Differential Alternative Splicing Genes and Isoform Regulation Networks of Rapeseed (Brassica napus L.) Infected with Sclerotinia sclerotiorum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transcriptome Analysis
2.2. AS Landscape and Differential AS (DAS) Analysis
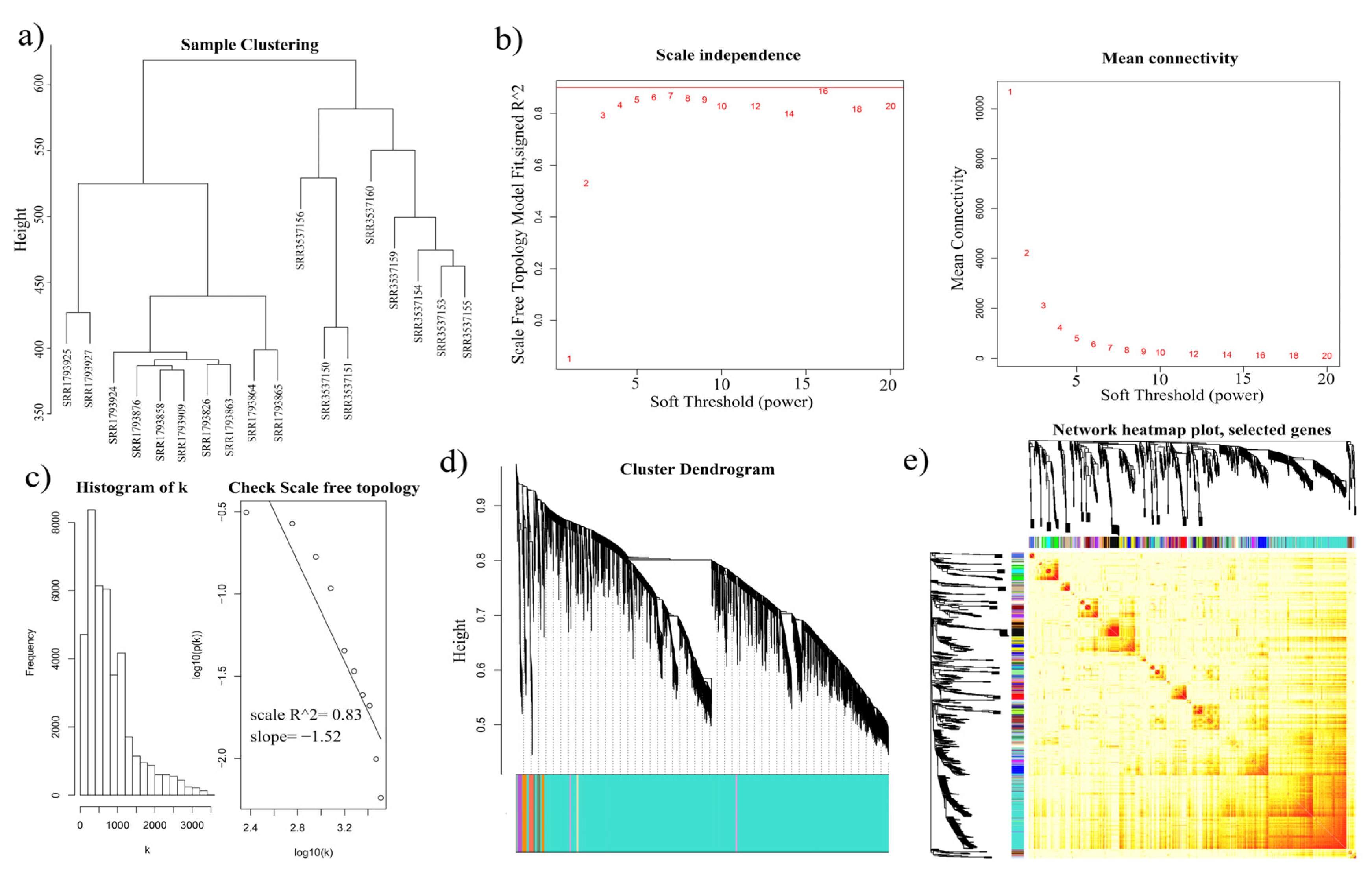
2.3. Weighted Gene CoExpression Network Analysis (WGCNA) of Isoforms
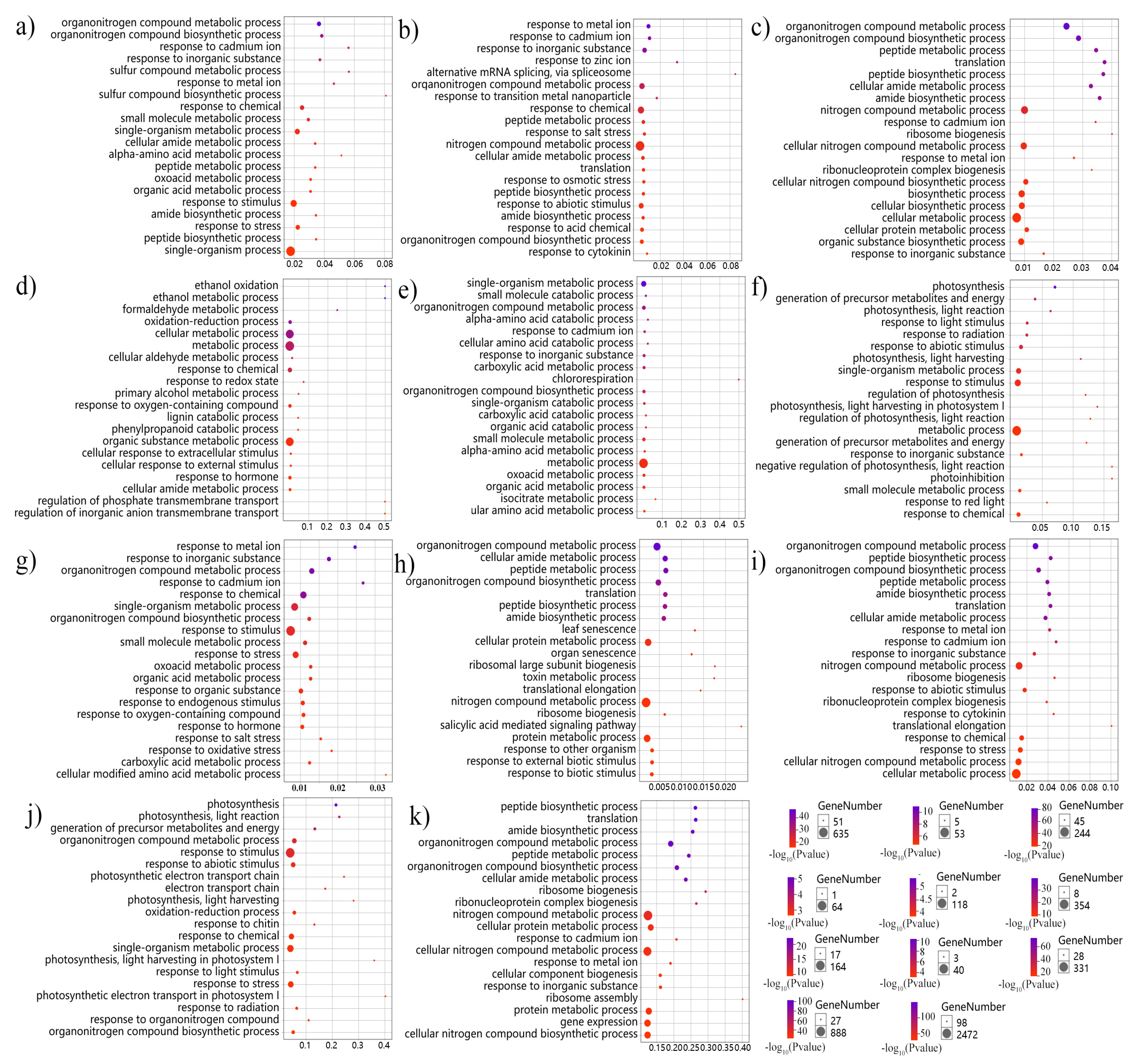
2.4. Functional Enrichment and Clustering
2.5. Homologous Gene Identification
3. Results
3.1. AS Landscape in B. napus in Response to S. sclerotiorum
3.2. Identification of DAS Genes
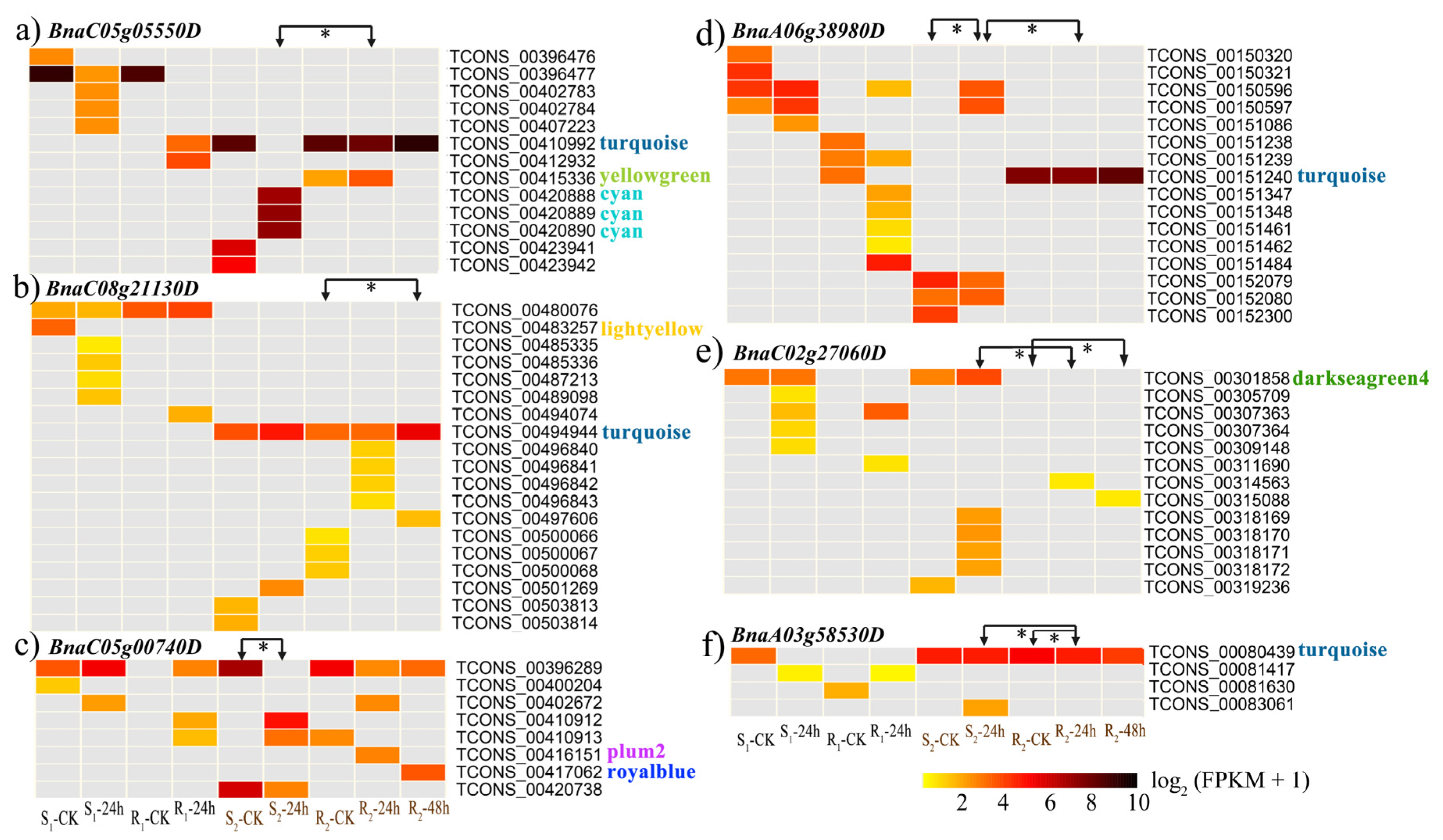
3.3. WGCNA of All Isoforms
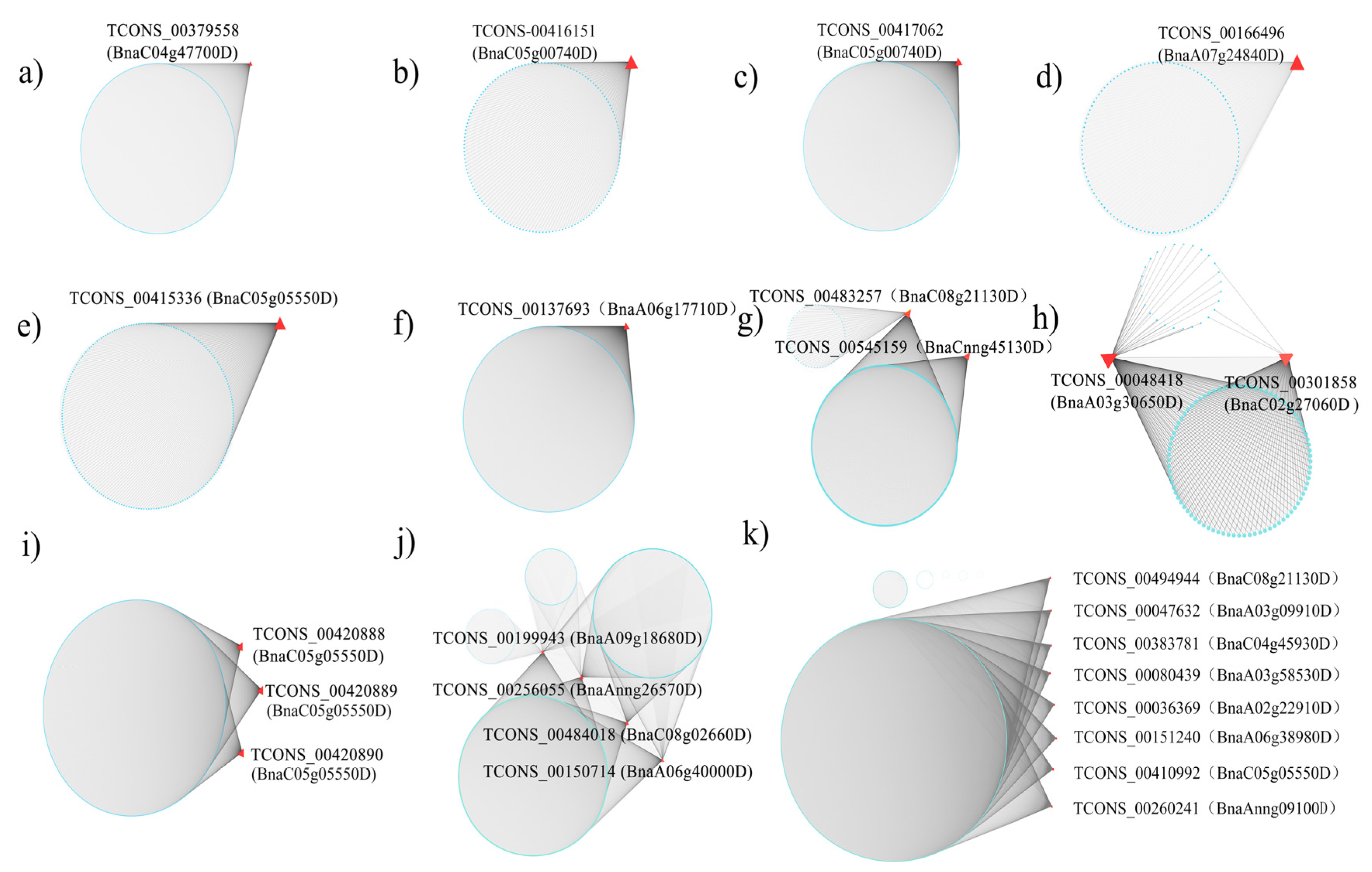
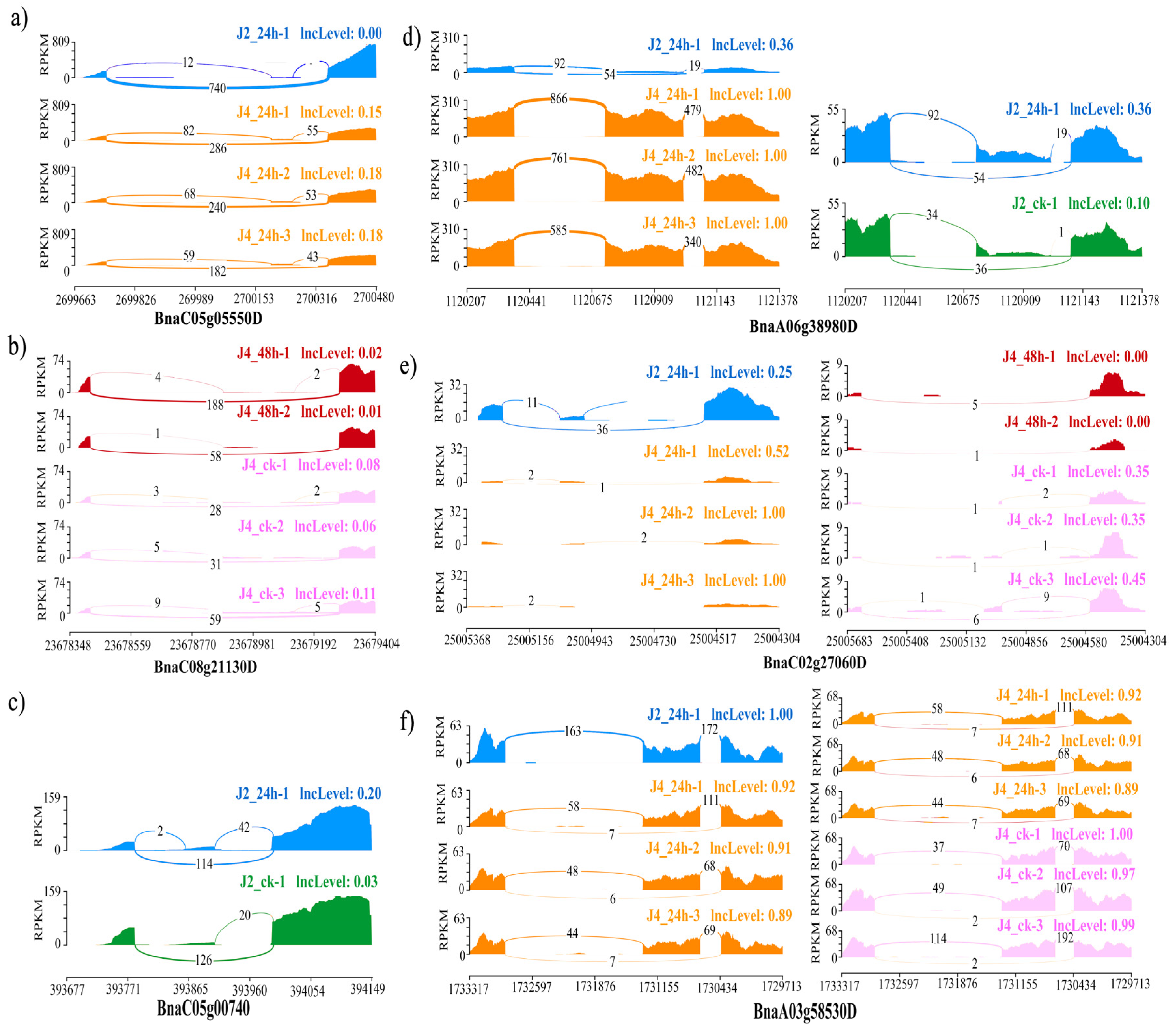
3.4. The Isoform Expression Landscape of Crucial DAS Genes
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science (New York) 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Free, S.J. Characterization of the Sclerotinia sclerotiorum cell wall proteome. Mol. Plant Pathol. 2015, 17, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 9 July 2020).
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, M.; Denton-Giles, M. The control of Sclerotinia stem rot on oilseed rape ( Brassica napus ): Current practices and future opportunities. Plant Pathol. 2016, 2016. 65, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ma, L.Y.; Cao, J.; Li, Y.L.; Ding, L.N.; Zhu, K.M.; Yang, Y.H.; Tan, X.L. Recent Advances in Mechanisms of Plant Defense to Sclerotinia sclerotiorum. Front. Plant Sci. 2019, 10, 1314. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 2006. 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Fenton, A.; Antonovics, J.; Brockhurst, M.A. Inverse-Gene-for-Gene Infection Genetics and Coevolutionary Dynamics. Am. Nat. 2009, 2009. 174, E230–E242. [Google Scholar] [CrossRef] [Green Version]
- Derbyshire, M.; Mbengue, M.; Barascud, M.; Navaud, O.; Raffaele, S. Small RNAs from the plant pathogenic fungus Sclerotinia sclerotiorum highlight host candidate genes associated with quantitative disease resistance. Mol. Plant Pathol. 2019, 20, 1279–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhao, Q.; Liu, S.; Shahid, M.; Lan, L.; Cai, G.; Zhang, C.; Fan, C.; Wang, Y.; Zhou, Y. Genome-wide Association Study Identifies New Loci for Resistance to Sclerotinia Stem Rot in Brassica napus. Front. Plant Sci. 2016, 7, 1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, D.; Mei, J.; Fu, Y.; Disi, J.O.; Li, J.; Qian, W. Quantitative trait loci analyses for resistance to Sclerotinia sclerotiorum and flowering time in Brassica napus. Mol. Breed. 2014, 34, 1797–1804. [Google Scholar] [CrossRef]
- Girard, I.J.; Tong, C.; Becker, M.G.; Mao, X.; Huang, J.; de Kievit, T.; Fernando, W.D.; Liu, S.; Belmonte, M.F. RNA sequencing of Brassica napus reveals cellular redox control of Sclerotinia infection. J. ExBot. 2017. [CrossRef] [PubMed] [Green Version]
- Joshi, R.K.; Megha, S.; Basu, U.; Rahman, M.H.; Kav, N.N.V. Genome Wide Identification and Functional Prediction of Long Non-Coding RNAs Responsive to Sclerotinia sclerotiorum Infection in Brassica napus. PLoS ONE 2016, 11, e0158784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calixto, C.P.G.; Guo, W.; James, A.B.; Tzioutziou, N.A.; Brown, J.W.S. Rapid and Dynamic Alternative Splicing Impacts the Arabidopsis Cold Response Transcriptome. Plant Cell 2018, 30, 1424–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, J.; Huang, B.O.; Xu, Y.M.; Li, J.; Huang, L.F.; Lin, J.; Zhang, J.; Min, Q.H.; Yang, W.M.; et al. Mechanism of alternative splicing and its regulation. Biomed. Rep. 2015, 3, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, N.H.; Kalyna, M.; Marquez, Y.; Barta, A.; Brown, J.W.S. Alternative splicing in plants--coming of age. Trends Plant Sci. 2012, 17, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Qin, J.; Tian, X.; Xu, S.; Wang, Y.; Li, H.; Wang, X.; Peng, H.; Yao, Y.; Hu, Z.; et al. Global profiling of alternative splicing landscape responsive to drought, heat and their combination in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2018, 16, 714–726. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.Q.; Wei, L.J.; Lin, A.; Zhang, C.; Sun, W.; Yang, B.; Lu, K.; Li, J.N. The Alternative Splicing Landscape of Brassica napus Infected with Leptosphaeria maculans. Genes 2019, 10, 296. [Google Scholar] [CrossRef] [Green Version]
- Mandadi, K.K.; Scholthof, K.B.G. Genome-Wide Analysis of Alternative Splicing Landscapes Modulated during Plant-Virus Interactions in Brachypodium distachyon. Plant Cell 2015, 27, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Gil, A. How did alternative splicing evolve? Nat. Rev. Genet. 2004, 5, 773–782. [Google Scholar] [CrossRef]
- Baralle, F.E.; Giudice, J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell Biol. 2017, 18, 437. [Google Scholar] [CrossRef]
- Breathnach, R.; Benoist, C.; O’Hare, K.; Gannon, F.; Chambon, P. Ovalbumin Gene: Evidence for a Leader Sequence in mRNA and DNA Sequences at the Exon-Intron Boundaries. Proc. Natl. Acad. Sci. 1978, 75, 4853–4857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staiger, D.; Brown, J.W.S. Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell 2013, 25, 3640–3656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.D.; Ares, M. Context-dependent control of alternative splicing by RNA-binding proteins. Nat. Rev. Genet. 2014, 15, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lin, W.-D.; Ray, P.; Lan, P.; Schmidt, W. Genome-Wide Detection of Condition-Sensitive Alternative Splicing in Arabidopsis Roots. Plant Physiol. 2013, 162, 1750–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczak, M.; Reiss, J.; Cooper, D.N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: Causes and consequences. Hum. Genet. 1992, 90, 41–54. [Google Scholar] [CrossRef]
- Yang, S.; Tang, F.; Zhu, H. Alternative Splicing in Plant Immunity. Int. J. Mol. Sci. 2014, 15, 10424–10445. [Google Scholar] [CrossRef] [Green Version]
- Dinesh-Kumar, S.P.; Baker, B.J. Alternatively spliced N resistance gene transcripts: Their possible role in tobacco mosaic virus resistance. Proc. Natl. Acad. Sci. USA 2000, 97, 1908–1913. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-C.; Gassmann, W. Alternative splicing and mRNA levels of the disease resistance gene RPS4 are induced during defense responses. Plant Physiol. 2007, 145, 1577–1587. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, X.; Liang, X.; Zhou, X.; Yang, F.; Liu, J.; He, S.Y.; Guo, Z. Alternative splicing of rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiol. 2016, 171, 1427–1442. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhao, Q.; Yang, Q.; Liu, H.; Li, Q.; Yi, X.; Cheng, Y.; Guo, L.; Fan, C.; Zhou, Y. Comparative transcriptomic analysis uncovers the complex genetic network for resistance to Sclerotinia sclerotiorum in Brassica napus. Sci. Rep. 2016, 6, 19007. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvain, F.; Michael, S. ASTALAVISTA: Dynamic and flexible analysis of alternative splicing events in custom gene datasets. Nucleic Acids Res. 2007, 35 (Suppl. 2), W297–W299. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Park, J.W.; Lu, Z.X.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. 2014, 111, E5593–E5601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Horvath, S. A General Framework For Weighted Gene Co-Expression Network Analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 17. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for Visualization and Analysis of Biological Networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenführer, J.; Lengauer, T. Improved scoring of functional groups from gene expression data by decorrelating go graph structure. Bioinformatics 2006, 22, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Schranz, M.E.; Lysak, M.A.; Mitchell-Olds, T. The ABC’s of comparative genomics in the Brassicaceae: Building blocks of crucifer genomes. Trends Plant Sci. 2006, 11, 535–542. [Google Scholar] [CrossRef]
- Murat, F.; Louis, A.; Maumus, F.; Armero, A.; Cooke, R.; Quesneville, H.; Crollius, H.R.; Salse, J. Understanding Brassicaceae evolution through ancestral genome reconstruction. Genome Biol. 2015, 16, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.-B.; Brendel, V. Genomewide comparative analysis of alternative splicing in plants. Proc. Natl. Acad. Sci. USA 2006, 103, 7175–7180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, T.; Lin, W.-D.; Chang, C.-L.; Matzke, M.; Matzke, A.J.M. A Genetic Screen Identifies PRP18a, a Putative Second Step Splicing Factor Important for Alternative Splicing and a Normal Phenotype in Arabidopsis thaliana. G3: Genes Genomes Genet. 2018, 8, 1367–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, Y.; Rosas-Diaz, T.; Caceres-Moreno, C.; Lozano-Duran, R.; Macho, A.P. The IMMUNE-ASSOCIATED NUCLEOTIDE-BINDING 9 Protein Is a Regulator of Basal Immunity in Arabidopsis thaliana. Mol. Plant. Microbe Interact. 2019, 32, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Li, M.; Dong, O.X.; Xia, S.; Liang, W.; Bao, Y.; Wasteneys, G.; Li, X. Differential regulation of TNL-mediated immune signaling by redundant helper CNLs. New Phytol. 2019, 222, 938–953. [Google Scholar] [CrossRef]
- Majewska, M.; Lipka, A.; Paukszto, L.; Jastrzebski, J.P.; Szeszko, K.; Gowkielewicz, M.; Lepiarczyk, E.; Jozwik, M.; Majewski, M.K. Placenta Transcriptome Profiling in Intrauterine Growth Restriction (IUGR). Int. J. Mol. Sci. 2019, 20, 1510. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, A.; Laiho, A.; Venäläinen, M.S.; McGlinchey, A.J.; Wang, N.; Elo, L.L. Systematic evaluation of differential splicing tools for RNA-seq studies. Brief. Bioinform. 2019. [Google Scholar] [CrossRef]
- Tan, S.; Wang, W.; Tian, C.; Niu, D.; Zhou, T.; Jin, Y.; Yang, Y.; Gao, D.; Dunham, R.; Liu, Z. Heat stress induced alternative splicing in catfish as determined by transcriptome analysis. Comp. Biochem. Physiol. Part D: Genom. Proteom. 2019, 29, 166–172. [Google Scholar] [CrossRef]
- Gu, J.; Li, W.; Wang, S.; Zhang, X.; Coules, A.; Ding, G.; Xu, F.; Ren, J.; Lu, C.; Shi, L. Differential Alternative Splicing Genes in Response to Boron Deficiency in Brassica napus. Genes 2019, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; He, F.; Berkowitz, O.; Liu, J.; Cao, P.; Tang, M.; Shi, H.; Wang, W.; Li, Q.; Shen, Z. Alternative Splicing Plays a Critical Role in Maintaining Mineral Nutrient Homeostasis in Rice (Oryza sativa). Plant Cell 2018, 30, 2267–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, J.P.; Phelps, K.; Whipps, J.M.; Young, C.S.; Smith, J.A.; Watling, M. Forecasting Sclerotinia Disease on Lettuce: A Predictive Model for Carpogenic Germination of Sclerotinia sclerotiorum Sclerotia. Phytopatholog 2007, 97, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Meena, P.D.; Verma, P.R.; Saharan, G.S.; Mehta, N.; Singh, D.; Kumar, A. Sclerotinia sclerotiorum (Lib.) de Bary causing Sclerotinia rot in oilseed Brassicas: A review. J. Oilseed Brassica 2016, 6, 1–44. [Google Scholar]
- Corwin, J.A.; Kliebenstein, D.J. Quantitative Resistance: More than just perception of a pathogen. Plant Cell 2017, 29, 655–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Berkowitz, G.A. Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytol. 2011, 190. [Google Scholar] [CrossRef]
- Arfaoui, A.; Hadrami, A.E.; Daayf, F. Pre-treatment of soybean plants with calcium stimulates ROS responses and mitigates infection by Sclerotinia sclerotiorum. Plant Physiol. Biochem. 2017, 122, 121–128. [Google Scholar] [CrossRef]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J. Nitric Oxide in the Offensive Strategy of Fungal and Oomycete Plant Pathogens. Front. Plant Sci. 2016, 7, 252. [Google Scholar] [CrossRef] [Green Version]
- Nováková, M.; Šašek, V.; Dobrev, P.I.; Valentová, O.; Burketová, L. Plant hormones in defense response of Brassica napus to Sclerotinia sclerotiorum—Reassessing the role of salicylic acid in the interaction with a necrotroph. Plant Physiol. Biochem. 2014, 80, 308–317. [Google Scholar] [CrossRef]
- Perchepied, L.; Balagué, C.; Riou, C.; Claudel-Renard, C.; Rivière, N.; Grezes-Besset, B.; Roby, D. Nitric oxide participates in the complex interplay of defense-related signaling pathways controlling disease resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana. Mol. Plant. Microbe Interact. 2010, 23, 846. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-P.; Yang, J.; Cai, X.-Z. Glycolate oxidase gene family in Nicotiana benthamiana: Genome-wide identification and functional analyses in disease resistance. Sci. Rep. 2018, 8, 8615. [Google Scholar] [CrossRef] [Green Version]
- Corwin, J.A.; Copeland, D.; Feusier, J.; Subedy, A.; Eshbaugh, R.; Palmer, C.; Maloof, J.; Kliebenstein, D.J. The Quantitative Basis of the Arabidopsis Innate Immune System to Endemic Pathogens Depends on Pathogen Genetics. PLoS Genet. 2016, 12, e1005789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joris, M.; Schloesser, M.; Baurain, D.; Hanikenne, M.; Muller, M.; Motte, P. Number of inadvertent RNA targets for morpholino knockdown in Danio rerio is largely underestimated: Evidence from the study of Ser/Arg-rich splicing factors. Nucleic Acids Res. 2017, 45, 9547–9557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, B.; Liu, D.; Qi, X. Splicing factor SR34b mutation reduces cadmium tolerance in Arabidopsis by regulating iron-regulated transporter 1 gene. Biochem. Biophys. Res. Commun. 2014, 455, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.F.; Carvalho, S.D.; Duque, P. The plant-specific SR45 protein negatively regulates glucose and ABA signaling during early seedling development in Arabidopsis. Plant Physiol. 2010, 154, 772–783. [Google Scholar] [CrossRef] [Green Version]
- Filichkin, S.A.; Priest, H.D.; Givan, S.A.; Shen, R.; Bryant, D.W.; Fox, S.E.; Wong, W.K.; Mockler, T.C. Genome-wide mapping of alternative splicing in Arabidopsis thaliana. Genome Res. 2010, 20, 45–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isshiki, M.; Tsumoto, A.; Shimamoto, K. The serine/arginine-rich protein family in rice plays important roles in constitutive and alternative splicing of pre-mRNA. Plant Cell 2006, 18, 146–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Heng, D.; Fang-ming, X.; Yong-sheng, L. Alterations of Alternative Splicing Patterns of Ser/Arg-Rich (SR) Genes in Response to Hormones and Stresses Treatments in Different Ecotypes of Rice (Oryza sativa). J. Integr. Agric. 2013, 12, 737–748. [Google Scholar] [CrossRef]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2005, 17, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35 (Suppl. 4), 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Mittler, R.; Feng, X.; Cohen, M. Post-transcriptional suppression of cytosolic ascorbate peroxidase expression during pathogen-induced programmed cell death in tobacco. Plant Cell 1998, 10, 461–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehra, A.; Meena, M.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Synergistic effects of plant defense elicitors and Trichoderma harzianum on enhanced induction of antioxidant defense system in tomato against Fusarium wilt disease. Bot. Stud. 2017, 58, 44. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, U.; Steinkellner, G.; Rozzell, J.D.; Glieder, A.; Winkler, M. Improved Fitness of Arabidopsis thaliana Nitrilase 2. ChemCatChem 2010, 2, 263–267. [Google Scholar] [CrossRef]
- Choi du, S.; Lim, C.W.; Hwang, B.K. Proteomics and functional analyses of Arabidopsis nitrilases involved in the defense response to microbial pathogens. Planta 2016, 244, 449–465. [Google Scholar] [CrossRef]
- Morris, C.A.; Owen, J.R.; Thomas, M.C.; EL-HITI, G.A.; Harwood, J.L.; Kille, P. Intracellular localization and induction of a dynamic RNA-editing event of macro-algal V-ATPase subunit A (VHA-A) in response to copper. Plant Cell Environ. 2014, 37, 189–203. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.H.; Chen, L.S.; Chen, R.B.; Zhang, F.Z.; Yang, L.T.; Tang, N. Expression of genes for two phosphofructokinases, tonoplast ATPase subunit A, and pyrophosphatase of tea roots in response to phosphorus-deficiency. J. Horticult. Sci. Biotechnol. 2010, 85, 449–453. [Google Scholar] [CrossRef]
- Rezaie, S.; Khodadadi, H.; Noorbakhsh, F.; Safari, M. Molecular Characterization of Subunit G of the Vacuolar ATPase in Pathogen Dermatophyte Trichophyton rubrum. Iran J. Public Health 2006, 35, 33–37. [Google Scholar]
- Tegeder, M.; Rentsch, D. Uptake and partitioning of amino acids and peptides. Mol Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef]
- Yang, H.; Postel, S.; Kemmerling, B.; Ludewig, U. Altered growth and improved resistance of Arabidopsis against Pseudomonas syringae by overexpression of the basic amino acid transporter AtCAT1. Plant Cell Environ. 2014, 37, 1404–1414. [Google Scholar] [CrossRef]
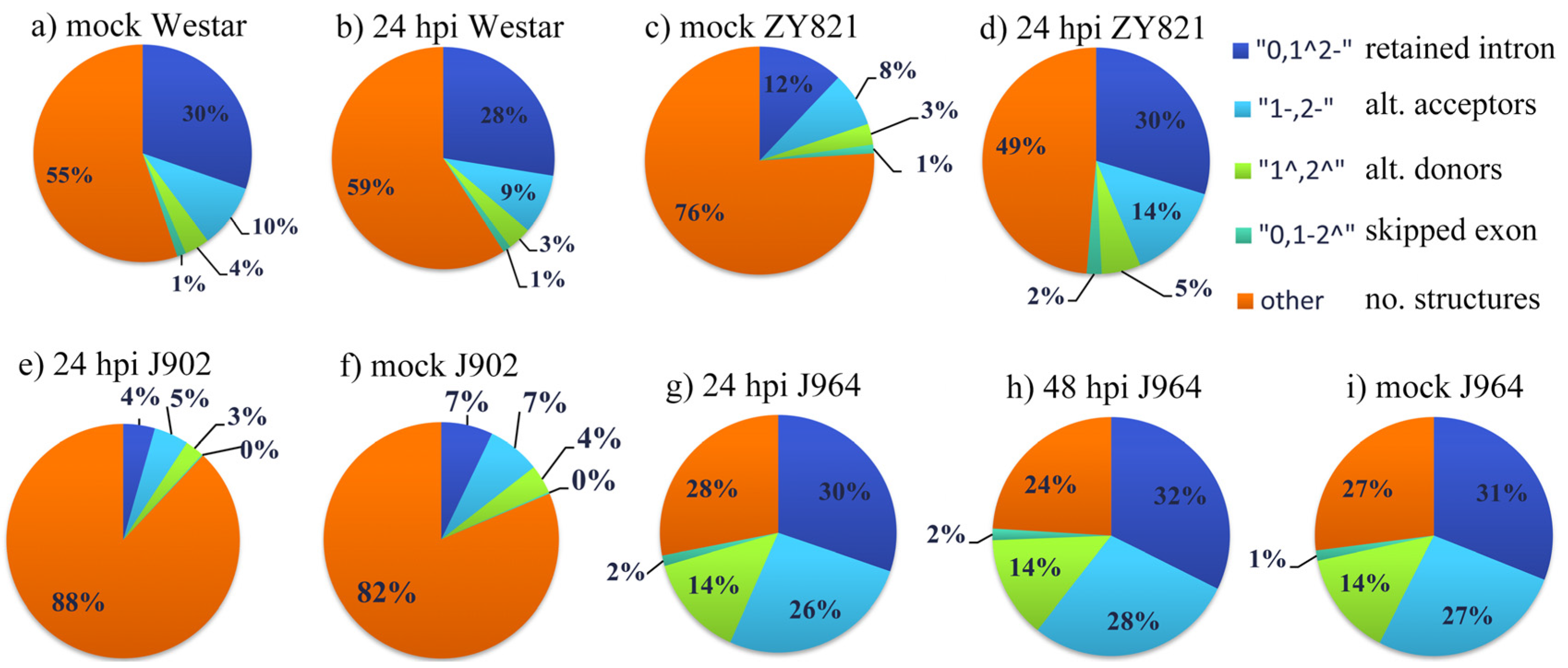
| Cultivar | Treatment | Dataset Name | Data Type | Number | Overall AS Events | Merged Transcript-Number | AS Event Ratio | AS Type | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR | AA | AD | ES | Other Event | ||||||||
| Westar | Mock | W_ck | SE | SRR3537150, SRR3537151 | 28,518 | 585,673 | 4.87% | 8638 | 2724 | 1049 | 380 | 15,727 |
| Westar | 24 hpi | W_24 h | SE | SRR3537153, SRR3537154, SRR3537155 | 44,388 | 784,570 | 5.66% | 12,230 | 3860 | 1538 | 518 | 26,242 |
| ZY 821 | Mock | Z_ck | SE | SRR3537156 | 7997 | 234,401 | 3.41% | 964 | 617 | 237 | 100 | 6079 |
| ZY 821 | 24 hpi | Z_24 h | SE | SRR3537159, SRR3537160 | 16,770 | 467,414 | 3.59% | 4993 | 2326 | 934 | 358 | 8159 |
| J902 | Mock | J2_ck | PE | SRR1793858 | 34,734 | 388,008 | 8.95% | 2470 | 2538 | 1371 | 79 | 28,276 |
| J902 | 24 hpi | J2_24 h | PE | SRR1793826 | 69,013 | 536,471 | 12.86% | 3038 | 3311 | 1792 | 130 | 60,742 |
| J964 | Mock | J4_ck | PE | SRR1793924, SRR1793925, SRR1793927 | 32,750 | 622,164 | 5.26% | 10,156 | 8688 | 4606 | 453 | 8847 |
| J964 | 24 hpi | J4_24 h | PE | SRR1793863, SRR1793864, SRR1793865 | 34,397 | 639,525 | 5.38% | 10,405 | 9084 | 4759 | 489 | 9660 |
| J964 | 48 hpi | J4_48 h | PE | SRR1793876, SRR1793909 | 15,202 | 387,918 | 3.92% | 4912 | 4308 | 2087 | 239 | 3656 |
| DAS Comparison | DAS Gene | At Ortholog | ASIP Synonym | % Identity | At Ortholog Family |
|---|---|---|---|---|---|
| J2_24 h vs. J2_ck | BnaC05g00740D | AT1G01170 | T25K16.16 | 92.50 | Protein of unknown function (DUF1138) |
| BnaA05g20710D | AT1G33970 | F12G12.21 | 82.22 | Immune-associated nucleotide binding 9 | |
| BnaA07g03760D | AT1G64610 | 57.47 | Transducin/WD40 repeat-like superfamily protein | ||
| BnaA07g25520D | AT1G65950 | F12P19.11 | 87.07 | Protein kinase superfamily protein | |
| BnaA09g18680D | AT2G01540 | 94.67 | Calcium-dependent lipid-binding protein | ||
| BnaA02g26350D | AT2G02360 | 61.11 | Phloem protein 2-B10 | ||
| BnaA07g13990D | AT2G28550 | T17D12.11 | 80.88 | (TF) Related to AP2.7 | |
| BnaC04g04760D | AT2G46090 | T3F17.26 | 84.18 | Diacylglycerol kinase protein | |
| BnaC03g25720D | AT2G46340 | 77.77 | SPA (suppressor of phyA-105) protein | ||
| BnaC01g36600D | AT3G14770 | T21E2.6 | 83.47 | Vacuolar sugar transporter SWEET2 | |
| BnaCnng45490D | AT4G16845 | FCAALL.23 | 80.65 | VEFS-Box of polycomb protein | |
| BnaCnn1978 | AT4G35785 | F4B14.7 | 68.05 | RNA-binding (RRM/RBD/RNP motifs) protein | |
| BnaA03g06980D | AT5G18230 | 88.12 | Transcription regulator NOT2/NOT3/NOT5 protein | ||
| BnaA10g14420D | AT5G21170 | T10F18.200 | 80.12 | 5’-AMP-activated protein kinase β-2 subunit protein | |
| BnaA03g09910D | AT5G58110 | 88.38 | ATPase activators | ||
| BnaA06g22260D | AT5G62760 | 71.72 | P-loop containing nucleoside triphosphate hydrolases superfamily | ||
| BnaC09g07670D | AT5G66890 | 73.10 | Leucine-rich repeat (LRR) protein | ||
| BnaC09g23900D | unknown | ||||
| J2_24 h vs. J2_ck\ J2_24 h vs. J4_24 h | BnaA09g50970D | AT1G03140 | 79.02 | Splicing factor Prp18 protein | |
| BnaC09g09280D | AT1G75660 | F10A5.15 | 68.57 | 5′-3′ exoribonuclease 3 | |
| BnaA06g38980D | AT3G44300 | 81.42 | Nitrilase 2 | ||
| BnaC04g40450D | AT5G65940 | K14B20.11 | 80.00 | β-hydroxyisobutyryl-CoA hydrolase 1 | |
| J2_24 h vs. J4_24 h | BnaCnng45130D | AT1G02305 | 78.82 | Cysteine proteinases superfamily protein | |
| BnaC05g05550D | AT1G07890 | F24B9.2 | 92.00 | Ascorbate peroxidase 1 | |
| BnaC05g22390D | AT1G29350 | 74.24 | RNA polymerase II degradation factor-like protein (DUF1296) | ||
| BnaA06n0344 | AT1G48840 | 50.79 | Plant protein of unknown function (DUF639) | ||
| BnaC06g04140D | AT1G50910 | 79.28 | Hypothetical protein | ||
| BnaA02g12910D | AT1G67170 | 69.08 | Sarcolemmal membrane-associated protein | ||
| BnaA07g24840D | AT1G68010 | T23K23.14 | 96.90 | Hydroxypyruvate reductase | |
| BnaCnng00710D | AT2G04560 | 82.68 | Transferases | ||
| BnaCnng63360D | AT2G19480 | F3P11.8 | 84.7 | Nucleosome assembly protein 1;2 | |
| BnaC04g33610D | AT2G21270 | 80.73 | Ubiquitin fusion degradation 1 | ||
| BnaAnng26570D | AT2G23010 | 77.02 | Serine carboxypeptidase-like 9 | ||
| BnaC04g45930D | AT2G26900 | 89.86 | Sodium Bile acid symporter family | ||
| BnaC04g47700D | AT2G41460 | 69.98 | Apurinic endonuclease-redox protein | ||
| BnaC04g03300D | AT2G44065 | 90.23 | Ribosomal protein L2 family | ||
| BnaA03g30650D | AT3G09500 | 94.31 | Ribosomal L29 protein | ||
| BnaAnng09240D | AT3G12130 | 78.88 | KH domain-containing protein/zinc finger (CCCH type) family | ||
| BnaC09g01340D | AT3G27100 | MOJ10.18 | 87.50 | ENY2 | |
| BnaC02g09410D | AT3G44280 | 73.15 | Peptidyl-prolyl cis-trans isomerase G | ||
| BnaA06g17710D | AT3G46970 | 95.01 | α-glucan phosphorylase 2 | ||
| BnaA04g04080D | AT3G54480 | T14E10.50 | 85.87 | SKP1/ASK-interacting protein 5 | |
| BnaC09g00230D | AT4G02780 | 85.71 | Terpenoid cyclases/Protein prenyltransferases superfamily | ||
| BnaA05n0101 | AT4G15090 | 84.82 | (TF) FRS (FAR1 Related Sequences) family | ||
| BnaA01g17760D | AT4G17300 | 90.49 | Class II aminoacyl-tRNA and biotin synthetases superfamily | ||
| BnaC03g02590D | AT5G06440 | MHF15.4 | 61.86 | Polyketide cyclase/dehydrase/lipid transport superfamily protein | |
| BnaA06g26720D | AT5G24150 | MLE8.1 | 77.76 | FAD/NAD(P)-binding oxidoreductase protein | |
| BnaA07g15350D | AT5G41350 | 79.44 | RING/U-box superfamily protein | ||
| BnaA02g22910D | AT5G42210 | 81.51 | Major facilitator superfamily protein | ||
| BnaA02g25070D | AT5G47180 | MQL5.3 | 94.09 | Plant VAMP (vesicle-associated membrane protein) family | |
| BnaC09g28380D | AT5G52380 | 69.28 | Vascular-related nac-domain 6 | ||
| BnaC03g14120D | AT5G55700 | 89.87 | β-amylase 4 | ||
| BnaC09g33270D | AT5G56740 | 77.87 | Histone acetyltransferase of the GNAT family 2 | ||
| BnaC02g10170D | AT5G59050 | K18B18.4 | 77.24 | G patch domain protein | |
| BnaC07g16670D | AT5G66550 | 80.10 | Maf-like protein | ||
| BnaCnng08340D | unknown | ||||
| BnaA06g40000D | unknown | ||||
| BnaC07n0282 | unknown | ||||
| BnaCnn1013 | unknown | ||||
| BnaC02g22380D | unknown | ||||
| BnaA06n0074 | unknown | ||||
| BnaC07n0181 | unknown | ||||
| BnaA03n0594 | unknown | ||||
| J2_24 h vs. J4_24 h\J4_24 h vs. J4_ck | BnaA09 g19610D | AT3G25840 | K9I22.6 | 73.57 | Protein kinase superfamily protein |
| BnaA03g58530D | AT4G21120 | 90.05 | Amino acid transporter 1 | ||
| J2_24 h vs. J4_24 h\J4_48 h vs. J4_ck | BnaC02g27060D | AT4G02620 | 87.23 | Vacuolar ATPase subunit F protein | |
| J4_24 h vs. J4_ck | BnaC06g34460D | AT1G73650 | F25P22.29 | 88.66 | Protein of unknown function (DUF1295) |
| BnaCnng43580D | AT3G21865 | 82.39 | Peroxin 22 | ||
| BnaCnng19550D | AT5G57860 | MTI20.11 | 87.37 | Ubiquitin-like superfamily protein | |
| BnaA04g17110D | AT5G65940 | K14B20.11 | 80.52 | β-hydroxyisobutyryl-CoA hydrolase 1 | |
| J4_48 h vs. J4_ck | BnaC08g42380D | AT1G10130 | T27I1.16 | 95.79 | Endoplasmic reticulum-type calcium-transporting ATPase 3 |
| BnaC03g69240D | AT1G52490 | 47.73 | F-box/associated interaction domain protein | ||
| BnaC09n0143 | AT1G62800 | F23N19.17 | 80.31 | Aspartate aminotransferase 4 | |
| BnaAnng09100D | AT1G75560 | 85.41 | Zinc knuckle (CCHC-type) protein | ||
| BnaC08g21130D | AT3G49430 | T9C5.30 | 85.21 | Ser/Arg-rich protein 34A | |
| BnaC07g01360D | AT5G03495 | 30.87 | RNA-binding (RRM/RBD/RNP motifs) protein | ||
| BnaC04g37090D | AT5G06480 | 69.11 | Immunoglobulin E-set superfamily protein | ||
| BnaC08g02660D | unknown | ||||
| BnaC09n0005 | unknown |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.-Q.; Xu, W.; Xu, F.; Lin, A.; Sun, W.; Jiang, H.-H.; Lu, K.; Li, J.-N.; Wei, L.-J. Differential Alternative Splicing Genes and Isoform Regulation Networks of Rapeseed (Brassica napus L.) Infected with Sclerotinia sclerotiorum. Genes 2020, 11, 784. https://doi.org/10.3390/genes11070784
Ma J-Q, Xu W, Xu F, Lin A, Sun W, Jiang H-H, Lu K, Li J-N, Wei L-J. Differential Alternative Splicing Genes and Isoform Regulation Networks of Rapeseed (Brassica napus L.) Infected with Sclerotinia sclerotiorum. Genes. 2020; 11(7):784. https://doi.org/10.3390/genes11070784
Chicago/Turabian StyleMa, Jin-Qi, Wen Xu, Fei Xu, Ai Lin, Wei Sun, Huan-Huan Jiang, Kun Lu, Jia-Na Li, and Li-Juan Wei. 2020. "Differential Alternative Splicing Genes and Isoform Regulation Networks of Rapeseed (Brassica napus L.) Infected with Sclerotinia sclerotiorum" Genes 11, no. 7: 784. https://doi.org/10.3390/genes11070784
APA StyleMa, J.-Q., Xu, W., Xu, F., Lin, A., Sun, W., Jiang, H.-H., Lu, K., Li, J.-N., & Wei, L.-J. (2020). Differential Alternative Splicing Genes and Isoform Regulation Networks of Rapeseed (Brassica napus L.) Infected with Sclerotinia sclerotiorum. Genes, 11(7), 784. https://doi.org/10.3390/genes11070784






