentiated | 21 (8.0) | 4 (21,1) | 2 (28.6) | 35 (14.8) | 2 (13.2) | 4 (20.0) | 35 (15.4) | 6 (18.2) | 6 (24.0) | 29 (51.7) | |
| Moderately differentiated | 100 (38.0) | 5 (26.3) | 2 (28.6) | 93 (39.2) | 7 (46.8) | 8 (40.0) | 85 (37.3) | 12 (36.4) | 9 (36.0) | 12 (21.4) | |
| Poorly differentiated | 101 (38.4) | 10 (52.6) | 1 (14.2) | 90 (38.0) | 6 (40.0) | 5 (25.0) | 90 (39.5) | 15 (45.4) | 6 (24.0) | 15 (15.9) | |
| Missing | 41 (15.6) | 0 (0.0) | 2 (28.6) | 19 (8.0) | 0 (0.0) | 3 (15.0) | 18 (7.8) | 0 (0.0) | 4 (16.0) | 0 (0.0) | |
| p-value * | 0.149 | 0.663 | 0.232 | ||||||||
| Stage | |||||||||||
| 0 | 23 (8.8) | 0 (0.0) | 0 (0.0) | 23 (9.7) | 0 (0.0) | 4 (20.0) | 19 (8.3) | 0 (0.0) | 4 (16.0 | 19 (9.3) | |
| I | 104 (39.5) | 9 (47.4) | 2 (28.6) | 93 (39.2) | 8 (53.4) | 6 (30.0) | 90 (39.5) | 16 (48.5) | 7 (28.0) | 81 (39.5) | |
| II | 65 (24.7) | 6 (31.6) | 3 (42.8) | 56 (23.6) | 3 (20.0) | 5 (25.0) | 57 (25.0) | 9 (27.2) | 7 (28.0) | 49 (23.9) | |
| III | 28 (10.7) | 1 (5.2) | 0 (0.0) | 27 (11.4) | 2 (13.3) | 1 (5.0) | 25 (11.0) | 3 (9.1) | 1 (4.0) | 24 (11.7) | |
| IV | 8 (3.0) | 0 (0.0) | 0 (0.0) | 8 (3.4) | 2 (13.3) | 0 (0.0) | 6 (2.6) | 2 (6.1) | 0 (0.0) | 6 (2.9) | |
| Missing | 35 (13.3) | 3 (15.8) | 2 (28.6) | 30 (12.7) | 0 (0.0) | 4 (20.0) | 31 (13.6) | 3 (9.1) | 6 (24.0) | 26 (12.7) | |
| p-value * | 0.619 | 0.134 | 0.288 | ||||||||
| Tumor invasiveness | |||||||||||
| In situ | 31 (11.8) | 0 (0.0) | 2 (28.6) | 29 (12.2) | 0 (0.0) | 6 (30.0) | 25 (11.0) | 0 (0.0) | 8 (32.0) | 23 (11.2) | |
| Invasive | 232 (88.2) | 19 (100.0) | 5 (71.4) | 208 (87.8) | 15 (100.0) | 14 (70.0) | 203 (89.0) | 33 (100.0) | 17 (68.0) | 182 (88.8) | |
| p-value | 0.106 | 0.014 | 0.001 | ||||||||
| Ki67 | |||||||||||
| High (≥14) | 146 (55.5) | 15 (78.9) | 3 (42.8) | 128 (54.0) | 13 (86.6) | 10 (40.0) | 123 (53.9) | 27 (81.8) | 11 (44.0) | 108 (52.7) | |
| Low (<14) | 56 (22.3) | 0 (0.0) | 2 (28.6) | 54 (22.8) | 1 (6.7) | 3 (15.0) | 52 (22.8) | 1 (3.0) | 5 (20.0) | 50 (24.4) | |
| Missing | 61 (23.2) | 4 (21.1) | 2 (28.6) | 55 (23.2) | 1 (6.7) | 7 (35.0) | 53 (23.3) | 5 (15.2) | 9 (36.0) | 47 (22.9) | |
| p-value * | 0.149 | 0.094 | 0.008 | ||||||||
| St. Gallen subtype | |||||||||||
| Luminal A | 78 (29.7) | 5 (26.3) | 2 (28.5) | 71 (30.0) | 11 (73.3) | 7 (35.0) | 60 (26.3) | 15 (45.5) | 7 (28.0) | 56 (27.3) | |
| Luminal B | 46 (17.5) | 0 (0.0) | 1 (14.3) | 45 (19.0) | 1 (6.7) | 2 (10.0) | 43 (18.8) | 1 (3.0) | 3 (12.0) | 42 (20.6) | |
| HER2 + | 13 (4.9) | 0 (0.0) | 0 (0.0) | 13 (5.5) | 0 (0.0) | 0 (0.0) | 13 (5.7) | 0 (0.0) | 0 (0.0) | 13 (6.3) | |
| Triple negative | 38 (14.4) | 10 (52.6) | 1 (14.3) | 27 (11.3) | 2 (13.3) | 3 (15.0) | 33 (14.5) | 12 (36.4) | 4 (16.0) | 22 (10.7) | |
| Missing | 88 (33.5) | 4 (21.1) | 3 (42.9) | 81 (34.2) | 1 (6.7) | 8 (40.0) | 79 (34.7) | 5 (15.1) | 11 (44.0) | 72 (35.1) | |
| p-value * | 0.001 | 0.02 | <0.001 | ||||||||
| Exitus | |||||||||||
| Living | 250 (95.1) | 17 (89.5) | 7 (100.0) | 226 (95.3) | 14 (93.3) | 18 (90.0) | 218 (95.6) | 30 (90.9) | 23 92.0) | 197 (96.1) | |
| Dead | 13 (4.9) | 2 (10.5) | 0 (0.0) | 11 (4.6) | 1 (6.7) | 2 (10.0) | 10 (4.4) | 3 (9.1) | 2 (8.0) | 8 (3.9) | |
| p-value * | 0.434 | 0.513 | 0.382 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglietta, J.; Ludovini, V.; Bianconi, F.; Pistola, L.; Reda, M.S.; Al-Refaie, A.; Tofanetti, F.R.; Mosconi, A.; Minenza, E.; Anastasi, P.; et al. Prevalence and Spectrum of BRCA Germline Variants in Central Italian High Risk or Familial Breast/Ovarian Cancer Patients: A Monocentric Study. Genes 2020, 11, 925. https://doi.org/10.3390/genes11080925
Foglietta J, Ludovini V, Bianconi F, Pistola L, Reda MS, Al-Refaie A, Tofanetti FR, Mosconi A, Minenza E, Anastasi P, et al. Prevalence and Spectrum of BRCA Germline Variants in Central Italian High Risk or Familial Breast/Ovarian Cancer Patients: A Monocentric Study. Genes. 2020; 11(8):925. https://doi.org/10.3390/genes11080925
Chicago/Turabian StyleFoglietta, Jennifer, Vienna Ludovini, Fortunato Bianconi, Lorenza Pistola, Maria Sole Reda, Antonella Al-Refaie, Francesca Romana Tofanetti, Annamaria Mosconi, Elisa Minenza, Paola Anastasi, and et al. 2020. "Prevalence and Spectrum of BRCA Germline Variants in Central Italian High Risk or Familial Breast/Ovarian Cancer Patients: A Monocentric Study" Genes 11, no. 8: 925. https://doi.org/10.3390/genes11080925
APA StyleFoglietta, J., Ludovini, V., Bianconi, F., Pistola, L., Reda, M. S., Al-Refaie, A., Tofanetti, F. R., Mosconi, A., Minenza, E., Anastasi, P., Molica, C., Stracci, F., & Roila, F. (2020). Prevalence and Spectrum of BRCA Germline Variants in Central Italian High Risk or Familial Breast/Ovarian Cancer Patients: A Monocentric Study. Genes, 11(8), 925. https://doi.org/10.3390/genes11080925





