Glia and Neural Stem and Progenitor Cells of the Healthy and Ischemic Brain: The Workplace for the Wnt Signaling Pathway
Abstract
:1. Introduction
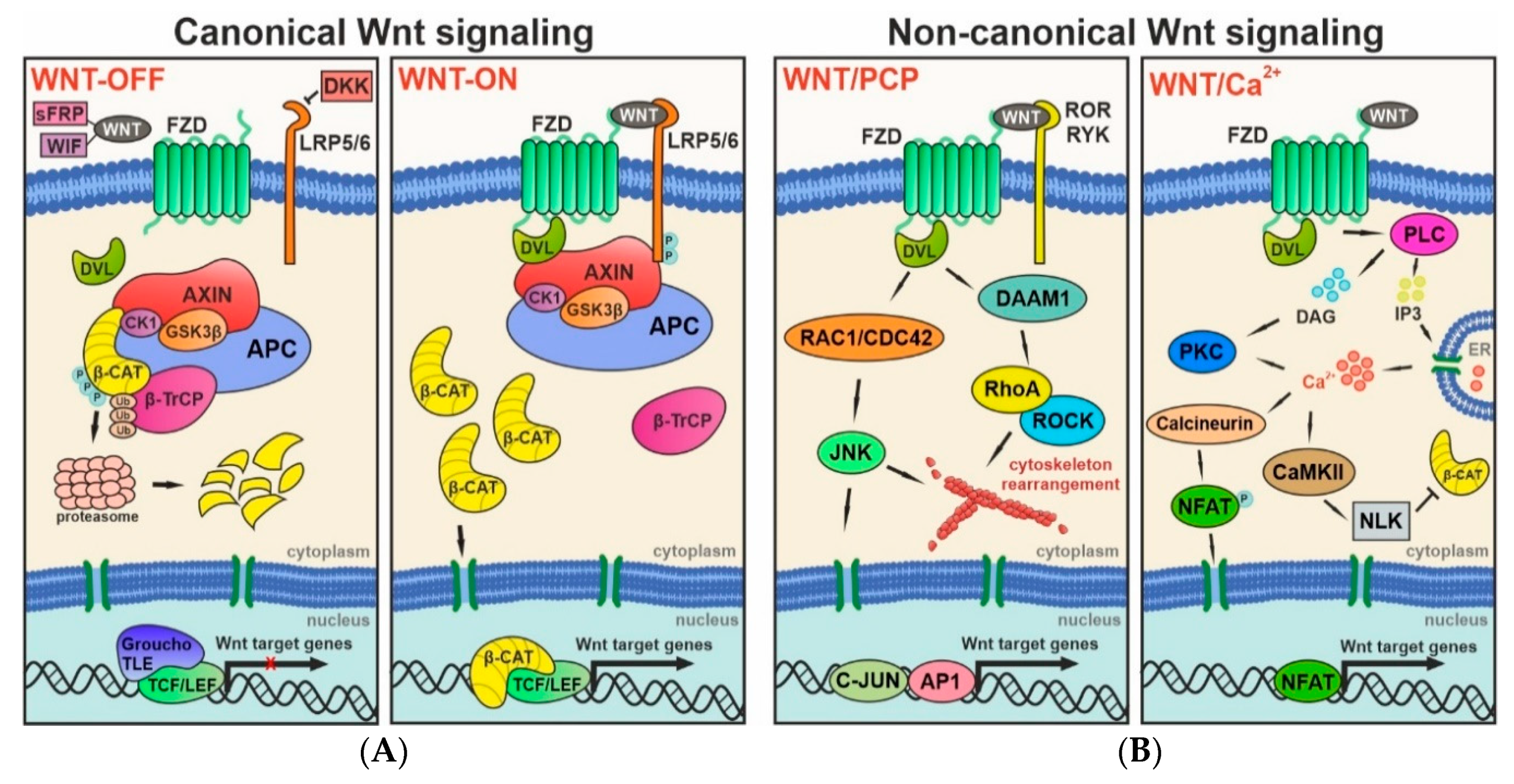
2. Wnt Signaling
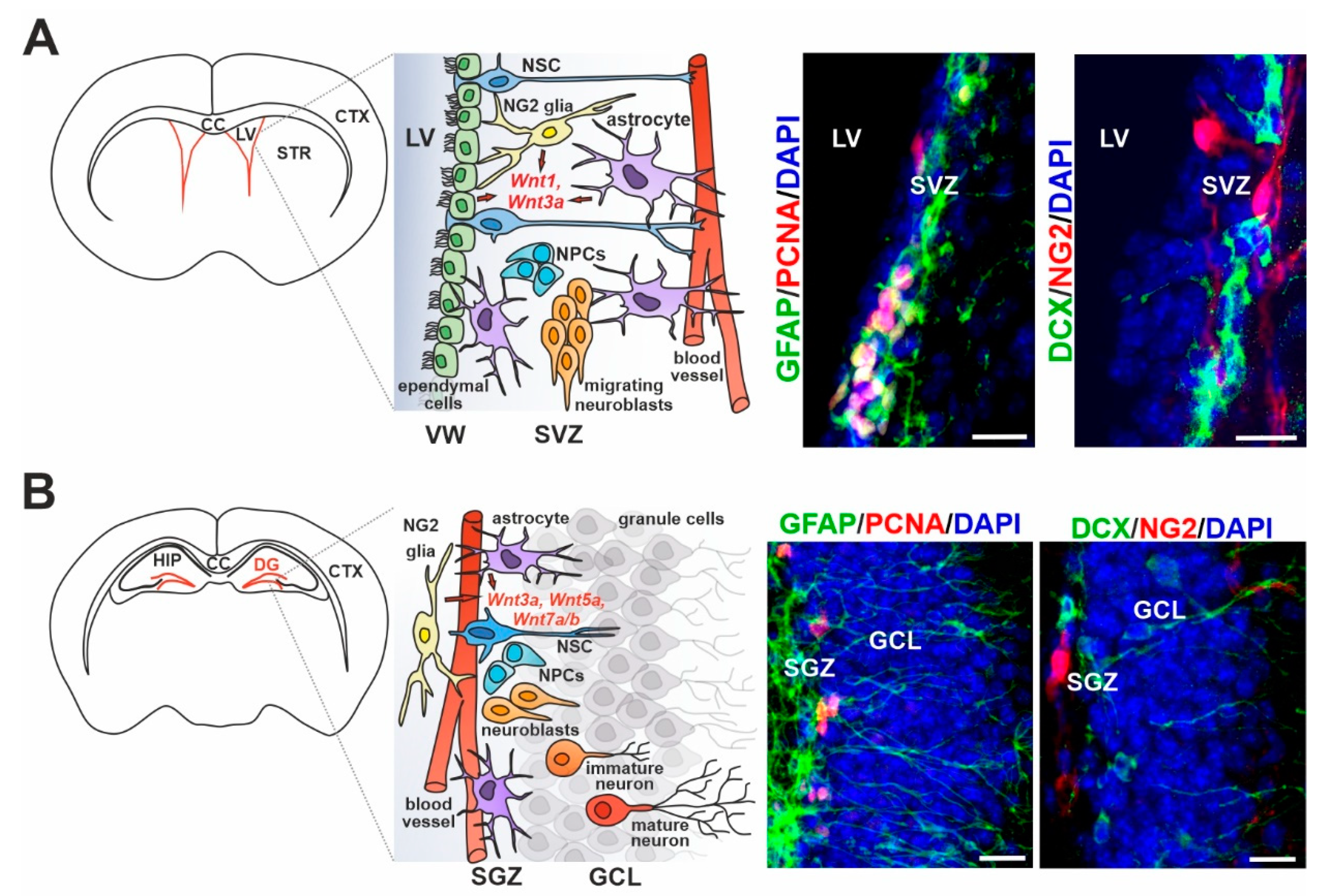
3. Wnt Signaling in Adult Neurogenesis of the Healthy Brain
4. Wnt Ligand Expression/Sensing in Particular Cell Types of the Adult Neurogenic Regions
5. Cerebral Ischemia
6. The Impact of Wnt Signaling on Different Cell Types during Ischemia
7. The Potential of Wnt Pathway Modulation in the Ischemic Stroke Therapy
8. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| AP1 | activator protein 1 |
| APC | adenomatous polyposis coli |
| ATP | adenosine triphosphate |
| ATP6AP2 | ATPase H+ transporting accessory protein 2 |
| AXIN | axis inhibition |
| β-CAT | β-catenin |
| β-TrCP | β-transducin repeats-containing protein |
| BBB | blood–brain barrier |
| Bcl2 | B-cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| BMP2/4 | bone morphogenetic proteins 2 and 4 |
| C-JUN | transcription factor C-JUN |
| Ca2+ | calcium |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CC | corpus callosum |
| CDC42 | GTPase CDC42 |
| CELSR1 | cadherin epidermal growth factor laminin G seven-pass G-type receptor 1 |
| CK1 | casein kinase 1 |
| Cl− | chloride |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| CTX | cortex |
| DAAM1 | DVL-associated activator of morphogenesis 1 |
| DAG | diacylglycerol |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DCX | doublecortin |
| DG | dentate gyrus |
| Disc1 | disrupted in schizophrenia 1 |
| DKK1/3 | dickkopf 1/3 |
| DVL | dishevelled |
| ER | endoplasmic reticulum |
| FACS | fluorescence-activated cell sorting |
| FCI | focal cerebral ischemia |
| FZD | frizzled |
| GCI | global cerebral ischemia |
| GCL | granule cell layer |
| GFAP | glial fibrillary acidic protein |
| GLI1,2,3 | glioma-associated oncogene 1,2,3 |
| GLP1 | glucagon-like peptide 1 |
| GSK3β | glycogen synthase kinase 3β |
| HIF1α | hypoxia-inducible factor 1α |
| HIP | hippocampus |
| Hipk1 | homeodomain interacting protein kinase 1 |
| IP3 | inositol trisphosphate |
| iPSCs | induced pluripotent stem cells |
| JNK | c-Jun N-terminal kinase |
| LGR5 | leucine-rich repeat-containing G protein-coupled receptor 5 |
| LiCl | lithium chloride |
| LINE1 | long interspersed nuclear elements 1 |
| LRP5/6 | low-density lipoprotein receptor-related protein 5/6 |
| LVs | lateral ventricles |
| MAPK | mitogen-activated protein kinase |
| MBP | myelin basic protein |
| MCAO | middle cerebral artery occlusion |
| Meg3 | maternally expressed gene 3 |
| miRNA | microRNA |
| MSCs | mesenchymal stem cells |
| Na+ | sodium |
| NeuroD1 | neurogenic differentiation 1 |
| NFAT | nuclear factor of activated T-cells |
| NG2 | neuron-glial antigen 2 |
| NLK | nemo-like kinase |
| NPCs | neural progenitor cells |
| NS/PCs | neural stem/progenitor cells |
| NSCs | neural stem cells |
| OPCs | oligodendrocyte precursor cells |
| P | phosphorylation |
| PCNA | proliferating cell nuclear antigen |
| PCP | planar cell polarity |
| PDGFRα | platelet-derived growth factor receptor α |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PKC | protein kinase C |
| PLC | phospholipase C |
| Prox1 | prospero-related homeodomain transcription factor 1 |
| RAC1 | Rac family small GTPase 1 |
| RhoA | Ras homolog family member A |
| ROCK | Rho-associated kinase |
| ROR | receptor tyrosine kinase ROR |
| ROS | reactive oxygen species |
| RT-PCR | reverse transcription polymerase chain reaction |
| RYK | receptor tyrosine kinase RYK |
| sFRPs | secreted FZD-related proteins |
| SGZ | subgranular zone |
| Shh | sonic hedgehog |
| siRNA | small interfering RNA |
| Sox2 | SRY-box transcription factor 2 |
| STR | striatum |
| SVZ | subventricular zone |
| TCF/LEF | T-cell factor/lymphoid enhancer-binding factor |
| TIMP1 | tissue inhibitor of metalloproteinases 1 |
| TLE | transducin-like enhancer of split |
| tPA | tissue plasminogen activator |
| Ub | ubiquitination |
| VEGF | vascular endothelial growth factor |
| VW | ventricular wall |
| WIF | Wnt inhibitory factor |
| Wip1 | wild-type p53-induced phosphatase 1 |
| WLS | Wntless |
References
- Hoseth, E.Z.; Krull, F.; Dieset, I.; Mørch, R.H.; Hope, S.; Gardsjord, E.S.; Steen, N.E.; Melle, I.; Brattbakk, H.R.; Steen, V.M.; et al. Exploring the Wnt signaling pathway in schizophrenia and bipolar disorder. Transl. Psychiatry 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Rojas, C.; Inestrosa, N. Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 1705. [Google Scholar] [CrossRef]
- L’Episcopo, F.; Tirolo, C.; Peruzzotti-Jametti, L.; Serapide, M.F.; Testa, N.; Caniglia, S.; Balzarotti, B.; Pluchino, S.; Marchetti, B. Neural stem cell grafts promote astroglia-driven neurorestoration in the aged parkinsonian brain via Wnt/β-catenin signaling. Stem Cells 2018, 36, 1179–1197. [Google Scholar] [CrossRef] [Green Version]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, L.; Liu, D.; Wen, H.; Hu, J.; Pang, T.; Sun, W.; Xu, E. Hypoxic postconditioning activates the Wnt/β-catenin pathway and protects against transient global cerebral ischemia through Dkk1 Inhibition and GSK-3β inactivation. FASEB J. 2019, 33, 9291–9307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Tang, J.; Li, S.Y.; Zhang, Y.Q.; Li, Y.; Dong, X.L. Involvement of the Wnt signaling pathway and cell apoptosis in the rat hippocampus following cerebral ischemia/reperfusion injury. Neural Regen. Res. 2013, 8, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kirdajova, D.; Anderova, M. NG2 cells and their neurogenic potential. Curr. Opin. Pharmacol. 2019, 50, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Valny, M.; Honsa, P.; Waloschkova, E.; Matuskova, H.; Kriska, J.; Kirdajova, D.; Androvic, P.; Valihrach, L.; Kubista, M.; Anderova, M. A single-cell analysis reveals multiple roles of oligodendroglial lineage cells during post-ischemic regeneration. Glia 2018, 66, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Bernstock, J.D.; Peruzzotti-Jametti, L.; Ye, D.; Gessler, F.A.; Maric, D.; Vicario, N.; Lee, Y.-J.; Pluchino, S.; Hallenbeck, J.M. Neural stem cell transplantation in ischemic stroke: A role for preconditioning and cellular engineering. J. Cereb. Blood Flow Metab. 2017, 37, 2314–2319. [Google Scholar] [CrossRef] [Green Version]
- Varela-Nallar, L.; Inestrosa, N.C. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Chae, W.-J.; Bothwell, A.L.M. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 2018, 39, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Komekado, H.; Yamamoto, H.; Chiba, T.; Kikuchi, A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells 2007, 12, 521–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bänziger, C.; Soldini, D.; Schütt, C.; Zipperlen, P.; Hausmann, G.; Basler, K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 2006, 125, 509–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Herreros, A.G.; Duñach, M. Intracellular signals activated by canonical Wnt ligands independent of GSK3 inhibition and β-catenin stabilization. Cells 2019, 8, 1148. [Google Scholar] [CrossRef] [Green Version]
- Van Amerongen, R. Alternative Wnt pathways and receptors. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [Green Version]
- Katoh, M. Canonical and non-canonical Wnt signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int. J. Oncol. 2017, 51, 1357–1369. [Google Scholar] [CrossRef] [Green Version]
- Hendrickx, G.; Boudin, E.; Verbeek, M.; Fransen, E.; Mortier, G.; Van Hul, W. Wnt16 requires Gα subunits as intracellular partners for both its canonical and non-canonical WNT signalling activity in osteoblasts. Calcif. Tissue Int. 2020, 106, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernández, E.; Velázquez, D.M.; Castañeda-Patlán, M.C.; Fuentes-García, G.; Fonseca-Camarillo, G.; Yamamoto-Furusho, J.K.; Romero-Avila, M.T.; García-Sáinz, J.A.; Robles-Flores, M. Canonical and non-canonical Wnt signaling are simultaneously activated by Wnts in colon cancer cells. Cell. Signal. 2020, 72. [Google Scholar] [CrossRef]
- Fan, J.; Wei, Q.; Liao, J.; Zou, Y.; Song, D.; Xiong, D.; Ma, C.; Hu, X.; Qu, X.; Chen, L.; et al. Noncanonical Wnt signaling plays an important role in modulating canonical Wnt-regulated stemness, proliferation and terminal differentiation of hepatic progenitors. Oncotarget 2017, 8, 27105–27119. [Google Scholar] [CrossRef] [Green Version]
- Brembeck, F.H.; Rosário, M.; Birchmeier, W. Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr. Opin. Genet. Dev. 2006, 16, 51–59. [Google Scholar] [CrossRef]
- Ladoux, B.; Nelson, W.J.; Yan, J.; Mège, R.M. The mechanotransduction machinery at work at adherens junctions. Integr. Biol. 2015, 7, 1109–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, M.S.; Singh, P.; Singh, G.; Jamwal, G.; Hussain, S.S.; Rana, A.; Akhter, Y.; Monga, S.P.; Dar, M.J. Terminal regions of β-catenin are critical for regulating its adhesion and transcription functions. Biochim. Biophys. Acta–Mol. Cell Res. 2016, 1863, 2345–2357. [Google Scholar] [CrossRef]
- Gao, J.; Liao, Y.; Qiu, M.; Shen, W. Wnt/β-catenin signaling in neural stem cell homeostasis and neurological diseases. Neuroscientist 2020. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Chen, Y.-G. Dishevelled: The hub of Wnt signaling. Cell. Signal. 2010, 22, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Cselenyi, C.S.; Jernigan, K.K.; Tahinci, E.; Thorne, C.A.; Lee, L.A.; Lee, E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of β-catenin. Proc. Natl. Acad. Sci. USA 2008, 105, 8032–8037. [Google Scholar] [CrossRef] [Green Version]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Krausova, M.; Korinek, V. Wnt signaling in adult intestinal stem cells and cancer. Cell. Signal. 2014, 26, 570–579. [Google Scholar] [CrossRef] [Green Version]
- The Wnt Homepage. Available online: http://web.stanford.edu/group/nusselab/cgi-bin/wnt/ (accessed on 25 June 2020).
- Bengoa-Vergniory, N.; Kypta, R.M. Canonical and noncanonical Wnt signaling in neural stem/progenitor cells. Cell. Mol. Life Sci. 2015, 72, 4157–4172. [Google Scholar] [CrossRef] [Green Version]
- Sugimura, R.; Li, L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res. C Embryo Today 2010, 90, 243–256. [Google Scholar] [CrossRef]
- Green, J.; Nusse, R.; Van Amerongen, R. The role of Ryk and Ror receptor tyrosine kinases in wnt signal transduction. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Ameyar, M.; Wisniewska, M.; Weitzman, J.B. A role for AP-1 in apoptosis: The case for and against. Biochimie 2003, 85, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, K.; McManus, E.J.; Hall, A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J. Cell Biol. 2007, 178, 355–361. [Google Scholar] [CrossRef]
- Ishitani, T.; Kishida, S.; Hyodo-Miura, J.; Ueno, N.; Yasuda, J.; Waterman, M.; Shibuya, H.; Moon, R.T.; Ninomiya-Tsuji, J.; Matsumoto, K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca2+ pathway to antagonize Wnt/β-catenin signaling. Mol. Cell. Biol. 2003, 23, 131–139. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, B.; Wu, W.; Davidson, G.; Marhold, J.; Li, M.; Mechler, B.M.; Dellus, H.; Hoppe, D.; Stannek, P.; Walter, C.; et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature 2002, 417, 664–667. [Google Scholar] [CrossRef]
- Okerlund, N.D.; Cheyette, B.N.R. Synaptic Wnt signaling—A contributor to major psychiatric disorders? J. Neurodev. Disord. 2011, 3, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Cselenyi, C.S.; Lee, E. Context-dependent activation or inhibition of Wnt-beta-Catenin signaling by kremen. Sci. Signal. 2008, 1, pe10. [Google Scholar] [CrossRef]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef] [Green Version]
- Prajerova, I.; Honsa, P.; Chvatal, A.; Anderova, M. Distinct effects of sonic hedgehog and Wnt-7a on differentiation of neonatal neural stem/progenitor cells in vitro. Neuroscience 2010, 171, 693–711. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, X.; Johnson, M.A.; Wang, Z.B.; LaVaute, T.; Zhang, S.C. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development 2009, 136, 4055–4063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballo, G.B.; Honorato, J.R.; De Lopes, G.P.F.; De Sampaio E Spohr, T.C.L. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, F.; Liu, Y.Y.; Zhao, C.H.; You, Y.; Wang, L.; Zhang, J.; Wei, B.; Ma, T.; Zhang, Q.; et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011, 21, 1534–1550. [Google Scholar] [CrossRef] [Green Version]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Mizrak, D.; Levitin, H.M.; Delgado, A.C.; Crotet, V.; Yuan, J.; Chaker, Z.; Silva-Vargas, V.; Sims, P.A.; Doetsch, F. Single-cell analysis of regional differences in adult V-SVZ neural stem cell lineages. Cell Rep. 2019, 26, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Berg, D.A.; Zhu, Y.; Shin, J.Y.; Song, J.; Bonaguidi, M.A.; Enikolopov, G.; Nauen, D.W.; Christian, K.M.; Ming, G.L.; et al. Single-cell RNA-seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell 2015, 17, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Urbán, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, T.; Hsieh, J.; Muotri, A.; Yeo, G.; Warashina, M.; Lie, D.C.; Moore, L.; Nakashima, K.; Asashima, M.; Gage, F.H. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009, 12, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Lie, D.C.; Colamarino, S.A.; Song, H.J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef]
- Wexler, E.M.; Paucer, A.; Kornblum, H.I.; Plamer, T.D.; Geschwind, D.H. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells 2009, 27, 1130–1141. [Google Scholar] [CrossRef]
- Qu, Q.; Sun, G.; Li, W.; Yang, S.; Ye, P.; Zhao, C.; Yu, R.T.; Gage, F.H.; Evans, R.M.; Shi, Y. Orphan nuclear receptor TLX activates Wnt/Β-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol. 2010, 12, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Adachi, K.; Mirzadeh, Z.; Sakaguchi, M.; Yamashita, T.; Nikolcheva, T.; Gotoh, Y.; Peltz, G.; Gong, L.; Kawase, T.; Alvarez-Buylla, A.; et al. β-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 2007, 25, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Ge, X.; Frank, C.L.; Madison, J.M.; Koehler, A.N.; Doud, M.K.; Tassa, C.; Berry, E.M.; Soda, T.; Singh, K.K.; et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell 2009, 136, 1017–1031. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Sun, G.; Murai, K.; Ye, P.; Li, W.; Asuelime, G.; Cheung, Y.-T.; Shi, Y. Wnt7a regulates multiple steps of neurogenesis. Mol. Cell. Biol. 2013, 33, 2551–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karalay, Ö.; Doberauer, K.; Vadodaria, K.C.; Knobloch, M.; Berti, L.; Miquelajauregui, A.; Schwark, M.; Jagasia, R.; Taketo, M.M.; Tarabykin, V.; et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- Marinaro, C.; Pannese, M.; Weinandy, F.; Sessa, A.; Bergamaschi, A.; Taketo, M.M.; Broccoli, V.; Comi, G.; Götz, M.; Martino, G.; et al. Wnt signaling has opposing roles in the developing and the adult brain that are modulated by Hipk1. Cereb. Cortex 2012, 22, 2415–2427. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Inoue, K.; Iwamura, H.; Terashima, K.; Soya, H.; Asashima, M.; Kuwabara, T. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J. 2011, 25, 3570–3582. [Google Scholar] [CrossRef]
- Solberg, N.; Machon, O.; Krauss, S. Effect of canonical Wnt inhibition in the neurogenic cortex, hippocampus, and premigratory dentate gyrus progenitor pool. Dev. Dyn. 2008, 237, 1799–1811. [Google Scholar] [CrossRef]
- Jang, M.-H.; Bonaguidi, M.A.; Kitabatake, Y.; Sun, J.; Song, J.; Kang, E.; Jun, H.; Zhong, C.; Su, Y.; Guo, J.U.; et al. Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 2013, 12, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Demidov, O.N.; Goh, A.M.; Virshup, D.M.; Lane, D.P.; Bulavin, D.V. Phosphatase WIP1 regulates adult neurogenesis and WNT signaling during aging. J. Clin. Investig. 2014, 124, 3263–3273. [Google Scholar] [CrossRef]
- Seib, D.R.M.; Corsini, N.S.; Ellwanger, K.; Plaas, C.; Mateos, A.; Pitzer, C.; Niehrs, C.; Celikel, T.; Martin-Villalba, A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell 2013, 12, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Marzo, A.; Galli, S.; Lopes, D.; McLeod, F.; Podpolny, M.; Segovia-Roldan, M.; Ciani, L.; Purro, S.; Cacucci, F.; Gibb, A.; et al. Reversal of synapse degeneration by restoring Wnt signaling in the adult hippocampus. Curr. Biol. 2016, 26, 2551–2561. [Google Scholar] [CrossRef] [Green Version]
- Kase, Y.; Otsu, K.; Shimazaki, T.; Okano, H. Involvement of p38 in age-related decline in adult neurogenesis via modulation of Wnt signaling. Stem Cell Rep. 2019, 12, 1313–1328. [Google Scholar] [CrossRef] [Green Version]
- Bevilaqua, L.R.M.; Kerr, D.S.; Medina, J.H.; Izquierdo, I.; Cammarota, M. Inhibition of hippocampal Jun N-terminal kinase enhances short-term memory but blocks long-term memory formation and retrieval of an inhibitory avoidance task. Eur. J. Neurosci. 2003, 17, 897–902. [Google Scholar] [CrossRef]
- Reinecke, K.; Herdegen, T.; Eminel, S.; Aldenhoff, J.B.; Schiffelholz, T. Knockout of c-Jun N-terminal kinases 1, 2 or 3 isoforms induces behavioural changes. Behav. Brain Res. 2013, 245, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Farías, G.G.; Alfaro, I.E.; Cerpa, W.; Grabowski, C.P.; Godoy, J.A.; Bonansco, C.; Inestrosa, N.C. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J. Biol. Chem. 2009, 284, 15857–15866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-Nallar, L.; Alfaro, I.E.; Serrano, F.G.; Parodi, J.; Inestrosa, N.C. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc. Natl. Acad. Sci. USA 2010, 107, 21164–21169. [Google Scholar] [CrossRef] [Green Version]
- Slater, P.G.; Ramirez, V.T.; Gonzalez-Billault, C.; Varela-Nallar, L.; Inestrosa, N.C. Frizzled-5 receptor is involved in neuronal polarity and morphogenesis of hippocampal neurons. PLoS ONE 2013, 8, e78892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raivich, G.; Bohatschek, M.; Da Costa, C.; Iwata, O.; Galiano, M.; Hristova, M.; Nateri, A.S.; Makwana, M.; Riera-Sans, L.; Wolfer, D.P.; et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 2004, 43, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengoa-Vergniory, N.; Gorroño-Etxebarria, I.; González-Salazar, I.; Kypta, R.M. A switch from canonical to noncanonical wnt signaling mediates early differentiation of human neural stem cells. Stem Cells 2014, 32, 3196–3208. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, S.B.; Guerrero, F.G.; Herrera-Soto, A.; Jensen-Flores, J.; Bustamante, D.B.; Oñate-Ponce, A.; Henny, P.; Varas-Godoy, M.; Inestrosa, N.C.; Varela-Nallar, L. Wnt5a promotes differentiation and development of adult-born neurons in the hippocampus by noncanonical Wnt signaling. Stem Cells 2020, 38, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Kim, J.H.; Song, G.S.; Jung, J.S. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol. Cell. Biochem. 2006, 288, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.T.; Han, J.; Pena, M.; Von Bohlen und Halbach, O.; Peters, J.; Gage, F.H. The Wnt adaptor protein ATP6AP2 regulates multiple stages of adult hippocampal neurogenesis. J. Neurosci. 2015, 35, 4983–4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavali, M.; Klingener, M.; Kokkosis, A.G.; Garkun, Y.; Felong, S.; Maffei, A.; Aguirre, A. Non-canonical Wnt signaling regulates neural stem cell quiescence during homeostasis and after demyelination. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H.; Loh, K.M.; Nusse, R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 2014, 62, 1377–1391. [Google Scholar] [CrossRef]
- Jäkel, S.; Dimou, L. Glial cells and their function in the adult brain: A journey through the history of their ablation. Front. Cell. Neurosci. 2017, 11, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Allen, N.D.; Skynner, M.; Gusterson, B.; Clark, A.J. Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia 2001, 34, 272–282. [Google Scholar] [CrossRef]
- Schreiner, B.; Romanelli, E.; Liberski, P.; Ingold-Heppner, B.; Sobottka-Brillout, B.; Hartwig, T.; Chandrasekar, V.; Johannssen, H.; Zeilhofer, H.U.; Aguzzi, A.; et al. Astrocyte depletion impairs redox homeostasis and triggers neuronal loss in the adult CNS. Cell Rep. 2015, 12, 1377–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Bolteus, A.J.; Balkin, D.M.; Henschel, O.; Bordey, A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia 2006, 54, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, T.; Andreu, Z.; Hortigüela, R.; Lie, D.C.; Mira, H. BMP and WNT signalling cooperate through LEF1 in the neuronal specification of adult hippocampal neural stem and progenitor cells. Sci. Rep. 2018, 8, 9241. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Estellés, M.; González-Gómez, P.; Hortigüela, R.; Díaz-Moreno, M.; San Emeterio, J.; Carvalho, A.L.; Fariñas, I.; Mira, H. Symmetric expansion of neural stem cells from the adult olfactory bulb is driven by astrocytes via WNT7A. Stem Cells 2012, 30, 2796–2809. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Götz, M. Glial control of neurogenesis. Curr. Opin. Neurobiol. 2017, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- ffrench-Constant, C.; Raff, M.C. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature 1986, 319, 499–502. [Google Scholar] [CrossRef]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Robins, S.C.; Villemain, A.; Liu, X.; Djogo, T.; Kryzskaya, D.; Storch, K.F.; Kokoeva, M.V. Extensive regenerative plasticity among adult NG2-glia populations is exclusively based on self-renewal. Glia 2013, 61, 1735–1747. [Google Scholar] [CrossRef]
- Birey, F.; Aguirre, A. Age-dependent netrin-1 signaling regulates NG2+ glial cell spatial homeostasis in normal adult gray matter. J. Neurosci. 2015, 35, 6946–6951. [Google Scholar] [CrossRef] [Green Version]
- Chari, D.M.; Crang, A.J.; Blakemore, W.F. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. J. Neuropathol. Exp. Neurol. 2003, 62, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.; Gruart, A.; Grade, S.; Zhang, Y.; Kröger, S.; Kirchhoff, F.; Eichele, G.; Delgado García, J.M.; Dimou, L. Decrease in newly generated oligodendrocytes leads to motor dysfunctions and changed myelin structures that can be rescued by transplanted cells. Glia 2016, 64, 2201–2218. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Kloc, M.; Chavali, M.; Hussein, I.; Wilson, M.; Christoffel, D.J.; Chen, T.; Frohman, M.A.; Robinson, J.K.; Russo, S.J.; et al. Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 2015, 88, 941–956. [Google Scholar] [CrossRef] [Green Version]
- Belachew, S.; Chittajallu, R.; Aguirre, A.A.; Yuan, X.; Kirby, M.; Anderson, S.; Gallo, V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J. Cell Biol. 2003, 161, 169–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, T.; Raff, M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 2000, 289, 1754–1757. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Fukaya, M.; Yang, J.K.; Rothstein, J.D.; Bergles, D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010, 68, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Rivers, L.E.; Young, K.M.; Rizzi, M.; Jamen, F.; Psachoulia, K.; Wade, A.; Kessaris, N.; Richardson, W.D. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008, 11, 1392–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Hill, R.A.; Dietrich, D.; Komitova, M.; Suzuki, R.; Nishiyama, A. Age-dependent fate and lineage restriction of single NG2 cells. Development 2011, 138, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, C.; Bergami, M.; Gascón, S.; Lepier, A.; Viganò, F.; Dimou, L.; Sutor, B.; Berninger, B.; Götz, M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014, 3, 1000–1014. [Google Scholar] [CrossRef] [Green Version]
- Honsa, P.; Pivonkova, H.; Dzamba, D.; Filipova, M.; Anderova, M. Polydendrocytes display large lineage plasticity following focal cerebral ischemia. PLoS ONE 2012, 7, e36816. [Google Scholar] [CrossRef] [Green Version]
- Honsa, P.; Valny, M.; Kriska, J.; Matuskova, H.; Harantova, L.; Kirdajova, D.; Valihrach, L.; Androvic, P.; Kubista, M.; Anderova, M. Generation of reactive astrocytes from NG2 cells is regulated by sonic hedgehog. Glia 2016, 64, 1518–1531. [Google Scholar] [CrossRef]
- Tatsumi, K.; Takebayashi, H.; Manabe, T.; Tanaka, K.F.; Makinodan, M.; Yamauchi, T.; Makinodan, E.; Matsuyoshi, H.; Okuda, H.; Ikenaka, K.; et al. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J. Neurosci. Res. 2008, 86, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ohayon, D.; Mckenzie, I.A.; Sinclair-Wilson, A.; Wright, J.L.; Fudge, A.D.; Emery, B.; Li, H.; Richardson, W.D. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 2016, 19, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.J.; Shen, W.; Ming, X.; Senatorov, V.V.; Chen, H.L.; Cheng, Y.; Hong, E.; Knoblach, S.; Gallo, V. SRY-Box containing gene 17 regulates the Wnt/β-catenin signaling pathway in oligodendrocyte progenitor cells. J. Neurosci. 2011, 31, 13921–13935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.A.; Patel, K.D.; Medved, J.; Reiss, A.M.; Nishiyama, A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J. Neurosci. 2013, 33, 14558–14566. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.-M.; Sun, S.; Wang, C.; Huang, H.; Hu, X.; Zhang, Z.; Lu, Q.R.; Qiu, M. Stage-specific regulation of oligodendrocyte development by Wnt/β-catenin signaling. J. Neurosci. 2014, 34, 8467–8473. [Google Scholar] [CrossRef]
- Ye, F.; Chen, Y.; Hoang, T.; Montgomery, R.L.; Zhao, X.; Bu, H.; Hu, T.; Taketo, M.M.; Van Es, J.H.; Clevers, H.; et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci. 2009, 12, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Feigenson, K.; Reid, M.; See, J.; Crenshaw, E.B.; Grinspan, J.B. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol. Cell. Neurosci. 2009, 42, 255–265. [Google Scholar] [CrossRef]
- Feigenson, K.; Reid, M.; See, J.; Crenshaw, E.B., III; Grinspan, J.B. Canonical Wnt signalling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN Neuro 2011, 3, e00061. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.; Maeda, Y.; Bannerman, P.; Xu, J.; Horiuchi, M.; Pleasure, D.; Guo, F. Adenomatous polyposis coli regulates oligodendroglial development. J. Neurosci. 2013, 33, 3113–3130. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Gascón, S.; Masserdotti, G.; Deshpande, A.; Simon, C.; Fischer, J.; Dimou, L.; Lie, D.C.; Schroeder, T.; Berninger, B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat. Cell Biol. 2013, 15, 602–613. [Google Scholar] [CrossRef] [Green Version]
- Azim, K.; Butt, A.M. GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 2011, 59, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Meffre, D.; Massaad, C.; Grenier, J. Lithium chloride stimulates plp and mbp expression in oligodendrocytes via wnt/β-catenin and akt/creb pathways. Neuroscience 2015, 284, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.N.; Van Amerongen, R.; Palmer, T.D.; Nusse, R. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin-responsive neural stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7324–7329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lie, D.C.; Dziewczapolski, G.; Willhoite, A.R.; Kaspar, B.K.; Shults, C.W.; Gage, F.H. The adult substantia nigra contains progenitor cells with neurogenic potential. J. Neurosci. 2002, 22, 6639–6649. [Google Scholar] [CrossRef]
- Shihabuddin, L.S.; Horner, P.J.; Ray, J.; Gage, F.H. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J. Neurosci. 2000, 20, 8727–8735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhonen, J.O.; Peterson, D.A.; Ray, J.; Gage, F.H. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 1996, 383, 624–627. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Martin-Villalba, A. Adult NSC diversity and plasticity: The role of the niche. Curr. Opin. Neurobiol. 2017, 42, 68–74. [Google Scholar] [CrossRef]
- Kriska, J.; Honsa, P.; Dzamba, D.; Butenko, O.; Kolenicova, D.; Janeckova, L.; Nahacka, Z.; Andera, L.; Kozmik, Z.; Taketo, M.M.; et al. Manipulating Wnt signaling at different subcellular levels affects the fate of neonatal neural stem/progenitor cells. Brain Res. 2016, 1651, 73–87. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, X.J.; Huang, H.; Guo, W.; Tang, T.; Xie, B.; Xu, X.; Zhang, Z.; Shen, Y.; Dai, Z.M.; et al. Wnt signaling represses astrogliogenesis via Ngn2-dependent direct suppression of astrocyte gene expression. Glia 2019, 67, 1333–1343. [Google Scholar] [CrossRef]
- Azim, K.; Akkermann, R.; Cantone, M.; Vera, J.; Jadasz, J.J.; Küry, P. Transcriptional profiling of ligand expression in cell specific populations of the adult mouse forebrain that regulates neurogenesis. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Corada, M.; Orsenigo, F.; Bhat, G.P.; Conze, L.L.; Breviario, F.; Cunha, S.I.; Claesson-Welsh, L.; Beznoussenko, G.V.; Mironov, A.A.; Bacigaluppi, M.; et al. Fine-tuning of Sox17 and canonical Wnt coordinates the permeability properties of the blood-brain barrier. Circ. Res. 2019, 124, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Laksitorini, M.D.; Yathindranath, V.; Xiong, W.; Hombach-Klonisch, S.; Miller, D.W. Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzadeh, Z.; Merkle, F.T.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paez-Gonzalez, P.; Abdi, K.; Luciano, D.; Liu, Y.; Soriano-Navarro, M.; Rawlins, E.; Bennett, V.; Garcia-Verdugo, J.M.; Kuo, C.T. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron 2011, 71, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Fernandez, C.; Arevalo-Martin, A.; Paniagua-Torija, B.; Ferrer, I.; Rodriguez, F.J.; Garcia-Ovejero, D. Wnts are expressed in the ependymal region of the adult spinal cord. Mol. Neurobiol. 2017, 54, 6342–6355. [Google Scholar] [CrossRef]
- Xing, L.; Anbarchian, T.; Tsai, J.M.; Plant, G.W.; Nusse, R. Wnt/β-catenin signaling regulates ependymal cell development and adult homeostasis. Proc. Natl. Acad. Sci. USA 2018, 115, E5954–E5962. [Google Scholar] [CrossRef] [Green Version]
- Ohata, S.; Nakatani, J.; Herranz-Pérez, V.; Cheng, J.G.; Belinson, H.; Inubushi, T.; Snider, W.D.; García-Verdugo, J.M.; Wynshaw-Boris, A.; Álvarez-Buylla, A. Loss of dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron 2014, 83, 558–571. [Google Scholar] [CrossRef] [Green Version]
- Sawamoto, K.; Wichterle, H.; Gonzalez-Perez, O.; Cholfin, J.A.; Yamada, M.; Spassky, N.; Murcia, N.S.; Garcia-Verdugo, J.M.; Marin, O.; Rubenstein, J.L.R.; et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 2006, 311, 629–632. [Google Scholar] [CrossRef]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.-C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Koh, S.H.; Park, H.H. Neurogenesis in stroke recovery. Transl. Stroke Res. 2017, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Brady, J.D.; Mohr, C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007, 10, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Rebai, O.; Amri, M. Chlorogenic acid prevents AMPA-mediated excitotoxicity in optic nerve oligodendrocytes through a PKC and caspase-dependent pathways. Neurotox. Res. 2018, 34, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Ischemic stroke and mitochondria: Mechanisms and targets. Protoplasma 2020, 257, 335–343. [Google Scholar] [CrossRef]
- Gelderblom, M.; Leypoldt, F.; Steinbach, K.; Behrens, D.; Choe, C.-U.; Siler, D.A.; Arumugam, T.V.; Orthey, E.; Gerloff, C.; Tolosa, E.; et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009, 40, 1849–1857. [Google Scholar] [CrossRef] [Green Version]
- Del Zoppo, G.J.; Hallenbeck, J.M. Advances in the vascular pathophysiology of ischemic stroke. Thromb. Res. 2000, 98, 73–81. [Google Scholar] [CrossRef]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.-B.; Zhuge, Q.; Zheng, W.; Shao, B.; Wang, B.; Sun, F.; Jin, K. Glial scar formation occurs in the human brain after ischemic stroke. Int. J. Med. Sci. 2014, 11, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Jansen, O.; Rohr, A. Neurothrombectomy in the treatment of acute ischaemic stroke. Nat. Rev. Neurol. 2013, 9, 645–652. [Google Scholar] [CrossRef]
- Stenman, J.M.; Rajagopal, J.; Carroll, T.J.; Ishibashi, M.; McMahon, J.; McMahon, A.P. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 2008, 322, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M.; et al. Wnt/β-catenin signaling controls development of the blood–brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Wang, R.; Wu, H.; Yang, S.; Qiu, Y. CPCGI confers neuroprotection by enhancing blood circulation and neurological function in cerebral ischemia/reperfusion rats. Mol. Med. Rep. 2019, 20, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, I. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J. Neurosci. 2005, 25, 2647–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastroiacovo, F.; Busceti, C.L.; Biagioni, F.; Moyanova, S.G.; Meisler, M.H.; Battaglia, G.; Caricasole, A.; Bruno, V.; Nicoletti, F. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Dashwood, W.M.; Zhong, X.; Nakagama, H.; Dashwood, R.H. Bcl-2 overexpression in PhIP-induced colon tumors: Cloning of the rat Bcl-2 promoter and characterization of a pathway involving β-catenin, c-Myc and E2F1. Oncogene 2007, 26, 6194–6202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Ge, M.; Han, Z.; Wang, S.; Yin, J.; Peng, L.; Xu, F.; Zhang, Q.; Dai, Z.; Xie, L.; et al. Wnt/β-catenin signaling pathway contributes to isoflurane postconditioning against cerebral ischemia-reperfusion injury and is possibly related to the transforming growth factorβ1/Smad3 signaling pathway. Biomed. Pharmacother. 2019, 110, 420–430. [Google Scholar] [CrossRef]
- Seifert-Held, T.; Pekar, T.; Gattringer, T.; Simmet, N.E.; Scharnagl, H.; Stojakovic, T.; Fazekas, F.; Storch, M.K. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis 2011, 218, 233–237. [Google Scholar] [CrossRef]
- Picard-Riera, N.; Nait-Oumesmar, B.; Baron-Van Evercooren, A. Endogenous adult neural stem cells: Limits and potential to repair the injured central nervous system. J. Neurosci. Res. 2004, 76, 223–231. [Google Scholar] [CrossRef]
- Piccin, D.; Morshead, C.M. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells 2011, 29, 528–538. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Zhang, J.Y.; Taylor, T.M.; Gu, X.; Zhao, Y.; Wei, L. Neuroprotective and regenerative roles of intranasal Wnt-3a administration after focal ischemic stroke in mice. J. Cereb. Blood Flow Metab. 2018, 38, 404–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shruster, A.; Ben-Zur, T.; Melamed, E.; Offen, D. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS ONE 2012, 7, e40843. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.N.; Zhang, L.M.; Sun, F.Y. β-Catenin siRNA inhibits ischemia-induced striatal neurogenesis in adult rat brain following a transient middle cerebral artery occlusion. Neurosci. Lett. 2008, 435, 108–112. [Google Scholar] [CrossRef]
- Lei, Z.-N.; Liu, F.; Zhang, L.-M.; Huang, Y.-L.; Sun, F.-Y. Bcl-2 increases stroke-induced striatal neurogenesis in adult brains by inhibiting BMP-4 function via activation of β-catenin signaling. Neurochem. Int. 2012, 61, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, Z.Z.; Zhang, J.Y.; Zhang, Y.; Won, S.; Sun, J.; Yu, S.P.; Li, J.; Wei, L. GSK-3β inhibition induced neuroprotection, regeneration, and functional recovery after intracerebral hemorrhagic stroke. Cell Transplant. 2017, 26, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.H.; Zhang, G.L.; Liu, X.Y.; Peng, A.; Ren, H.Y.; Huang, S.H.; Liu, T.; Wang, X.J. CELSR1 promotes neuroprotection in cerebral ischemic injury mainly through the Wnt/PKC signaling pathway. Int. J. Mol. Sci. 2020, 21, 1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Cheng, C.; Liu, Y.; Liu, N.; Lo, E.H.; Wang, X. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. 2018, 9, 945. [Google Scholar] [CrossRef]
- Qi, C.; Zhang, J.; Chen, X.; Wan, J.; Wang, J.; Zhang, P.; Liu, Y. Hypoxia stimulates neural stem cell proliferation by increasing HIF-1α expression and activating Wnt/β-catenin signaling. Cell. Mol. Biol. (Noisy-le-grand) 2017, 63, 12–19. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Yan, T.; Wu, H.; Feng, J.; Chen, H.; Gao, C.; Peng, T.; Yang, D.; Shen, J. Peroxynitrite enhances self-renewal, proliferation and neuronal differentiation of neural stem/progenitor cells through activating HIF-1α and Wnt/β-catenin signaling pathway. Free Radic. Biol. Med. 2018, 117, 158–167. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Shan, G. MiR-148b regulates proliferation and differentiation of neural stem cells via Wnt/β-catenin signaling in rat ischemic stroke model. Front. Cell. Neurosci. 2017, 11, 329. [Google Scholar] [CrossRef]
- Qiu, C.W.; Liu, Z.Y.; Hou, K.; Liu, S.Y.; Hu, Y.X.; Zhang, L.; Zhang, F.L.; Lv, K.Y.; Kang, Q.; Hu, W.Y.; et al. Wip1 knockout inhibits neurogenesis by affecting the Wnt/β-catenin signaling pathway in focal cerebral ischemia in mice. Exp. Neurol. 2018, 309, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liang, Y.; Zheng, S.; Zhang, H. Inhibition of myeloperoxidase by N-Acetyl lysyltyrosylcysteine amide reduces oxidative stress-mediated inflammation, neuronal damage, and neural stem cell injury in a murine model of stroke. J. Pharmacol. Exp. Ther. 2018, 364, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, D.; You, H. Repression of long non-coding RNA MEG3 restores nerve growth and alleviates neurological impairment after cerebral ischemia-reperfusion injury in a rat model. Biomed. Pharmacother. 2019, 111, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ko, H.M.; Kwon, K.J.; Lee, J.; Han, S.-H.; Han, D.W.; Cheong, J.H.; Ryu, J.H.; Shin, C.Y. tPA regulates neurite outgrowth by phosphorylation of LRP5/6 in neural progenitor cells. Mol. Neurobiol. 2014, 49, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.C.; Zhang, Z.G.; Wang, Y.; Zhang, R.L.; Gregg, S.; Liu, X.S.; Chopp, M. Wnt expression in the adult rat subventricular zone after stroke. Neurosci. Lett. 2007, 418, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, X.; Wu, Y.; Zhao, B.; Liu, X.; Pan, Y.; Liu, Y.; Ding, Y.; Qiu, M.; Wang, Y.Z.; et al. Wnt/β-catenin signaling mediates the seizure-facilitating effect of postischemic reactive astrocytes after pentylenetetrazole-kindling. Glia 2016, 64, 1083–1091. [Google Scholar] [CrossRef]
- Busceti, C.L.; Di Menna, L.; Bianchi, F.; Mastroiacovo, F.; Di Pietro, P.; Traficante, A.; Bozza, G.; Niehrs, C.; Battaglia, G.; Bruno, V.; et al. Dickkopf-3 causes neuroprotection by inducing vascular endothelial growth factor. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, Z.; Man, J.; Cui, K.; Fu, X.; Yu, L.; Gao, Y.; Liao, L.; Xiao, Q.; Guo, R.; et al. Wnt-3a alleviates neuroinflammation after ischemic stroke by modulating the responses of microglia/macrophages and astrocytes. Int. Immunopharmacol. 2019, 75. [Google Scholar] [CrossRef]
- Shang, Y.C.; Chong, Z.Z.; Hou, J.; Maiese, K. Wnt1, FoxO3a, and NF-κB oversee microglial integrity and activation during oxidant stress. Cell. Signal. 2010, 22, 1317–1329. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Zhang, X.; Chen, J.; Liu, X.; Xue, J.; Zhang, L.; Lan, X. Wnt canonical pathway activator TWS119 drives microglial anti-inflammatory activation and facilitates neurological recovery following experimental stroke. J. Neuroinflammation 2019, 16. [Google Scholar] [CrossRef]
- Halleskog, C.; Dijksterhuis, J.P.; Kilander, M.B.C.; Becerril-Ortega, J.; Villaescusa, J.C.; Lindgren, E.; Arenas, E.; Schulte, G. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J. Neuroinflammation 2012, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Shen, W.; Jin, L.; Pan, J.; Zhou, Y.; Pan, G.; Xie, Q.; Hu, Q.; Wu, S.; Zhang, H.; et al. Treadmill exercise promotes neurogenesis and myelin repair via upregulating Wnt/β–catenin signaling pathways in the juvenile brain following focal cerebral ischemia/reperfusion. Int. J. Mol. Med. 2020, 45, 1447–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicaise, A.M.; Johnson, K.M.; Willis, C.M.; Guzzo, R.M.; Crocker, S.J. TIMP-1 promotes oligodendrocyte differentiation through receptor-mediated signaling. Mol. Neurobiol. 2019, 56, 3380–3392. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Geng, J.; Qu, M.; Yuan, F.; Wang, Y.; Pan, J.; Li, Y.; Ma, Y.; Zhou, P.; Zhang, Z.; et al. Oligodendrocyte precursor cells transplantation protects blood–brain barrier in a mouse model of brain ischemia via Wnt/β-catenin signaling. Cell Death Dis. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 26 June 2020).
- Marei, H.E.; Hasan, A.; Rizzi, R.; Althani, A.; Afifi, N.; Cenciarelli, C.; Caceci, T.; Shuaib, A. Potential of stem cell-based therapy for ischemic stroke. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, D.; Ottani, A.; Neri, L.; Zaffe, D.; Grieco, P.; Jochem, J.; Cavallini, G.M.; Catania, A.; Guarini, S. Multiple beneficial effects of melanocortin MC4 receptor agonists in experimental neurodegenerative disorders: Therapeutic perspectives. Prog. Neurobiol. 2017, 148, 40–56. [Google Scholar] [CrossRef]
- Spaccapelo, L.; Galantucci, M.; Neri, L.; Contri, M.; Pizzala, R.; D’Amico, R.; Ottani, A.; Sandrini, M.; Zaffe, D.; Giuliani, D.; et al. Up-regulation of the canonical Wnt-3A and Sonic hedgehog signaling underlies melanocortin-induced neurogenesis after cerebral ischemia. Eur. J. Pharmacol. 2013, 707, 78–86. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, X.; Zhao, K.; Cui, L.; Wang, L.; Dong, L.; Li, Y.; Liu, Z.; Wang, C.; Zhang, X.; et al. Beneficial effects of sulindac in focal cerebral ischemia: A positive role in Wnt/β-catenin pathway. Brain Res. 2012, 1482, 71–80. [Google Scholar] [CrossRef]
- He, W.; Tian, X.; Lv, M.; Wang, H. Liraglutide protects neurite outgrowth of cortical neurons under oxidative stress though activating the Wnt pathway. J. Stroke Cerebrovasc. Dis. 2018, 27, 2696–2702. [Google Scholar] [CrossRef]
- Hu, Q.; Liang, X.; Chen, D.; Chen, Y.; Doycheva, D.; Tang, J.; Tang, J.; Zhang, J.H. Delayed hyperbaric oxygen therapy promotes neurogenesis through reactive oxygen species/hypoxia-inducible factor-1α/β-catenin pathway in middle cerebral artery occlusion rats. Stroke 2014, 45, 1807–1814. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.; Yao, Y. HBO promotes the differentiation of neural stem cells via interactions between the Wnt3/β-catenin and BMP2 signaling pathways. Cell Transplant. 2019, 28, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, J.; Chen, C.; Wang, W.; Wen, L.; Gao, K.; Chen, X.; Xiong, S.; Zhao, H.; Li, S. Wnt/β-catenin coupled with HIF-1α/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Sci. Rep. 2015, 5, 16151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, C.W.; Liu, Z.Y.; Zhang, F.L.; Zhang, L.; Li, F.; Liu, S.Y.; He, J.Y.; Xiao, Z.C. Post-stroke gastrodin treatment ameliorates ischemic injury and increases neurogenesis and restores the Wnt/β-Catenin signaling in focal cerebral ischemia in mice. Brain Res. 2019, 1712, 7–15. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Kang, Z.; Xu, Y.; Jiang, W.; Zhang, S. Cornin increases angiogenesis and improves functional recovery after stroke via the Ang1/Tie2 axis and the Wnt/β-catenin pathway. Arch. Pharm. Res. 2016, 39, 133–142. [Google Scholar] [CrossRef]
- Chen, B.; Tao, J.; Lin, Y.; Lin, R.; Liu, W.; Chen, L. Electro-acupuncture exerts beneficial effects against cerebral ischemia and promotes the proliferation of neural progenitor cells in the cortical peri-infarct area through the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2015, 36, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Lambert, C.; Cisternas, P.; Inestrosa, N.C. Role of Wnt signaling in central nervous system injury. Mol. Neurobiol. 2016, 53, 2297–2311. [Google Scholar] [CrossRef]
- Scott, E.L.; Brann, D.W. Estrogen regulation of Dkk1 and Wnt/β-Catenin signaling in neurodegenerative disease. Brain Res. 2013, 1514, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, M.; Wang, Y.; Li, Q.; Deng, G.; Wan, J.; Yang, Q.; Chen, Q.; Wang, J. GSK-3β inhibitor TWS119 attenuates rtPA-induced hemorrhagic transformation and activates the Wnt/β-catenin signaling pathway after acute ischemic stroke in rats. Mol. Neurobiol. 2016, 53, 7028–7036. [Google Scholar] [CrossRef]
- Libro, R.; Bramanti, P.; Mazzon, E. The role of the Wnt canonical signaling in neurodegenerative diseases. Life Sci. 2016, 158, 78–88. [Google Scholar] [CrossRef]
- Tran, F.H.; Zheng, J.J. Modulating the wnt signaling pathway with small molecules. Protein Sci. 2017, 26, 650–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knotek, T.; Janeckova, L.; Kriska, J.; Korinek, V.; Anderova, M. Glia and Neural Stem and Progenitor Cells of the Healthy and Ischemic Brain: The Workplace for the Wnt Signaling Pathway. Genes 2020, 11, 804. https://doi.org/10.3390/genes11070804
Knotek T, Janeckova L, Kriska J, Korinek V, Anderova M. Glia and Neural Stem and Progenitor Cells of the Healthy and Ischemic Brain: The Workplace for the Wnt Signaling Pathway. Genes. 2020; 11(7):804. https://doi.org/10.3390/genes11070804
Chicago/Turabian StyleKnotek, Tomas, Lucie Janeckova, Jan Kriska, Vladimir Korinek, and Miroslava Anderova. 2020. "Glia and Neural Stem and Progenitor Cells of the Healthy and Ischemic Brain: The Workplace for the Wnt Signaling Pathway" Genes 11, no. 7: 804. https://doi.org/10.3390/genes11070804
APA StyleKnotek, T., Janeckova, L., Kriska, J., Korinek, V., & Anderova, M. (2020). Glia and Neural Stem and Progenitor Cells of the Healthy and Ischemic Brain: The Workplace for the Wnt Signaling Pathway. Genes, 11(7), 804. https://doi.org/10.3390/genes11070804





