Genetic and Genomic Landscape of Secondary and Therapy-Related Acute Myeloid Leukemia
Abstract
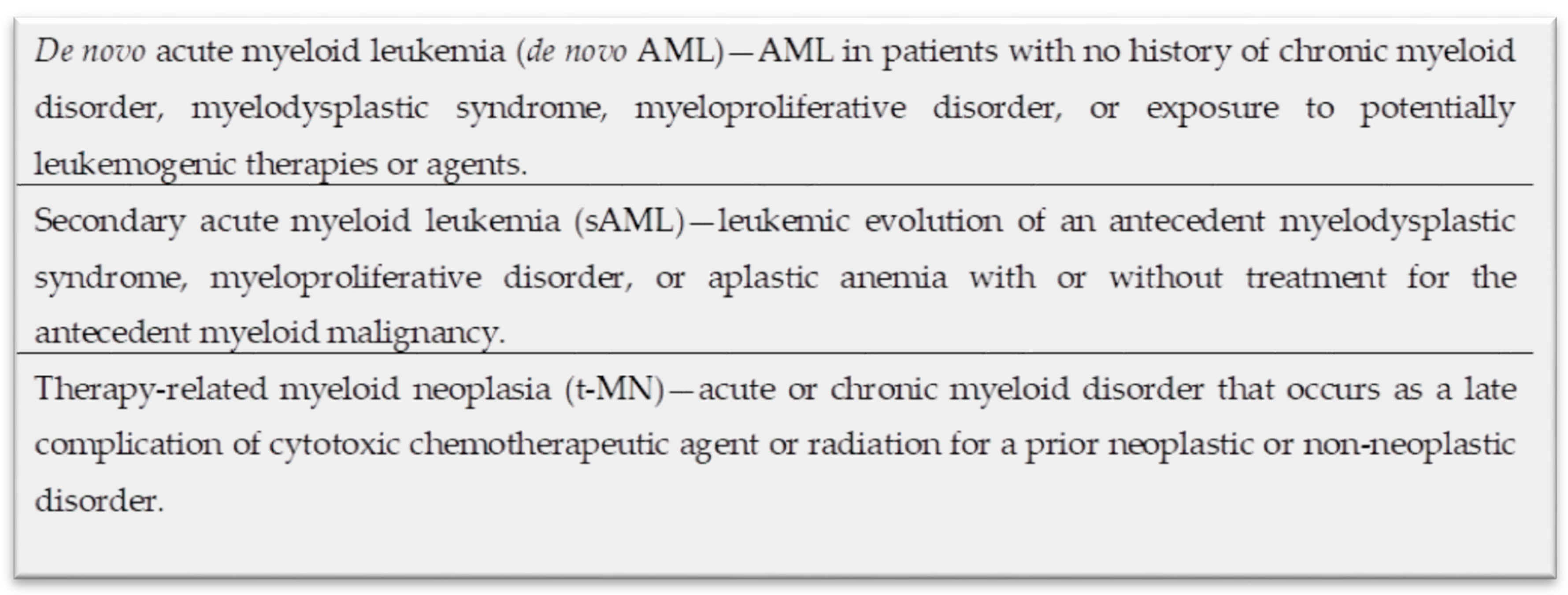
1. Introduction
2. Secondary AML Arising from Myelodysplastic Syndrome (MDS) and MPN in Blast Phase (MPN-BP)
| Complex Karyotype (CK) | Unbalanced Abnormalities | Balanced Abnormalities |
|---|---|---|
| 3 or more abnormalities | −7/del(7q) | t(11;16)(q23.3;p13.3) |
| del(5q)/t(5q) | t(3;21)(q26.2;q22.1) | |
| i(17q)/t(17p) | t(1;3)(p36.3;q21.2) | |
| -13/del(13q) | t(2;11)(p21;q23.3) | |
| del(11q) | t(5;12)(q32;p13.2) | |
| del(12p)/t(12p) | t(5;7)(q32;q11.2) | |
| idic(X)(q13) | t(5;17)(q32;p13.2) | |
| t(5;10)(q32;q21.2) | ||
| t(3;5)(q25.3;q35.1) |
3. Mutational Landscape of Secondary AML Arising from Antecedent Myeloid Neoplasms
3.1. Mutations in Epigenetic Regulators
3.2. Mutations in the Transcriptional Regulator Genes
3.3. Mutations in the RNA Splicing Factors (SF)
3.4. Mutations in the Signaling Pathways
3.5. Genetic Risk Factors for Leukemic Progression from Chronic Myelomonocytic Leukemia
4. Mutational Landscape of Therapy-Related Myeloid Neoplasms
5. Biology of Therapy-Related Myeloid Neoplasm
| Functional Group | Genetic Abnormality | Frequency in de novo AML (%) | Frequency in t-MN (%) | Reference |
|---|---|---|---|---|
| Cytogenetics | Del(5q) | 5–16 | 42 | [116,117,118,119] |
| Del7(q)/-7 | 4–14 | 49 | ||
| Del 17p/-17 | 4 | 20 | ||
| CK | 5–17 | 48 | ||
| Diploid karyotype | 41–48 | 8 | ||
| Epigenetic regulation | ASXL1 | 10 | 4 | [105,116,120] |
| DNMT3A | 30 | 20 | ||
| TET2 | 17 | 10 | ||
| IDH1 | 8–10 | 3–5 | ||
| IDH2 | 9–10 | 0–5 | ||
| Signaling pathway | FLT3 | 24–28 | 8–16 | |
| KIT | 4–6 | 0–3 | ||
| Splicing factor | SF3B1 | 10 | 3 | |
| DNA damage response | TP53 | 2–12 | 13–37 | |
| CEBPA | 9 | 3 | ||
| NPM1 | 34 | 18 |
6. The Role of Clonal Hematopoiesis in the Development of Myeloid Malignancies
7. Treatment of Secondary Acute Myeloid Leukemia and Therapy-Related Myeloid Neoplasms
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Granfeldt Ostgard, L.S.; Medeiros, B.C.; Sengelov, H.; Norgaard, M.; Andersen, M.K.; Dufva, I.H.; Friis, L.S.; Kjeldsen, E.; Marcher, C.W.; Preiss, B.; et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: A national population-based cohort study. J. Clin. Oncol. 2015, 33, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Hulegardh, E.; Nilsson, C.; Lazarevic, V.; Garelius, H.; Antunovic, P.; Rangert Derolf, A.; Mollgard, L.; Uggla, B.; Wennstrom, L.; Wahlin, A.; et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: A report from the Swedish Acute Leukemia Registry. Am. J. Hematol. 2015, 90, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Valentini, C.G.; Fianchi, L.; Voso, M.T.; Caira, M.; Leone, G.; Pagano, L. Incidence of acute myeloid leukemia after breast cancer. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011069. [Google Scholar] [CrossRef]
- Craig, B.M.; Rollison, D.E.; List, A.F.; Cogle, C.R. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol. Biomark. Prev. 2012, 21, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Dohner, K.; Krauter, J.; Kohne, C.H.; Horst, H.A.; Held, G.; von Lilienfeld-Toal, M.; Wilhelm, S.; Kundgen, A.; Gotze, K.; et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood 2011, 117, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.; Lin, E.; Jain, N.; Estey, E.E.; Cortes, J.E.; O’Brien, S.; Faderl, S.; Ravandi, F.; Pierce, S.; Kantarjian, H. Survival is poorer in patients with secondary core-binding factor acute myelogenous leukemia compared with de novo core-binding factor leukemia. Cancer 2009, 115, 3217–3221. [Google Scholar] [CrossRef]
- Boddu, P.; Kantarjian, H.M.; Garcia-Manero, G.; Ravandi, F.; Verstovsek, S.; Jabbour, E.; Borthakur, G.; Konopleva, M.; Bhalla, K.N.; Daver, N.; et al. Treated secondary acute myeloid leukemia: A distinct high-risk subset of AML with adverse prognosis. Blood Adv. 2017, 1, 1312–1323. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Atenafu, E.G.; Messner, H.A.; Craddock, K.J.; Brandwein, J.M.; Lipton, J.H.; Minden, M.D.; Schimmer, A.D.; Schuh, A.C.; Yee, K.W.; et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood 2013, 121, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, R.C.; Mar, B.G.; Mazzola, E.; Grauman, P.V.; Shareef, S.; Allen, S.L.; Pigneux, A.; Wetzler, M.; Stuart, R.K.; Erba, H.P.; et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015, 125, 1367–1376. [Google Scholar] [CrossRef]
- Ostgard, L.S.; Kjeldsen, E.; Holm, M.S.; Brown Pde, N.; Pedersen, B.B.; Bendix, K.; Johansen, P.; Kristensen, J.S.; Norgaard, J.M. Reasons for treating secondary AML as de novo AML. Eur. J. Haematol. 2010, 85, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Haase, D.; Feuring-Buske, M.; Schafer, C.; Schoch, C.; Troff, C.; Gahn, B.; Hiddemann, W.; Wormann, B. Cytogenetic analysis of CD34+ subpopulations in AML and MDS characterized by the expression of CD38 and CD117. Leukemia 1997, 11, 674–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blau, O.; Hofmann, W.K.; Baldus, C.D.; Thiel, G.; Serbent, V.; Schumann, E.; Thiel, E.; Blau, I.W. Chromosomal aberrations in bone marrow mesenchymal stroma cells from patients with myelodysplastic syndrome and acute myeloblastic leukemia. Exp. Hematol. 2007, 35, 221–229. [Google Scholar] [CrossRef]
- Raza, A.; Gezer, S.; Mundle, S.; Gao, X.Z.; Alvi, S.; Borok, R.; Rifkin, S.; Iftikhar, A.; Shetty, V.; Parcharidou, A.; et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes. Blood 1995, 86, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Horny, H.P.; Bennett, J.M.; Fonatsch, C.; Germing, U.; Greenberg, P.; Haferlach, T.; Haase, D.; Kolb, H.J.; Krieger, O.; et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: Consensus statements and report from a working conference. Leuk. Res. 2007, 31, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Dicker, F.; Haferlach, C.; Sundermann, J.; Wendland, N.; Weiss, T.; Kern, W.; Haferlach, T.; Schnittger, S. Mutation analysis for RUNX1, MLL-PTD, FLT3-ITD, NPM1 and NRAS in 269 patients with MDS or secondary AML. Leukemia 2010, 24, 1528–1532. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Schanz, J.; Tuchler, H.; Sole, F.; Mallo, M.; Luno, E.; Cervera, J.; Granada, I.; Hildebrandt, B.; Slovak, M.L.; Ohyashiki, K.; et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J. Clin. Oncol. 2012, 30, 820–829. [Google Scholar] [CrossRef]
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 2011, 364, 2496–2506. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.A.; Verstovsek, S.; Cervantes, F.; Barosi, G.; Reilly, J.T.; Dupriez, B.; Levine, R.; Le Bousse-Kerdiles, M.C.; Wadleigh, M.; Campbell, P.J.; et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk. Res. 2007, 31, 737–740. [Google Scholar] [CrossRef]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, P.; Karow, A.; Nienhold, R.; Looser, R.; Hao-Shen, H.; Nissen, I.; Girsberger, S.; Lehmann, T.; Passweg, J.; Stern, M.; et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 2014, 123, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M.; Lasho, T.L.; Guglielmelli, P.; Biamonte, F.; Pardanani, A.; Pereira, A.; Finke, C.; Score, J.; Gangat, N.; Mannarelli, C.; et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013, 27, 1861–1869. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Guglielmelli, P.; Finke, C.M.; Rotunno, G.; Elala, Y.; Pacilli, A.; Hanson, C.A.; Pancrazzi, A.; Ketterling, R.P.; et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016, 1, 21–30. [Google Scholar] [CrossRef]
- Tefferi, A.; Vannucchi, A.M. Genetic Risk Assessment in Myeloproliferative Neoplasms. Mayo Clin. Proc. 2017, 92, 1283–1290. [Google Scholar] [CrossRef]
- Ortmann, C.A.; Kent, D.G.; Nangalia, J.; Silber, Y.; Wedge, D.C.; Grinfeld, J.; Baxter, E.J.; Massie, C.E.; Papaemmanuil, E.; Menon, S.; et al. Effect of mutation order on myeloproliferative neoplasms. N. Engl. J. Med. 2015, 372, 601–612. [Google Scholar] [CrossRef]
- Crisa, E.; Venturino, E.; Passera, R.; Prina, M.; Schinco, P.; Borchiellini, A.; Giai, V.; Ciocca Vasino, M.A.; Bazzan, M.; Vaccarino, A.; et al. A retrospective study on 226 polycythemia vera patients: Impact of median hematocrit value on clinical outcomes and survival improvement with anti-thrombotic prophylaxis and non-alkylating drugs. Ann. Hematol. 2010, 89, 691–699. [Google Scholar] [CrossRef]
- Cerquozzi, S.; Tefferi, A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: A literature review of incidence and risk factors. Blood Cancer J. 2015, 5, e366. [Google Scholar] [CrossRef]
- Barbui, T.; Thiele, J.; Passamonti, F.; Rumi, E.; Boveri, E.; Ruggeri, M.; Rodeghiero, F.; d’Amore, E.S.; Randi, M.L.; Bertozzi, I.; et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: An international study. J. Clin. Oncol. 2011, 29, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Vallapureddy, R.R.; Mudireddy, M.; Penna, D.; Lasho, T.L.; Finke, C.M.; Hanson, C.A.; Ketterling, R.P.; Begna, K.H.; Gangat, N.; Pardanani, A.; et al. Leukemic transformation among 1306 patients with primary myelofibrosis: Risk factors and development of a predictive model. Blood Cancer J. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Caramazza, D.; Vaidya, R.; George, G.; Begna, K.; Schwager, S.; Van Dyke, D.; Hanson, C.; Wu, W.; Pardanani, A.; et al. DIPSS plus: A refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. 2011, 29, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Grinfeld, J.; Nangalia, J.; Baxter, E.J.; Wedge, D.C.; Angelopoulos, N.; Cantrill, R.; Godfrey, A.L.; Papaemmanuil, E.; Gundem, G.; MacLean, C.; et al. Classification and Personalized Prognosis in Myeloproliferative Neoplasms. N. Engl. J. Med. 2018, 379, 1416–1430. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Larson, D.R.; Finke, C.; Wassie, E.A.; Pieri, L.; Gangat, N.; Fjerza, R.; Belachew, A.A.; Lasho, T.L.; et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014, 124, 2507–2513. [Google Scholar] [CrossRef]
- Courtier, F.; Carbuccia, N.; Garnier, S.; Guille, A.; Adelaide, J.; Cervera, N.; Gelsi-Boyer, V.; Mozziconacci, M.J.; Rey, J.; Vey, N.; et al. Genomic analysis of myeloproliferative neoplasms in chronic and acute phases. Haematologica 2017, 102, e11–e14. [Google Scholar] [CrossRef]
- Harutyunyan, A.; Klampfl, T.; Cazzola, M.; Kralovics, R. p53 lesions in leukemic transformation. N. Engl. J. Med. 2011, 364, 488–490. [Google Scholar] [CrossRef]
- Rampal, R.; Ahn, J.; Abdel-Wahab, O.; Nahas, M.; Wang, K.; Lipson, D.; Otto, G.A.; Yelensky, R.; Hricik, T.; McKenney, A.S.; et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc. Natl. Acad. Sci. USA 2014, 111, E5401–E5410. [Google Scholar] [CrossRef] [PubMed]
- Klampfl, T.; Harutyunyan, A.; Berg, T.; Gisslinger, B.; Schalling, M.; Bagienski, K.; Olcaydu, D.; Passamonti, F.; Rumi, E.; Pietra, D.; et al. Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood 2011, 118, 167–176. [Google Scholar] [CrossRef]
- Jager, R.; Gisslinger, H.; Passamonti, F.; Rumi, E.; Berg, T.; Gisslinger, B.; Pietra, D.; Harutyunyan, A.; Klampfl, T.; Olcaydu, D.; et al. Deletions of the transcription factor Ikaros in myeloproliferative neoplasms. Leukemia 2010, 24, 1290–1298. [Google Scholar] [CrossRef]
- Puda, A.; Milosevic, J.D.; Berg, T.; Klampfl, T.; Harutyunyan, A.S.; Gisslinger, B.; Rumi, E.; Pietra, D.; Malcovati, L.; Elena, C.; et al. Frequent deletions of JARID2 in leukemic transformation of chronic myeloid malignancies. Am. J. Hematol. 2012, 87, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Bjorkholm, M.; Derolf, A.R.; Hultcrantz, M.; Kristinsson, S.Y.; Ekstrand, C.; Goldin, L.R.; Andreasson, B.; Birgegard, G.; Linder, O.; Malm, C.; et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J. Clin. Oncol. 2011, 29, 2410–2415. [Google Scholar] [CrossRef] [PubMed]
- Yogarajah, M.; Tefferi, A. Leukemic Transformation in Myeloproliferative Neoplasms: A Literature Review on Risk, Characteristics, and Outcome. Mayo Clin. Proc. 2017, 92, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.J.; Shen, D.; Ding, L.; Shao, J.; Koboldt, D.C.; Chen, K.; Larson, D.E.; McLellan, M.D.; Dooling, D.; Abbott, R.; et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Shukron, O.; Vainstein, V.; Kundgen, A.; Germing, U.; Agur, Z. Analyzing transformation of myelodysplastic syndrome to secondary acute myeloid leukemia using a large patient database. Am. J. Hematol. 2012, 87, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Makishima, H.; Yoshizato, T.; Yoshida, K.; Sekeres, M.A.; Radivoyevitch, T.; Suzuki, H.; Przychodzen, B.; Nagata, Y.; Meggendorfer, M.; Sanada, M.; et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat. Genet. 2017, 49, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Friesen, I.; Damm, F.; Yun, H.; Weissinger, E.M.; Krauter, J.; Wagner, K.; Chaturvedi, A.; Sharma, A.; Wichmann, M.; et al. Prognostic significance of ASXL1 mutations in patients with myelodysplastic syndromes. J. Clin. Oncol. 2011, 29, 2499–2506. [Google Scholar] [CrossRef]
- Kakosaiou, K.; Panitsas, F.; Daraki, A.; Pagoni, M.; Apostolou, P.; Ioannidou, A.; Vlachadami, I.; Marinakis, T.; Giatra, C.; Vasilatou, D.; et al. ASXL1 mutations in AML are associated with specific clinical and cytogenetic characteristics. Leuk. Lymphoma 2018, 59, 2439–2446. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Hanson, C.A.; Hodnefield, J.M.; Lasho, T.L.; Finke, C.M.; Knudson, R.A.; Ketterling, R.P.; Pardanani, A.; Tefferi, A. Differential prognostic effect of IDH1 versus IDH2 mutations in myelodysplastic syndromes: A Mayo Clinic study of 277 patients. Leukemia 2012, 26, 101–105. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Patnaik, M.M.; Saeed, L.; Mudireddy, M.; Idossa, D.; Finke, C.; Ketterling, R.P.; Pardanani, A.; Gangat, N. Targeted next-generation sequencing in myelodysplastic syndromes and prognostic interaction between mutations and IPSS-R. Am. J. Hematol. 2017, 92, 1311–1317. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Malcovati, L.; Galli, A.; Pellagatti, A.; Karimi, M.; Sato-Otsubo, A.; Sato, Y.; Suzuki, H.; Yoshizato, T.; Yoshida, K.; et al. Gene expression and risk of leukemic transformation in myelodysplasia. Blood 2017, 130, 2642–2653. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Jabbour, E.; Wang, X.; Luthra, R.; Bueso-Ramos, C.; Patel, K.; Pierce, S.; Yang, H.; Wei, Y.; Daver, N.; et al. Dynamic acquisition of FLT3 or RAS alterations drive a subset of patients with lower risk MDS to secondary AML. Leukemia 2013, 27, 2081–2083. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Chen, X.; Zhang, Y.; Wang, M.; Liu, H.; Cao, P.; Ma, X.; Wang, T.; Zhang, J.; et al. CSF3R Mutations are frequently associated with abnormalities of RUNX1, CBFB, CEBPA, and NPM1 genes in acute myeloid leukemia. Cancer 2018, 124, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017, 129, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.T.; Simonds, E.F.; Jones, C.; Hale, M.B.; Goltsev, Y.; Gibbs, K.D.J.; Merker, J.D.; Zehnder, J.L.; Nolan, G.P.; Gotlib, J. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood 2010, 116, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Lasho, T.; Finke, C.; Oh, S.T.; Gotlib, J.; Tefferi, A. LNK mutation studies in blast-phase myeloproliferative neoplasms, and in chronic-phase disease with TET2, IDH, JAK2 or MPL mutations. Leukemia 2010, 24, 1713–1718. [Google Scholar] [CrossRef]
- Grand, F.H.; Hidalgo-Curtis, C.E.; Ernst, T.; Zoi, K.; Zoi, C.; McGuire, C.; Kreil, S.; Jones, A.; Score, J.; Metzgeroth, G.; et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood 2009, 113, 6182–6192. [Google Scholar] [CrossRef]
- Milosevic, J.D.; Puda, A.; Malcovati, L.; Berg, T.; Hofbauer, M.; Stukalov, A.; Klampfl, T.; Harutyunyan, A.S.; Gisslinger, H.; Gisslinger, B.; et al. Clinical significance of genetic aberrations in secondary acute myeloid leukemia. Am. J. Hematol. 2012, 87, 1010–1016. [Google Scholar] [CrossRef]
- Stegelmann, F.; Bullinger, L.; Griesshammer, M.; Holzmann, K.; Habdank, M.; Kuhn, S.; Maile, C.; Schauer, S.; Dohner, H.; Dohner, K. High-resolution single-nucleotide polymorphism array-profiling in myeloproliferative neoplasms identifies novel genomic aberrations. Haematologica 2010, 95, 666–669. [Google Scholar] [CrossRef]
- Delhommeau, F.; Dupont, S.; Della Valle, V.; James, C.; Trannoy, S.; Masse, A.; Kosmider, O.; Le Couedic, J.P.; Robert, F.; Alberdi, A.; et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 2009, 360, 2289–2301. [Google Scholar] [CrossRef]
- Stegelmann, F.; Bullinger, L.; Schlenk, R.F.; Paschka, P.; Griesshammer, M.; Blersch, C.; Kuhn, S.; Schauer, S.; Dohner, H.; Dohner, K. DNMT3A mutations in myeloproliferative neoplasms. Leukemia 2011, 25, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Abdel-Wahab, O.; Guglielmelli, P.; Patel, J.; Caramazza, D.; Pieri, L.; Finke, C.M.; Kilpivaara, O.; Wadleigh, M.; et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia 2010, 24, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Beer, P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N. Engl. J. Med. 2010, 362, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Carbuccia, N.; Murati, A.; Trouplin, V.; Brecqueville, M.; Adelaide, J.; Rey, J.; Vainchenker, W.; Bernard, O.A.; Chaffanet, M.; Vey, N.; et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia 2009, 23, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Nagata, Y.; Kao, H.W.; Sanada, M.; Okuno, Y.; Huang, C.F.; Liang, D.C.; Kuo, M.C.; Lai, C.L.; Lee, E.H.; et al. Clonal leukemic evolution in myelodysplastic syndromes with TET2 and IDH1/2 mutations. Haematologica 2014, 99, 28–36. [Google Scholar] [CrossRef]
- Thol, F.; Weissinger, E.M.; Krauter, J.; Wagner, K.; Damm, F.; Wichmann, M.; Gohring, G.; Schumann, C.; Bug, G.; Ottmann, O.; et al. IDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosis. Haematologica 2010, 95, 1668–1674. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Biamonte, F.; Score, J.; Hidalgo-Curtis, C.; Cervantes, F.; Maffioli, M.; Fanelli, T.; Ernst, T.; Winkelman, N.; Jones, A.V.; et al. EZH2 mutational status predicts poor survival in myelofibrosis. Blood 2011, 118, 5227–5234. [Google Scholar] [CrossRef]
- Gelsi-Boyer, V.; Trouplin, V.; Adelaide, J.; Bonansea, J.; Cervera, N.; Carbuccia, N.; Lagarde, A.; Prebet, T.; Nezri, M.; Sainty, D.; et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br. J. Haematol. 2009, 145, 788–800. [Google Scholar] [CrossRef]
- Tefferi, A.; Abdel-Wahab, O.; Cervantes, F.; Crispino, J.D.; Finazzi, G.; Girodon, F.; Gisslinger, H.; Gotlib, J.; Kiladjian, J.J.; Levine, R.L.; et al. Mutations with epigenetic effects in myeloproliferative neoplasms and recent progress in treatment: Proceedings from the 5th International Post-ASH Symposium. Blood Cancer J. 2011, 1, e7. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.; Gao, J.; Adli, M.; Dey, A.; Trimarchi, T.; Chung, Y.R.; Kuscu, C.; Hricik, T.; Ndiaye-Lobry, D.; Lafave, L.M.; et al. Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. J. Exp. Med. 2013, 210, 2641–2659. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.L.; Williams, D.M.; O’Keefe, C.; Rogers, O.; Ingersoll, R.G.; Spivak, J.L.; Verma, A.; Maciejewski, J.P.; McDevitt, M.A.; Moliterno, A.R. Disruption of the ASXL1 gene is frequent in primary, post-essential thrombocytosis and post-polycythemia vera myelofibrosis, but not essential thrombocytosis or polycythemia vera: Analysis of molecular genetics and clinical phenotypes. Haematologica 2011, 96, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kitaura, J.; Matsui, H.; Hou, H.A.; Chou, W.C.; Nagamachi, A.; Kawabata, K.C.; Togami, K.; Nagase, R.; Horikawa, S.; et al. SETBP1 mutations drive leukemic transformation in ASXL1-mutated MDS. Leukemia 2015, 29, 847–857. [Google Scholar] [CrossRef]
- Tsai, S.C.; Shih, L.Y.; Liang, S.T.; Huang, Y.J.; Kuo, M.C.; Huang, C.F.; Shih, Y.S.; Lin, T.H.; Chiu, M.C.; Liang, D.C. Biological Activities of RUNX1 Mutants Predict Secondary Acute Leukemia Transformation from Chronic Myelomonocytic Leukemia and Myelodysplastic Syndromes. Clin. Cancer Res. 2015, 21, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Miller, C.B.; Radtke, I.; Phillips, L.A.; Dalton, J.; Ma, J.; White, D.; Hughes, T.P.; Le Beau, M.M.; Pui, C.H.; et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 2008, 453, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014, 28, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Graubert, T.A.; Shen, D.; Ding, L.; Okeyo-Owuor, T.; Lunn, C.L.; Shao, J.; Krysiak, K.; Harris, C.C.; Koboldt, D.C.; Larson, D.E.; et al. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat. Genet. 2011, 44, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Martignoles, J.A.; Delhommeau, F.; Hirsch, P. Genetic Hierarchy of Acute Myeloid Leukemia: From Clonal Hematopoiesis to Molecular Residual Disease. Int. J. Mol. Sci. 2018, 19, 3850. [Google Scholar] [CrossRef]
- Shih, L.Y.; Huang, C.F.; Wu, J.H.; Wang, P.N.; Lin, T.L.; Dunn, P.; Chou, M.C.; Kuo, M.C.; Tang, C.C. Heterogeneous patterns of FLT3 Asp(835) mutations in relapsed de novo acute myeloid leukemia: A comparative analysis of 120 paired diagnostic and relapse bone marrow samples. Clin. Cancer Res. 2004, 10, 1326–1332. [Google Scholar] [CrossRef]
- Mughal, T.I.; Cross, N.C.; Padron, E.; Tiu, R.V.; Savona, M.; Malcovati, L.; Tibes, R.; Komrokji, R.S.; Kiladjian, J.J.; Garcia-Manero, G.; et al. An International MDS/MPN Working Group’s perspective and recommendations on molecular pathogenesis, diagnosis and clinical characterization of myelodysplastic/myeloproliferative neoplasms. Haematologica 2015, 100, 1117–1130. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Tefferi, A. Cytogenetic and molecular abnormalities in chronic myelomonocytic leukemia. Blood Cancer J. 2016, 6, e393. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Wassie, E.A.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.; Tefferi, A. Blast transformation in chronic myelomonocytic leukemia: Risk factors, genetic features, survival, and treatment outcome. Am. J. Hematol. 2015, 90, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Wassie, E.A.; Itzykson, R.; Lasho, T.L.; Kosmider, O.; Finke, C.M.; Hanson, C.A.; Ketterling, R.P.; Solary, E.; Tefferi, A.; Patnaik, M.M. Molecular and prognostic correlates of cytogenetic abnormalities in chronic myelomonocytic leukemia: A Mayo Clinic-French Consortium Study. Am. J. Hematol. 2014, 89, 1111–1115. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Gelsi-Boyer, V.; Meggendorfer, M.; Morabito, M.; Berthon, C.; Ades, L.; Fenaux, P.; Beyne-Rauzy, O.; et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J. Clin. Oncol. 2013, 31, 2428–2436. [Google Scholar] [CrossRef]
- Coltro, G.; Mangaonkar, A.A.; Lasho, T.L.; Finke, C.M.; Pophali, P.; Carr, R.; Gangat, N.; Binder, M.; Pardanani, A.; Fernandez-Zapico, M.; et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia (CMML)—A study of 1084 patients. Leukemia 2019, 34, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Bera, R.; Chiu, M.C.; Huang, Y.J.; Lin, T.H.; Kuo, M.C.; Shih, L.Y. RUNX1 mutations promote leukemogenesis of myeloid malignancies in ASXL1-mutated leukemia. J. Hematol. Oncol. 2019, 12, 104. [Google Scholar] [CrossRef]
- Kosmider, O.; Gelsi-Boyer, V.; Ciudad, M.; Racoeur, C.; Jooste, V.; Vey, N.; Quesnel, B.; Fenaux, P.; Bastie, J.N.; Beyne-Rauzy, O.; et al. TET2 gene mutation is a frequent and adverse event in chronic myelomonocytic leukemia. Haematologica 2009, 94, 1676–1681. [Google Scholar] [CrossRef]
- Meggendorfer, M.; Bacher, U.; Alpermann, T.; Haferlach, C.; Kern, W.; Gambacorti-Passerini, C.; Haferlach, T.; Schnittger, S. SETBP1 mutations occur in 9% of MDS/MPN and in 4% of MPN cases and are strongly associated with atypical CML, monosomy 7, isochromosome i(17)(q10), ASXL1 and CBL mutations. Leukemia 2013, 27, 1852–1860. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Lasho, T.L.; Finke, C.M.; Hanson, C.A.; Hodnefield, J.M.; Knudson, R.A.; Ketterling, R.P.; Pardanani, A.; Tefferi, A. Spliceosome mutations involving SRSF2, SF3B1, and U2AF35 in chronic myelomonocytic leukemia: Prevalence, clinical correlates, and prognostic relevance. Am. J. Hematol. 2013, 88, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Lasho, T.L.; Vijayvargiya, P.; Finke, C.M.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Tefferi, A. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J. 2016, 6, e385. [Google Scholar] [CrossRef]
- Kuo, M.C.; Liang, D.C.; Huang, C.F.; Shih, Y.S.; Wu, J.H.; Lin, T.L.; Shih, L.Y. RUNX1 mutations are frequent in chronic myelomonocytic leukemia and mutations at the C-terminal region might predict acute myeloid leukemia transformation. Leukemia 2009, 23, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Routbort, M.J.; Loghavi, S.; Tang, Z.; Medeiros, L.J.; Wang, S.A. Characterization of chronic myelomonocytic leukemia with TP53 mutations. Leuk. Res. 2018, 70, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A. Etiology and Management of Therapy-Related Myeloid Leukemia. Hematology 2007, 2007, 453–459. [Google Scholar] [CrossRef]
- Rowley, J.D.; Olney, H.J. International workshop on the relationship of prior therapy to balanced chromosome aberrations in therapy-related myelodysplastic syndromes and acute leukemia: Overview report. Genes Chromosomes Cancer 2002, 33, 331–345. [Google Scholar] [CrossRef]
- Mauritzson, N.; Albin, M.; Rylander, L.; Billstrom, R.; Ahlgren, T.; Mikoczy, Z.; Bjork, J.; Stromberg, U.; Nilsson, P.G.; Mitelman, F.; et al. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976-1993 and on 5098 unselected cases reported in the literature 1974-2001. Leukemia 2002, 16, 2366–2378. [Google Scholar] [CrossRef]
- Oliai, C.; Schiller, G. How to address second and therapy-related acute myelogenous leukaemia. Br. J. Haematol. 2020, 188, 116–128. [Google Scholar] [CrossRef]
- Marusyk, A.; Porter, C.C.; Zaberezhnyy, V.; DeGregori, J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010, 8, e1000324. [Google Scholar] [CrossRef]
- Nadiminti, K.; Sidiqi, M.H.; Meleveedu, K.; Alkhateeb, H.B.; Al-Kali, A.; Hogan, W.J.; Litzow, M.; Kumar, S.K.; Patnaik, M.M.; Gertz, M.A.; et al. Characteristics and Outcomes of Therapy Related Myeloid Neoplasms in Patients with Multiple Myeloma Following Autologous Stem Cell Transplantation. Blood 2019, 134, 4560. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Halfdanarson, T.R.; Hilal, T. Assessment of Therapy-Related Myeloid Neoplasms in Patients With Neuroendocrine Tumors After Peptide Receptor Radionuclide Therapy: A Systematic Review. JAMA Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Heyssel, R.; Brill, A.B.; Woodbury, L.A.; Edwin, T.; Ghose, N.T.; Hoshino, T.; Yamasaki, M. Leukemia in Hiroshima atomic bomb survivors. Blood. 1960;15(3):313-331. Blood 2016, 127, 2165. [Google Scholar] [CrossRef]
- Morton, L.M.; Dores, G.M.; Schonfeld, S.J.; Linet, M.S.; Sigel, B.S.; Lam, C.J.K.; Tucker, M.A.; Curtis, R.E. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern Era. JAMA Oncol. 2019, 5, 318–325. [Google Scholar] [CrossRef]
- Super, H.J.; McCabe, N.R.; Thirman, M.J.; Larson, R.A.; Le Beau, M.M.; Pedersen-Bjergaard, J.; Philip, P.; Diaz, M.O.; Rowley, J.D. Rearrangements of the MLL gene in therapy-related acute myeloid leukemia in patients previously treated with agents targeting DNA-topoisomerase II. Blood 1993, 82, 3705–3711. [Google Scholar] [CrossRef]
- Micallef, I.N.M.; Lillington, D.M.; Apostolidis, J.; Amess, J.A.L.; Neat, M.; Matthews, J.; Clark, T.; Foran, J.M.; Salam, A.; Lister, T.A.; et al. Therapy-related myelodysplasia and secondary acute myelogenous leukemia after high-dose therapy with autologous hematopoietic progenitor-cell support for lymphoid malignancies. J. Clin. Oncol. 2000, 18, 947. [Google Scholar] [CrossRef] [PubMed]
- Awada, H.; Kuzmanovic, T.; Kishtagari, A.; Durrani, J.; Durmaz, A.; Hong, S.; Kerr, C.M.; Adema, V.; Guan, Y.; Sandhu, S.; et al. Mutational patterns and clonal architecture of therapy-related acute myeloid Leukemia. Blood 2019, 134, 1405. [Google Scholar] [CrossRef]
- Wong, T.N.; Ramsingh, G.; Young, A.L.; Miller, C.A.; Touma, W.; Welch, J.S.; Lamprecht, T.L.; Shen, D.; Hundal, J.; Fulton, R.S.; et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015, 518, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.S.; Ley, T.J.; Link, D.C.; Miller, C.A.; Larson, D.E.; Koboldt, D.C.; Wartman, L.D.; Lamprecht, T.L.; Liu, F.; Xia, J.; et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 2012, 150, 264–278. [Google Scholar] [CrossRef]
- Bondar, T.; Medzhitov, R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 2010, 6, 309–322. [Google Scholar] [CrossRef]
- Dudgeon, C.; Shreeram, S.; Tanoue, K.; Mazur, S.J.; Sayadi, A.; Robinson, R.C.; Appella, E.; Bulavin, D.V. Genetic variants and mutations of PPM1D control the response to DNA damage. Cell Cycle 2013, 12, 2656–2664. [Google Scholar] [CrossRef]
- Hsu, J.I.; Dayaram, T.; Tovy, A.; De Braekeleer, E.; Jeong, M.; Wang, F.; Zhang, J.; Heffernan, T.P.; Gera, S.; Kovacs, J.J.; et al. PPM1D Mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell 2018, 23, 700–713.e706. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Coombs, C.C.; Zehir, A.; Devlin, S.M.; Kishtagari, A.; Syed, A.; Jonsson, P.; Hyman, D.M.; Solit, D.B.; Robson, M.E.; Baselga, J.; et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 2017, 21, 374–382.e374. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Kahler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef]
- Soerensen, J.F.; Aggerholm, A.; Kerndrup, G.B.; Hansen, M.C.; Ewald, I.K.L.; Bill, M.; Ebbesen, L.H.; Rosenberg, C.A.; Hokland, P.; Ludvigsen, M.; et al. Clonal hematopoiesis predicts development of therapy-related myeloid neoplasms post-autologous stem cell transplantation. Blood Adv. 2020, 4, 885–892. [Google Scholar] [CrossRef]
- Churpek, J.E.; Marquez, R.; Neistadt, B.; Claussen, K.; Lee, M.K.; Churpek, M.M.; Huo, D.; Weiner, H.; Bannerjee, M.; Godley, L.A.; et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer 2016, 122, 304–311. [Google Scholar] [CrossRef]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy-related myeloid neoplasms: When genetics and environment collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Le Beau, M.M.; Huo, D.; Karrison, T.; Sobecks, R.M.; Anastasi, J.; Vardiman, J.W.; Rowley, J.D.; Larson, R.A. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: The University of Chicago series. Blood 2003, 102, 43–52. [Google Scholar] [CrossRef]
- Frohling, S.; Schlenk, R.F.; Kayser, S.; Morhardt, M.; Benner, A.; Dohner, K.; Dohner, H. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: Results from AMLSG trial AML HD98-B. Blood 2006, 108, 3280–3288. [Google Scholar] [CrossRef]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom medical research council trials. Blood 2010, 116, 354–365. [Google Scholar] [CrossRef]
- Kuzmanovic, T.; Patel, B.J.; Sanikommu, S.R.; Nagata, Y.; Awada, H.; Kerr, C.M.; Przychodzen, B.P.; Jha, B.K.; Hiwase, D.; Singhal, D.; et al. Genomics of therapy-related myeloid neoplasms. Haematologica 2020, 105, e98–e101. [Google Scholar] [CrossRef]
- Desai, P.; Mencia-Trinchant, N.; Savenkov, O.; Simon, M.S.; Cheang, G.; Lee, S.; Samuel, M.; Ritchie, E.K.; Guzman, M.L.; Ballman, K.V.; et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat. Med. 2018, 24, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Tong, R.S.; Birmann, B.M.; Druley, T.E. Clonal hematopoiesis and risk of acute myeloid leukemia. Haematologica 2019, 104, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Challen, G.A.; Birmann, B.M.; Druley, T.E. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 2016, 7, 12484. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Papula, A.L.; Poon, G.Y.P.; Wong, W.H.; Young, A.L.; Druley, T.E.; Fisher, D.S.; Blundell, J.R. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 2020, 367, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.J.; Lindsley, R.C.; Tchekmedyian, V.; Mar, B.G.; Shi, J.; Jaiswal, S.; Bosworth, A.; Francisco, L.; He, J.; Bansal, A.; et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J. Clin. Oncol. 2017, 35, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Mouhieddine, T.H.; Park, J.; Redd, R.; Gibson, C.J.; Manier, S.; Nassar, A.; Hornburg, K.; Capelletti, M.; Huynh, D.; Pistofidis, R.S.; et al. Abstract 2954: Immunomodulator maintenance post autologous stem cell transplant predicts better outcome in multiple myeloma patients with clonal hematopoiesis of indeterminate potential. Cancer Res. 2018, 78, 2954. [Google Scholar] [CrossRef]
- Husby, S.; Favero, F.; Nielsen, C.; Sørensen, B.S.; Bæch, J.; Grell, K.; Hansen, J.W.; Rodriguez-Gonzalez, F.G.; Haastrup, E.K.; Fischer-Nielsen, A.; et al. Clinical impact of clonal hematopoiesis in patients with lymphoma undergoing ASCT: A national population-based cohort study. Leukemia 2020. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Puig, N.; Cedena, M.T.; Goicoechea, I.; Valdes-Mas, R.; Vazquez, I.; Chillon, M.C.; Aguirre, P.; Sarvide, S.; Gracia-Aznárez, F.J.; et al. Biological and clinical significance of dysplastic hematopoiesis in patients with newly diagnosed multiple myeloma. Blood 2020, 135, 2375–2387. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, A.; Wang, J.; Fernald, A.A.; Davis, E.M.; Johnson, C.R.; Hu, C.; Cheng, J.X.; McNerney, M.E.; Beau, M.M.L. Cytotoxic Therapy–Induced Effects on Both Hematopoietic and Marrow Stromal Cells Promotes Therapy-Related Myeloid Neoplasms. Blood Cancer Discov. 2020. [Google Scholar] [CrossRef]
- Takahashi, K.; Wang, F.; Kantarjian, H.; Doss, D.; Khanna, K.; Thompson, E.; Zhao, L.; Patel, K.; Neelapu, S.; Gumbs, C.; et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: A case-control study. Lancet Oncol. 2017, 18, 100–111. [Google Scholar] [CrossRef]
- Gillis, N.K.; Ball, M.; Zhang, Q.; Ma, Z.; Zhao, Y.; Yoder, S.J.; Balasis, M.E.; Mesa, T.E.; Sallman, D.A.; Lancet, J.E.; et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: A proof-of-concept, case-control study. Lancet Oncol. 2017, 18, 112–121. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.; Joo, Y.D.; Lee, W.S.; Bae, S.H.; Zang, D.Y.; Kwon, J.; Kim, M.K.; Lee, J.; Lee, G.W.; et al. Prospective Randomized Comparison of Idarubicin and High-Dose Daunorubicin in Induction Chemotherapy for Newly Diagnosed Acute Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 2754–2763. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Cortes, J.E.; Hogge, D.E.; Tallman, M.S.; Kovacsovics, T.J.; Damon, L.E.; Komrokji, R.; Solomon, S.R.; Kolitz, J.E.; Cooper, M.; et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs. cytarabine/daunorubicin in older adults with untreated AML. Blood 2014, 123, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Di Agostino, S. New therapeutic strategies to treat human cancers expressing mutant p53 proteins. J. Exp. Clin. Cancer Res. 2018, 37, 30. [Google Scholar] [CrossRef]
- Kahn, J.D.; Miller, P.G.; Silver, A.J.; Sellar, R.S.; Bhatt, S.; Gibson, C.; McConkey, M.; Adams, D.; Mar, B.; Mertins, P.; et al. PPM1D-truncating mutations confer resistance to chemotherapy and sensitivity to PPM1D inhibition in hematopoietic cells. Blood 2018, 132, 1095–1105. [Google Scholar] [CrossRef]
- Litzow, M.R.; Tarima, S.; Perez, W.S.; Bolwell, B.J.; Cairo, M.S.; Camitta, B.M.; Cutler, C.S.; de Lima, M.; Dipersio, J.F.; Gale, R.P.; et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood 2010, 115, 1850–1857. [Google Scholar] [CrossRef]
- Sengsayadeth, S.; Labopin, M.; Boumendil, A.; Finke, J.; Ganser, A.; Stelljes, M.; Ehninger, G.; Beelen, D.; Niederwieser, D.; Blaise, D.; et al. Transplant outcomes for secondary acute myeloid leukemia: Acute leukemia working party of the european society for blood and bone marrow transplantation study. Biol. Blood Marrow Transpl. 2018, 24, 1406–1414. [Google Scholar] [CrossRef]
- Cutler, C. Transplantation for therapy-related, TP53-mutated myelodysplastic syndrome—Not because we can, but because we should. Haematologica 2017, 102, 1970–1971. [Google Scholar] [CrossRef][Green Version]
- Aldoss, I.; Pham, A.; Li, S.M.; Gendzekhadze, K.; Afkhami, M.; Telatar, M.; Hong, H.; Padeganeh, A.; Bedell, V.; Cao, T.; et al. Favorable impact of allogeneic stem cell transplantation in patients with therapy-related myelodysplasia regardless of TP53 mutational status. Haematologica 2017, 102, 2030–2038. [Google Scholar] [CrossRef]
- Yoshizato, T.; Nannya, Y.; Atsuta, Y.; Shiozawa, Y.; Iijima-Yamashita, Y.; Yoshida, K.; Shiraishi, Y.; Suzuki, H.; Nagata, Y.; Sato, Y.; et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: Impact on outcome of stem cell transplantation. Blood 2017, 129, 2347–2358. [Google Scholar] [CrossRef]
- Bejar, R.; Stevenson, K.E.; Caughey, B.; Lindsley, R.C.; Mar, B.G.; Stojanov, P.; Getz, G.; Steensma, D.P.; Ritz, J.; Soiffer, R.; et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J. Clin. Oncol. 2014, 32, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Chilkulwar, A.; Saliba, R.M.; Chen, J.; Rondon, G.; Patel, K.P.; Khogeer, H.; Shah, A.R.; Randolph, B.V.; Perez, J.M.R.; et al. Prognostic factors influencing survival after allogeneic transplantation for AML/MDS patients with TP53 mutations. Blood 2018, 131, 2989–2992. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.; Hunter, A.; Talati, C.; Sallman, D.A.; Asghari, H.; Song, J.; Hussaini, M.; Bejanyan, N.; Elmariah, H.; Kuykendall, A.T.; et al. Impact of TP53 gene Mutation Clearance and Conditioning Intensity on Outcome in MDS or AML Patients Prior to Allogeneic Stem Cell Transplantation. Blood 2019, 134, 149. [Google Scholar] [CrossRef]
| Epigenetic Regulators | RNA Splicing Factors | Transcriptional Regulator Genes | Activated Signaling Pathways |
|---|---|---|---|
| TET2 | SF3B1 | RUNX1 | CBL |
| IDH1/2 | SRSF2 | ETV6 | NRAS |
| DNMT3A | U2AF1 | IKZF1 | KIT |
| EZH2 | ZRSR2 | CUX1 | JAK2 |
| ASXL1 | TP53 | MPL | |
| SETBP1 | PHF6 | FLT3 | |
| NF1 |
| Functional Group | Gene | Location | Type of Mutation | Protein Function | Frequency in MDS (%) | Frequency in sAML (%) | HR for sAML | Ref. |
|---|---|---|---|---|---|---|---|---|
| Transcriptional regulators | RUNX1 | 21q22.3 | Nonsense/missense/indel | Transcription factor in hematopoiesis | 13 | 25–30 | 2.9 | [16] |
| KMT2A | 11q23 | Partial tandem duplication | Histone methyltransferase, transcription factor | 4 | 14 | 3.1 | [16] | |
| TP53 | 17p13.1 | Missense/indel | Regulate cell cycle, DNA repair, apoptosis | 10 | 15 | [10] | ||
| Epigenetic regulators | ASXL1 | 20q11 | Frameshift | Chromatin-binding associated w/PRC1/2 | 20 | 35 | 2.4 | [47,48] |
| EZH2 | 7q35-q36 | Missense, indel | LOF H3K27 methyltransferase | 4 | 9 | [10] | ||
| IDH1 | 2q34 | Missense, hotspot | Enzyme, cellular protection from oxidative stress | 5–10 | 11 | 7.0 | [46,49] | |
| IDH2 | 15q26.1 | Missense, hotspot | 5 | 11 | 3.8 | [50] | ||
| RNA splicing factors | SRSF2 | 17q25.1 | Missense/hotspot | RNA splicing factor | 15 | 20 | 2.8–3.9 | [50] |
| Activated signaling pathways | FLT3 | 13q12 | ITD | Cytokine receptor | <1 | 12–20 | 3.76 | [16,46] |
| RAS | multiple | Missense/activation | ERK/MAPK signaling | 5 | 11–23 | 3.77 | [16,46,51,52] | |
| CSF3R | 1p34.3 | Nonsense | Cytokine, controls the production, differentiation, and function of granulocytes | 3 | 8 | 6.0 | [50,53] |
| Functional Group | Gene | Location | Type of Mutation | Protein Function | Frequency in MPN | Frequency in sAML (%) | HR of sAML | Ref |
|---|---|---|---|---|---|---|---|---|
| Activated signaling pathways | FLT3 | 13q12 | FLT3-ITD | Cytokine receptor | <3% MPN | 13 | [36] | |
| SH2B3 | 12q24 | Missense (LOF), deletion | Negative regulator of JAK2 | 1% ET; 2% PMF | 13 | [55,56] | ||
| CBL | 11q23.3 | Missense (LOF) | Cytokine receptor internalization | 4% PMF | 8 | [57,58] | ||
| NRAS | 1p13.2 | Missense (activation) | ERK/MAPK signaling | Rare PMF | 8 | >2 | [34,38] | |
| NF1 | 17q11 | Missense deletion | ERK/MAPK signaling | Rare PMF | 8 | [59] | ||
| Epigenetic regulators | TET2 | 4q24 | Missense, nonsense deletion | Active 5-methyl-cytosine demethylation | 10–20% MPN | 21 | >2 | [34,60] |
| DNMT3A | 2p23.3 | Missense, hotspot | DNA methylase | 5–10% MPN | 18 | [61] | ||
| IDH1 | 2q34 | Missense, hotspot | Enzyme, cellular protection from oxidative stress | <2% PV/ET 1–4% PMF | 15–30 | 4 | [32,62,63] | |
| IHD2 | 15q26.1 | Missense, hotspot | 15–30 | 2–55 | [26,34,63] | |||
| EZH2 | 7q35-36 | Missense, indel | LOF H3K27 methyltransferase | 3% PV 5–10% PMF | 13 | 146 | [26,41] | |
| ASXL1 | 20q11.1 | Nonsense/indel | Chromatin-binding associated w/PRC1/2 | 1–3% ET/PV; 25% PMF | 25 | 2 | [32,64] | |
| Transcriptional regulators | TP53 | 17p13.1 | Missense/indel | Transcription factor, regulate cell cycle, DNA repair, apoptosis | <5% MPN | 10–20 | 15–82 | [26,34,37] |
| MDM4 | 1q32.1 | Amplification 1q | inhibits p53-mediated transcriptional activation | <1% MPN | 18 | [37] | ||
| CUX1 | 7q22 | Deletion 7q | Transcription factor regulating TP53 & ATM | <3% MPN | 17 | [39] | ||
| IKZF1 | 7p12.2 | Deletion 7p, indel | Transcription factor in lymphopoiesis | <3% MPN | 10 | [39] | ||
| RUNX1 | 21q22.3 | Nonsense/missense/indel | Transcription factor in hematopoiesis | <3% MPN | 10–15 | >2 | [34,39] | |
| RNA splicing | SRSF2 | 17q25.1 | Missense, hotspot | RNA splicing factor | <2% ET; 15% PMF | 15 | 3–74 | [26,32,65] |
| U2AF1 | 21q22.3 | Missense | RNA splicing factor | 10–15% PMF | 13 | [36,65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higgins, A.; Shah, M.V. Genetic and Genomic Landscape of Secondary and Therapy-Related Acute Myeloid Leukemia. Genes 2020, 11, 749. https://doi.org/10.3390/genes11070749
Higgins A, Shah MV. Genetic and Genomic Landscape of Secondary and Therapy-Related Acute Myeloid Leukemia. Genes. 2020; 11(7):749. https://doi.org/10.3390/genes11070749
Chicago/Turabian StyleHiggins, Alexandra, and Mithun Vinod Shah. 2020. "Genetic and Genomic Landscape of Secondary and Therapy-Related Acute Myeloid Leukemia" Genes 11, no. 7: 749. https://doi.org/10.3390/genes11070749
APA StyleHiggins, A., & Shah, M. V. (2020). Genetic and Genomic Landscape of Secondary and Therapy-Related Acute Myeloid Leukemia. Genes, 11(7), 749. https://doi.org/10.3390/genes11070749





