Prognostic Significance of RAS Mutations and P53 Expression in Cutaneous Squamous Cell Carcinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection, Sample Selection, and Clinicopathological Characterization
2.2. DNA Extraction and Mutation Analysis
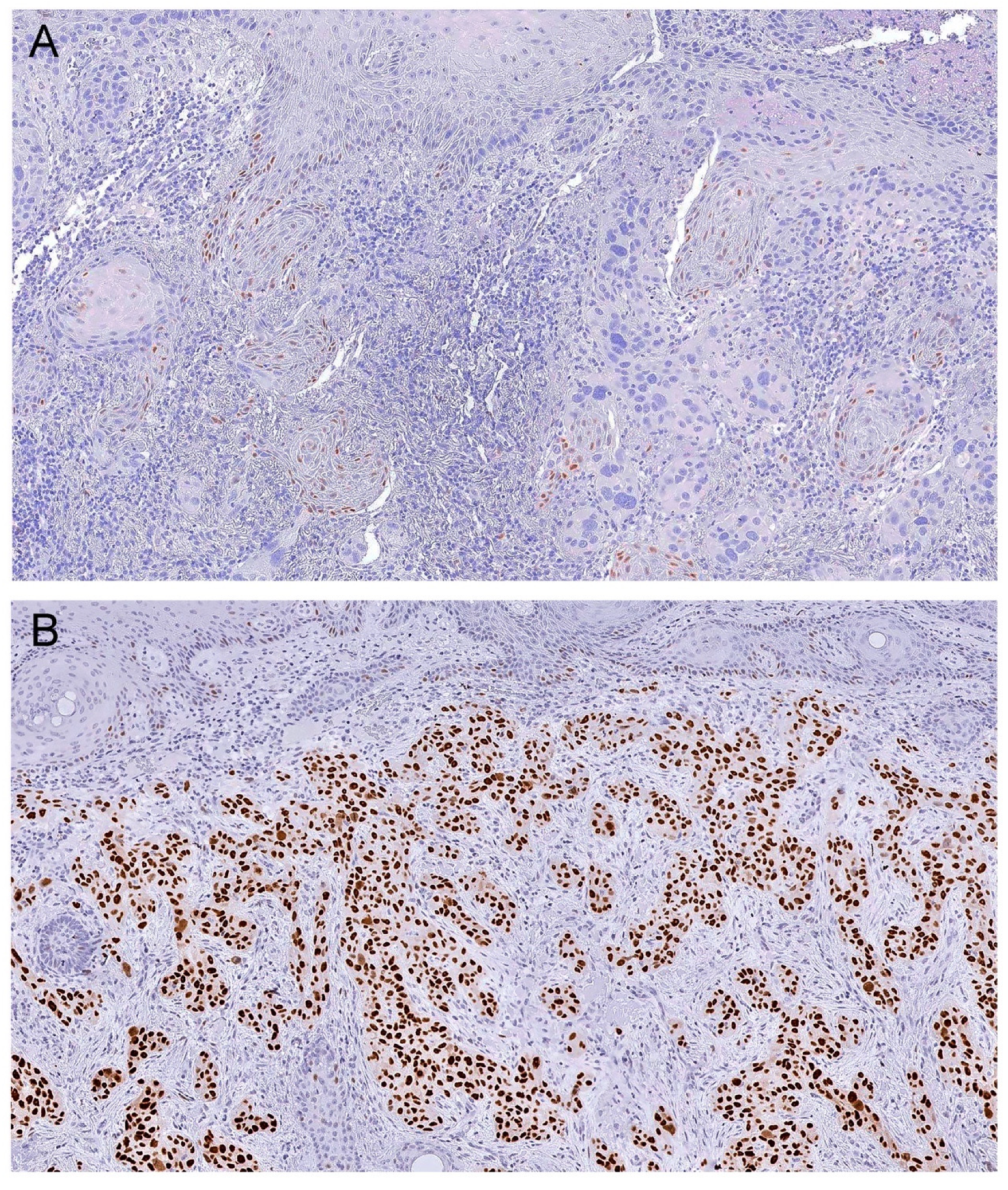
2.3. Immunohistochemistry Protocol and Analysis
2.4. Statistical Analysis
3. Results
3.1. Relationship between RAS Mutations, P53 Expression, and Clinicopathological Features
3.2. Relationship between RAS Mutation, P53 Expression, and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Ullrich, S.E. Mechanisms underlying UV-induced immune suppression. Mutat. Res. 2005, 571, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Criscione, V.D.; Weinstock, M.A.; Naylor, M.F.; Luque, C.; Eide, M.J.; Bingham, S.F.; Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009, 115, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ebrahimi, A.; Low, T.H.; Gao, K.; Palme, C.E.; Sydney, C.; Ashford, B.G.; Iyer, N.G.; Clark, J.R.; Gupta, R. Predictive value of the 8th edition American Joint Commission Cancer (AJCC) nodal staging system for patients with cutaneous squamous cell carcinoma of the head and neck. J. Surg. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef]
- Rowe, D.E.; Carroll, R.J.; Day, C.L., Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef]
- Reschly, M.J.; Messina, J.L.; Zaulyanov, L.L.; Cruse, W.; Fenske, N.A. Utility of sentinel lymphadenectomy in the management of patients with high-risk cutaneous squamous cell carcinoma. Dermatol. Surg. 2003, 29, 135–140. [Google Scholar]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Macedo, S.; Fernandes, M.; Pestana, A.; Pardal, J.; Batista, R.; Vinagre, J.; Sanches, A.; Baptista, A.; Lopes, J.M.; et al. TERT promoter mutations are associated with poor prognosis in cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018. [Google Scholar] [CrossRef]
- Hou, P.; Liu, D.; Shan, Y.; Hu, S.; Studeman, K.; Condouris, S.; Wang, Y.; Trink, A.; El-Naggar, A.K.; Tallini, G.; et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin. Cancer Res. 2007, 13, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hou, P.; Ji, M.; Guan, H.; Studeman, K.; Jensen, K.; Vasko, V.; El-Naggar, A.K.; Xing, M. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J. Clin. Endocrinol. Metab. 2008, 93, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; El-Naggar, A.K.; Cote, G.J.; Myers, J.N.; Sherman, S.I. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2008, 93, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Florence, M.E.; Massuda, J.Y.; Soares, T.C.; Stelini, R.F.; Poppe, L.M.; Brocker, E.B.; Metze, K.; Cintra, M.L.; de Souza, E.M. p53 immunoexpression in stepwise progression of cutaneous squamous cell carcinoma and correlation with angiogenesis and cellular proliferation. Pathol. Res. Pract. 2015, 211, 782–788. [Google Scholar] [CrossRef]
- Barzilai, A.; Lyakhovitsky, A.; Trau, H.; Fogel, M.; Huszar, M. Expression of p53 in the evolution of squamous cell carcinoma: Correlation with the histology of the lesion. J. Am. Acad. Dermatol. 2007, 57, 669–676. [Google Scholar] [CrossRef]
- Kubo, Y.; Urano, Y.; Yoshimoto, K.; Iwahana, H.; Fukuhara, K.; Arase, S.; Itakura, M. p53 gene mutations in human skin cancers and precancerous lesions: Comparison with immunohistochemical analysis. J. Investig. Dermatol. 1994, 102, 440–444. [Google Scholar] [CrossRef][Green Version]
- Campbell, C.; Quinn, A.G.; Ro, Y.S.; Angus, B.; Rees, J.L. p53 mutations are common and early events that precede tumor invasion in squamous cell neoplasia of the skin. J. Investig. Dermatol. 1993, 100, 746–748. [Google Scholar] [CrossRef]
- Oram, Y.; Orengo, I.; Baer, S.C.; Ocal, T. p53 Protein expression in squamous cell carcinomas from sun-exposed and non-sun-exposed sites. J. Am. Acad. Dermatol. 1994, 31, 417–422. [Google Scholar] [CrossRef]
- Almquist, L.M.; Karagas, M.R.; Christensen, B.C.; Welsh, M.M.; Perry, A.E.; Storm, C.A.; Nelson, H.H. The role of TP53 and MDM2 polymorphisms in TP53 mutagenesis and risk of non-melanoma skin cancer. Carcinogenesis 2011, 32, 327–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nan, H.; Qureshi, A.A.; Hunter, D.J.; Han, J. A functional SNP in the MDM2 promoter, pigmentary phenotypes, and risk of skin cancer. Cancer Causes Control 2009, 20, 171–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Willis, A.; Jung, E.J.; Wakefield, T.; Chen, X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene 2004, 23, 2330–2338. [Google Scholar] [CrossRef]
- Leffell, D.J. The scientific basis of skin cancer. J. Am. Acad. Dermatol. 2000, 42, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Pierceall, W.E.; Goldberg, L.H.; Tainsky, M.A.; Mukhopadhyay, T.; Ananthaswamy, H.N. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol. Carcinog. 1991, 4, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Durinck, S.; Ho, C.; Wang, N.J.; Liao, W.; Jakkula, L.R.; Collisson, E.A.; Pons, J.; Chan, S.W.; Lam, E.T.; Chu, C.; et al. Temporal dissection of tumorigenesis in primary cancers. Cancer Discov. 2011, 1, 137–143. [Google Scholar] [CrossRef]
- South, A.P.; Purdie, K.J.; Watt, S.A.; Haldenby, S.; den Breems, N.; Dimon, M.; Arron, S.T.; Kluk, M.J.; Aster, J.C.; McHugh, A.; et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J. Investig. Dermatol. 2014, 134, 2630–2638. [Google Scholar] [CrossRef]
- Ribas, A.; Flaherty, K.T. BRAF targeted therapy changes the treatment paradigm in melanoma. Nat. Rev. Clin. Oncol. 2011, 8, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Cammareri, P.; Rose, A.M.; Vincent, D.F.; Wang, J.; Nagano, A.; Libertini, S.; Ridgway, R.A.; Athineos, D.; Coates, P.J.; McHugh, A.; et al. Inactivation of TGFbeta receptors in stem cells drives cutaneous squamous cell carcinoma. Nat. Commun. 2016, 7, 12493. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, R.; Li, J.; Wang, Y.; Boone, C.W.; Lubet, R.A.; You, M. Induction of invasive mouse skin carcinomas in transgenic mice with mutations in both H-ras and p53. Mol. Cancer Res. 2005, 3, 563–574. [Google Scholar] [CrossRef]
- Farasat, S.; Yu, S.S.; Neel, V.A.; Nehal, K.S.; Lardaro, T.; Mihm, M.C.; Byrd, D.R.; Balch, C.M.; Califano, J.A.; Chuang, A.Y.; et al. A new American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma: Creation and rationale for inclusion of tumor (T) characteristics. J. Am. Acad. Dermatol. 2011, 64, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- SB, E. American Joint Committee on Cancer, American Cancer Society. In AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual, 7th ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bramer, G.R. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat. Q 1988, 41, 32–36. [Google Scholar] [PubMed]
- Brantsch, K.D.; Meisner, C.; Schonfisch, B.; Trilling, B.; Wehner-Caroli, J.; Rocken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Marcuzzo, T.; Giudici, F.; Ober, E.; Rizzardi, C.; Bottin, C.; Zanconati, F. Her2 immunohistochemical evaluation by traditional microscopy and by digital analysis, and the consequences for FISH testing. Pathol. Res. Pract. 2016, 212, 911–918. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, N.J.; Nikolaishvili-Feinberg, N.; Midkiff, B.R.; Conway, K.; Millikan, R.C.; Geradts, J. Rational Manual and Automated Scoring Thresholds for the Immunohistochemical Detection of TP53 Missense Mutations in Human Breast Carcinomas. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Ferrandiz-Pulido, C.; Masferrer, E.; Toll, A.; Hernandez-Losa, J.; Mojal, S.; Pujol, R.M.; Ramon y Cajal, S.; de Torres, I.; Garcia-Patos, V. mTOR signaling pathway in penile squamous cell carcinoma: pmTOR and peIF4E over expression correlate with aggressive tumor behavior. J. Urol. 2013, 190, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Detre, S.; Saclani Jotti, G.; Dowsett, M. A “quickscore” method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. J. Clin. Pathol. 1995, 48, 876–878. [Google Scholar] [CrossRef]
- Lipozencic, J.; Celic, D.; Strnad, M.; Toncic, R.J.; Pasic, A.; Rados, J.; Znaor, A. Skin cancers in Croatia, 2003–2005: Epidemiological study. Coll. Antropol. 2010, 34, 865–869. [Google Scholar]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- Mauerer, A.; Herschberger, E.; Dietmaier, W.; Landthaler, M.; Hafner, C. Low incidence of EGFR and HRAS mutations in cutaneous squamous cell carcinomas of a German cohort. Exp. Dermatol. 2011, 20, 848–850. [Google Scholar] [CrossRef]
- Biddle, A.; Liang, X.; Gammon, L.; Fazil, B.; Harper, L.J.; Emich, H.; Costea, D.E.; Mackenzie, I.C. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011, 71, 5317–5326. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Castillo, E.; Harewood, L.; Ostano, P.; Reymond, A.; Dummer, R.; Raffoul, W.; Hoetzenecker, W.; Hofbauer, G.F.; Dotto, G.P. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell 2012, 149, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.G.; Offidani, A.; Lucarini, G.; Simonelli, L.; Amati, S.; Cellini, A.; Biagini, G.; Ricotti, G. Advances in the understanding of malignant transformation of keratinocytes: An immunohistochemical study. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Gusterson, B.A.; Anbazhagan, R.; Warren, W.; Midgely, C.; Lane, D.P.; O’Hare, M.; Stamps, A.; Carter, R.; Jayatilake, H. Expression of p53 in premalignant and malignant squamous epithelium. Oncogene 1991, 6, 1785–1789. [Google Scholar]
- Nelson, M.A.; Einspahr, J.G.; Alberts, D.S.; Balfour, C.A.; Wymer, J.A.; Welch, K.L.; Salasche, S.J.; Bangert, J.L.; Grogan, T.M.; Bozzo, P.O. Analysis of the p53 gene in human precancerous actinic keratosis lesions and squamous cell cancers. Cancer Lett. 1994, 85, 23–29. [Google Scholar] [CrossRef]
- Onodera, H.; Nakamura, S.; Sugai, T. Cell proliferation and p53 protein expressions in cutaneous epithelial neoplasms. Am. J. Dermatopathol. 1996, 18, 580–588. [Google Scholar] [CrossRef]
- Clayman, G.L.; Lee, J.J.; Holsinger, F.C.; Zhou, X.; Duvic, M.; El-Naggar, A.K.; Prieto, V.G.; Altamirano, E.; Tucker, S.L.; Strom, S.S.; et al. Mortality risk from squamous cell skin cancer. J. Clin. Oncol. 2005, 23, 759–765. [Google Scholar] [CrossRef]
- Leibovitch, I.; Huilgol, S.C.; Selva, D.; Hill, D.; Richards, S.; Paver, R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J. Am. Acad. Dermatol. 2005, 53, 253–260. [Google Scholar] [CrossRef]
- Eroglu, A.; Berberoglu, U.; Berreroglu, S. Risk factors related to locoregional recurrence in squamous cell carcinoma of the skin. J. Surg. Oncol. 1996, 61, 124–130. [Google Scholar] [CrossRef]
- Brougham, N.D.; Dennett, E.R.; Cameron, R.; Tan, S.T. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J. Surg. Oncol. 2012, 106, 811–815. [Google Scholar] [CrossRef]
- Mourouzis, C.; Boynton, A.; Grant, J.; Umar, T.; Wilson, A.; Macpheson, D.; Pratt, C. Cutaneous head and neck SCCs and risk of nodal metastasis—UK experience. J. Craniomaxillofac. Surg. 2009, 37, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Faut, M.; Wevers, K.P.; van Ginkel, R.J.; Diercks, G.F.; Hoekstra, H.J.; Kruijff, S.; Been, L.B.; van Leeuwen, B.L. Nodular Histologic Subtype and Ulceration are Tumor Factors Associated with High Risk of Recurrence in Sentinel Node-Negative Melanoma Patients. Ann. Surg. Oncol. 2017, 24, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.J.; Saunders, A. Risk factors for positive margins after wide local excision of cutaneous squamous cell carcinoma. J. Dermatolog. Treat. 2018, 29, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.R.; Skinner, R.; Dueck, A.C.; Sekulic, A.; Pockaj, B.A. Risk Factors Predicting Positive Margins at Primary Wide Local Excision of Cutaneous Melanoma. Dermatol. Surg. 2016, 42, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, A.; McKee, P.H.; Simpson, J.A.; McGuire, B.; Hobbs, C. Prognostic significance of Ki-67 and p53 immunoreactivity in cutaneous squamous cell carcinomas. Am. J. Dermatopathol. 1996, 18, 351–357. [Google Scholar] [CrossRef]
- Senovilla, L.; Vacchelli, E.; Galon, J.; Adjemian, S.; Eggermont, A.; Fridman, W.H.; Sautes-Fridman, C.; Ma, Y.; Tartour, E.; Zitvogel, L.; et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology 2012, 1, 1323–1343. [Google Scholar] [CrossRef]
- Azimi, F.; Scolyer, R.A.; Rumcheva, P.; Moncrieff, M.; Murali, R.; McCarthy, S.W.; Saw, R.P.; Thompson, J.F. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J. Clin. Oncol. 2012, 30, 2678–2683. [Google Scholar] [CrossRef]
- Sondergaard, K.; Schou, G. Survival with primary cutaneous malignant melanoma, evaluated from 2012 cases. A multivariate regression analysis. Virchows Arch. A Pathol. Anat. Histopathol. 1985, 406, 179–195. [Google Scholar] [CrossRef]
- Kim, C.; Ko, C.J.; Leffell, D.J. Cutaneous squamous cell carcinomas of the lower extremity: A distinct subset of squamous cell carcinomas. J. Am. Acad. Dermatol. 2014, 70, 70–74. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Curado, M.P.; Filho, J.B. Cutaneous squamous cell carcinoma of the lower limbs in Goiania, Goias, Brazil. Int J. Dermatol. 2006, 45, 1039–1042. [Google Scholar] [CrossRef]
- McCall, C.O.; Chen, S.C. Squamous cell carcinoma of the legs in African Americans. J. Am. Acad. Dermatol. 2002, 47, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Kobel, M.; Ronnett, B.M.; Singh, N.; Soslow, R.A.; Gilks, C.B.; McCluggage, W.G. Interpretation of P53 Immunohistochemistry in Endometrial Carcinomas: Toward Increased Reproducibility. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S123–S131. [Google Scholar] [CrossRef] [PubMed]
| All Tumors | In Situ cSCCs | Invasive cSCCs | ||
|---|---|---|---|---|
| Clinical and molecular features | Number of cases | 162 | 31 | 131 |
| Age at diagnosis (mean (±SD)) | 77.6 ± 12.2 | 79.5 ± 7.4 | 77.1 ± 13.0 | |
| Male | 74.9 ± 12.2 | 78.8 ± 6.3 | 74.1 ± 12.9 | |
| Female | 81.6 ± 11.0 | 80.3 ± 8.6 | 82.0 ± 11.7 | |
| Gender (n (%)) | ||||
| Male | 97 (59.9) | 16 (51.6) | 81 (61.8) | |
| Female | 65 (40.1) | 15 (48.4) | 50 (38.2) | |
| Sun exposure (n (%)) | ||||
| Chronic | 110 (67.9) | 11 (35.5) | 99 (75.6) | |
| Intermittent | 49 (30.2) | 19 (61.3) | 30 (22.9) | |
| Undetermined | 3 (1.9) | 1 (3.2) | 2 (1.5) | |
| Location (n (%)) | ||||
| Face | 108 (66.7) | 10 (32.3) | 98 (74.8) | |
| Trunk | 9 (5.6) | 5 (16.1) | 4 (3.1) | |
| Upper limb | 20 (12.3) | 4 (12.9) | 16 (12.2) | |
| Lower limb | 22 (13.6) | 11 (35.5) | 11 (8.4) | |
| Not specified | 3 (1.9) | 1 (3.2) | 2 (1.5) | |
| Follow-up (months) | 41.6 ± 28.9 | 38.9 ± 21.5 | 42.2 ± 30.3 | |
| Progression-free survival (months) | 38.7 ± 29.2 | 37.6 ± 21.7 | 38.9 ± 30.7 | |
| Recurrence | ||||
| No | 142 (87.7) | 28 (90.3) | 114 (87.0) | |
| Yes | 20 (12.3) | 3 (9.7) | 17 (13.0) | |
| Metastases | ||||
| No | 154 (95.1) | 31 (100) | 123 (93.9) | |
| Yes | 8 (4.9) | 0 | 8 (6.1) | |
| p53 immunohistochemistry | ||||
| Cells counted | 4224,4 ± 2223.7 | 3041,1 ± 1066.0 | 4497,5 ± 2332.1 | |
| Mean h-score | 91.6 ± 5.9 | 109.6 ± 16.3 | 87.9 ± 6.2 | |
| Wild type | 29 (17.9) | 8 (25.8) | 21 (16.0) | |
| Overexpression (h-score 1+) | 78 (48.1) | 12 (38.7) | 66 (50.4) | |
| Overexpression (h-score 2+) | 45 (27.8) | 8 (25.2) | 37 (28.2) | |
| Overexpression (h-score 3+) | 10 (6.2) | 3 (9.7) | 7 (5.3) | |
| RAS mutations | ||||
| Wild type | 147 (90.7) | 30 (96.8) | 117 (89.3) | |
| Mutation | 15 (9.3) | 1 (3.2) | 14 (10.7) | |
| HRAS mutations | ||||
| Wild type | 149 (92.0) | 30 (96.8) | 119 (90.8) | |
| Mutation | 13 (8.0) | 1 (3.2) | 12 (9.2) | |
| KRAS mutations | ||||
| Wild type | 160 (98.8) | 31 (100) | 129 (98.5) | |
| Mutation | 2 (1.2) | 0 (0) | 2 (1.5) | |
| TERTp mutations | ||||
| Wild type | 98 (60.5) | 21 (67.7) | 77 (58.8) | |
| Mutation | 48 (29.6) | 6 (19.4) | 42 (32.1) | |
| Maximum tumor thickness | Maximum tumor size | |||
| <2 cm | 75 (46.3) | 12 (38.7) | 63 (48.1) | |
| ≥2 cm | 39 (24.1) | 9 (29.0) | 30 (22.9) | |
| Not assessed | 48 (29.6) | 10 (32.3) | 38 (29.0) | |
| Superficial margins (mm) | 2.1 ± 2.8 | 1.7 ± 1.8 | 2.2 ± 2.9 | |
| Deep margins (mm) | 2.4 ±2.3 | 3.3 ± 1.7 | 2.2 ± 2.4 | |
| Ulceration | ||||
| No | 53 (32.7) | 11 (35.5) | 42 (32.1) | |
| Yes | 101 (62.3) | 19 (61.3) | 82 (62.6) | |
| Undetermined | 8 (4.9) | 1 (3.2) | 7 (5.3) | |
| Actinic Keratosis | ||||
| No | 56 (34.6) | 6 (19.4) | 50 (38.2) | |
| Yes | 96 (59.3) | 25 (80.6) | 71 (54.2) | |
| Undetermined | 10 (6.6) | 10 (7.6) | ||
| Invasion | ||||
| Non-invasive | 31 (19.1) | |||
| Invasive | 131 (80.9) | |||
| Histologic type | ||||
| Acantholytic | 10 (7.6) | |||
| Spindle cell | 1 (0.8) | |||
| Verrucous | 2 (1.5) | |||
| Bowenoid | 1 (0.8) | |||
| Not otherwise specified (NOS) | 117 (89.3) | |||
| Histological grade | ||||
| Well differentiated | 46 (35.1) | |||
| Moderately differentiated | 68 (51.9) | |||
| Poorly differentiated | 13 (9.9) | |||
| Not assessed | 4 (3.1) | |||
| Pattern of invasion | ||||
| Expansive | 70 (53.4) | |||
| Infiltrative | 57 (43.5) | |||
| Not assessed | 4 (3.1) | |||
| Level of invasion | ||||
| Papillary dermis | 39 (29.8) | |||
| Reticular dermis | 60 (45.8) | |||
| Subcutaneous tissue | 26 (19.8) | |||
| Not assessed | 6 (4.6) | |||
| Maximum tumor thickness | 3.8 ± 3.0 | |||
| Maximum tumor thickness | ||||
| < 6 mm | 103 (78.6) | |||
| ≥ 6 mm | 22 (16.8) | |||
| Not assessed | 6 (4.6) | |||
| Intratumoral infiltrate | ||||
| Moderate–intense | 13 (9.9) | |||
| Few–absent | 118 (90.1) | |||
| Peritumoral infiltrate | ||||
| Moderate–intense | 74 (56.5) | |||
| Few–absent | 57 (43.5) | |||
| Lymphovascular invasion | ||||
| Not present | 126 (96.2) | |||
| Present | 5 (3.8) | |||
| Perineural invasion | ||||
| Not present | 128 (97.7) | |||
| Present | 3 (2.3) | |||
| Recurrence | Metastasis | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Odds Ratio (OR) (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Mean age (years) | ||||||||
| < 80 | 1 | 0.008 | 1 | 0.019 | NA | NA | ||
| ≥ 80 | 16.00 (2.05–124.71) | 12.17 (1.51–97.82) | ||||||
| Ulceration | ||||||||
| No | 1 | 0.049 | 1 | 0.261 | 1 | 0.196 | ||
| Yes | 2.92 (1.00–8.51) | 1.97 (0.60–6.41) | 2.77 (0.59–13.01) | |||||
| Level of invasion | ||||||||
| Dermis | 1 | 0.829 | 1 | 0.028 | 1 | 0.315 | ||
| Subcutaneous tissue | 0.86 (0.23–3.29) | 5.82 (1.21–27.89) | 2.68 (0.39–18.27) | |||||
| RAS | ||||||||
| Wild type | 1 | 0.878 | 1 | 0.864 | ||||
| Mutation | 1.13 (0.23–5.57) | 1.21 (0.14–10.62) | ||||||
| p53 overexpression * | ||||||||
| 1.01 (1.00–1.02) | 0.045 | 1.01 (1.00–1.02) | 0.145 | 1.01 (1.00–1.02) | 0.281 | |||
| Superficial margins * | ||||||||
| 1.14 (1.00–1.31) | 0.059 | 1.18 (1.02–1.37) | 0.026 | 1.03 (0.85–1.25) | 0.753 | |||
| Max. tumor thickness * | ||||||||
| 1.09 (0.94–1.27) | 0.238 | 1.25 (1.05–1.40) | 0.011 | 1.17 (0.90–1.51) | 0.247 | |||
| Peritumoral infiltrate | ||||||||
| Moderate–intense | 1 | 0.178 | 1 | 0.032 | 1 | 0.069 | ||
| Few–absent | 2.04 (0.72–5.74) | 10.22 (1.22–85.65) | 8.00 (0.85–75.30) | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, M.A.; Macedo, S.; Fernandes, M.S.; Pestana, A.; Pardal, J.; Batista, R.; Vinagre, J.; Sanches, A.; Baptista, A.; Lopes, J.M.; et al. Prognostic Significance of RAS Mutations and P53 Expression in Cutaneous Squamous Cell Carcinomas. Genes 2020, 11, 751. https://doi.org/10.3390/genes11070751
Campos MA, Macedo S, Fernandes MS, Pestana A, Pardal J, Batista R, Vinagre J, Sanches A, Baptista A, Lopes JM, et al. Prognostic Significance of RAS Mutations and P53 Expression in Cutaneous Squamous Cell Carcinomas. Genes. 2020; 11(7):751. https://doi.org/10.3390/genes11070751
Chicago/Turabian StyleCampos, Manuel António, Sofia Macedo, Margarida Sá Fernandes, Ana Pestana, Joana Pardal, Rui Batista, João Vinagre, Agostinho Sanches, Armando Baptista, José Manuel Lopes, and et al. 2020. "Prognostic Significance of RAS Mutations and P53 Expression in Cutaneous Squamous Cell Carcinomas" Genes 11, no. 7: 751. https://doi.org/10.3390/genes11070751
APA StyleCampos, M. A., Macedo, S., Fernandes, M. S., Pestana, A., Pardal, J., Batista, R., Vinagre, J., Sanches, A., Baptista, A., Lopes, J. M., & Soares, P. (2020). Prognostic Significance of RAS Mutations and P53 Expression in Cutaneous Squamous Cell Carcinomas. Genes, 11(7), 751. https://doi.org/10.3390/genes11070751







