Abstract
Introduction: Smoking is considered the leading cause of preventable morbidity and mortality worldwide. Studies have sought to identify predictors of response to smoking cessation treatments. The aim of this study was to analyze a possible association of target gene expression for smoking cessation with varenicline. Methods: We included 74 smokers starting treatment with varenicline. Gene expression analysis was performed through the custom RT² Profiler qPCR array assay, including 17 genes. Times for sample collection were before the start of therapy (T0) and two weeks (T2) and four weeks (T4) after the start of treatment. Results: For gene expression analysis, we selected 14 patients who had success and 13 patients resistant to varenicline treatment. Success was considered to be when a patient achieved tobacco abstinence until the fourth week of treatment and resistant was when a patient had not stopped smoking as of the fourth week of treatment. We observed a significant difference for CHRNA7 gene expression: in the resistant group, samples from T2 and T4 had lower expression compared with T0 (fold change: 0.38, P = 0.007; fold change: 0.67, P = 0.004; respectively). Conclusion: This exploratory clinical study, searching for a possible predictor of effectiveness for varenicline, reaffirmed the association of the α7 nAChR subunit for nicotine dependence and smoking therapy effectiveness with varenicline.
1. Introduction
Smoking is considered the leading cause of preventable morbidity and mortality worldwide. Heart and cerebrovascular diseases, about one fifth of all cancers, lung diseases such as apnea and emphysema and prenatal disorders are associated with tobacco use [1,2,3,4].
Nicotine is the main component of addictive tobacco and acts on nicotinic acetylcholine receptors (nAChR), promoting the release of several neurotransmitters and neuroregulators such as dopamine, acetylcholine, epinephrine, norepinephrine, serotonin, β-endorphin, vasopressin and γ -aminobutyric acid (GABA). The main neurotransmitter studied is dopamine. Nicotine increases levels of dopamine in the brain’s mesocorticolimbic region, especially in the circuit known as the “reward system” (ventral tegmental area, nucleus accumbens and prefrontal cortex), generating feelings of pleasure and well-being [5,6]. The process of nicotine addiction is complex and not all mechanisms have been elucidated. In addition to the activation of the reward system, there is a formation of associative memories of hedonic effect with environments and situations related to the use of tobacco, resulting in positive reinforcement related to its consumption [7,8].
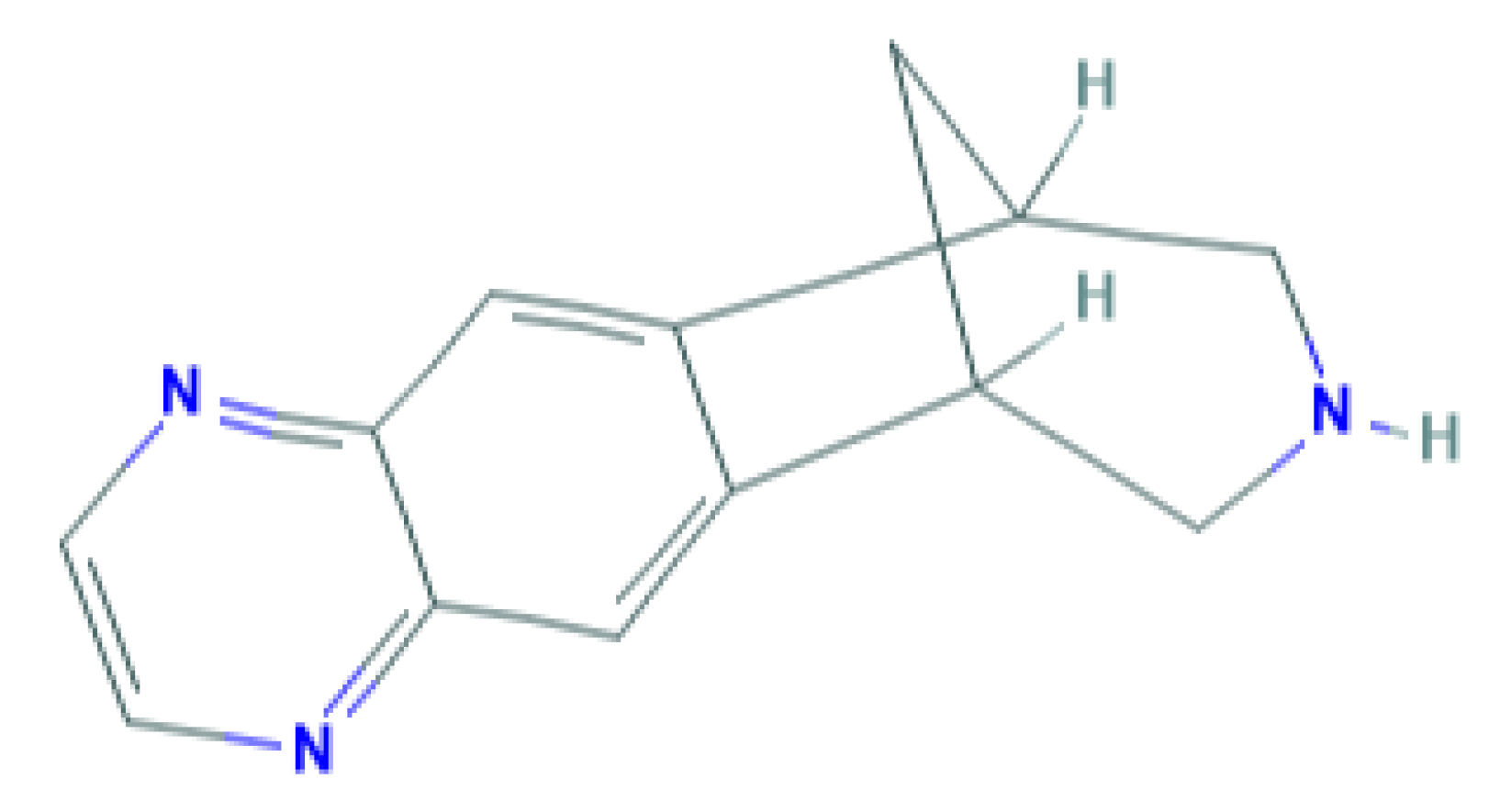
Varenicline is the newest smoking cessation drug, which has been specifically developed to act on nAChR. It was synthesized based on the chemical structure of cytisine, an alkaloid used in therapy for smoking cessation that is extracted from the Cytisus laburnum L plant [9]. It acts as a partial agonist on nAChRs composed of α4β2 subunits [10], but the drug also acts on other nAChRs, such as nAChR (α3β4), acts weakly on receptors containing α6 subunit and nAChR (α3β2), and exerts full agonist activity on α7 receptors [11]. Figure 1 shows the chemical structure of varenicline.
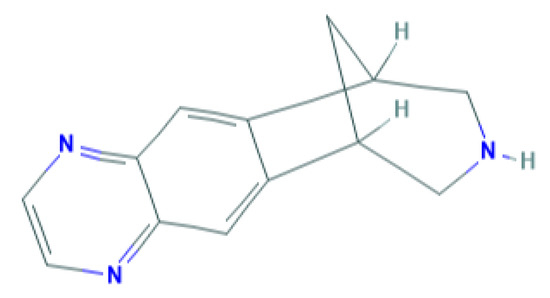
Figure 1.
Chemical structure of varenicline. Source: PubChem database.
Interindividual variability in response to drugs for smoking cessation suggests that treatments may be more effective in subgroups of smokers [12,13,14,15]. In the context of personalized medicine, researchers have sought to identify predictors of response to smoking cessation treatments, which are generally involved in pharmacokinetic and/or pharmacodynamic pathways, such as genetic factors, gene expression profiles, proteins and metabolites [16,17,18,19].
In studies of gene expression, obtaining central nervous system samples of patients is not feasible. As an alternative, studies have proposed the analysis of gene expression in peripheral blood [20,21,22]. Although not the target tissue of pharmacodynamics of drugs for smoking cessation, blood has many transcriptional similarities to multiple brain regions [23]. In this context, the main aim of this study was to analyze a possible association of target gene expression in samples from peripheral blood mononuclear cells from patients treated with varenicline.
2. Materials and Methods
2.1. Patient Sample
A prospective cohort study enrolled 74 patients, men and women, smokers, aged ≥ 18 years, body mass index ≥ 18.5 and <30 kg/m2, from the Smoking Assistance Program (PAF), Instituto do Coracao (InCor), Hospital das Clinicas, Faculdade de Medicina, Universidade de Sao Paulo (HCFMUSP), Sao Paulo, SP, Brazil. The protocol was approved by the Ethics Committee for Human Medical Research of the Clinical Hospital of the School of Medicine, University of São Paulo (CAAE 60133816.0.0000.0068; SDC 4341/16/007). Patients were informed of the study and, if they consented, were included and signed an informed consent form in accordance with the Declaration of Helsinki, and all methods were performed according to good clinical practice guidelines.
Exclusion criteria were: patients with hepatic, renal and gastrointestinal disorders that compromised drug metabolism and elimination; patients who had taken cytochrome P450 enzyme-inducing or inhibiting drugs in the previous 6 weeks; alcoholic patients and drug users; those with unstable psychiatric illnesses; women at risk of pregnancy; patients with contraindications to the treatment with varenicline mentioned above.
The patients were followed by the InCor-PAF team, and pharmacological treatment with varenicline was conducted for 12 weeks in dosages according to the package insert (from the 1st to the 3rd day, 0.5 mg once a day; from the 4th to the 7th day, 0.5 mg twice daily; and from the eighth day until the end of treatment, 1 mg twice daily). Patient data were collected in four visits at the following times: T1 (initial visit), T0 (before the start of pharmacological treatment), T2 and T4 (two and four weeks after the start of pharmacological treatment, respectively).
The following treatment outcomes were considered: success, when the patient achieved tobacco abstinence until T4, and resistant, when the patient could not stop smoking by the end of T4. Smoking abstinence was confirmed by the measurement of exhaled carbon monoxide in all visits. Patients with carbon monoxide values <4 ppm were considered abstinent.
The customized assay for the analysis of gene expression was a major financial investment in this research and, therefore, we used it to analyze only the samples of patients treated with varenicline, since it is the newest, most effective and most costly drug in the anti-smoking treatment. The 17 genes with the greatest potential for association with response to treatment with varenicline were chosen, according to previous studies [16,18,24,25].
Patients who started treatment with varenicline underwent blood collection for RNA in three time periods: T0, T2 and T4. For blood collection, PAXgene Blood RNA tubes (PreAnalytiX GmbH, Feldbachstrasse, Hombrechtikon, Switzerland) were used, and, after, they were incubated for 2 h at room temperature to ensure complete lysis of blood cells and then stored at −20°C until RNA was extracted from the samples.
As for the sample number for the gene expression assay, of the 74 patients treated with varenicline, samples were chosen from 27 patients (14 in the success group and 13 in the resistant group), a number based on previous studies [26,27], who correctly followed fasting, took the medication at the agreed times and attended all collection times. Altogether, there were 81 samples (from the 27 patients) due to the 3 treatment times (T0, T2 and T4). However, some samples did not show good results for the analysis of gene expression and we performed the expression test for 60 samples of different times. Eight samples did not show amplification results, and we ended up with 52 samples in all (9 resistant from time T0, 7 resistant from T2, 8 resistant from T4, 9 successes from T0, 8 successes from T2 and 11 successes from T4) from 14 patients in the success group and 13 in the resistant group. This final sample size for each analysis group is comparable to previous studies [27].
2.2. RNA Extraction, cDNA Synthesis and gene Expression Assay
The RNA purification and concentration process was performed using the PAXgene Blood RNA Kit (PreAnalytiX GmbH, Feldbachstrasse, Hombrechtikon, Switzerland). The cDNA synthesis process was performed using the RT2 HT First Strand Kit (Qiagen, Frederick, Maryland, USA) as described by the manufacturer. All cDNA samples were analyzed for quality by quality-control assay (QC disc, Qiagen).
The expression protocol was performed by real-time PCR using the Rotor Gene 6000 (Corbett) equipment with a custom RT² Profiler PCR Array assay with RT² FAST SYBR Green/ROX qPCR Master Mix (Qiagen, Frederick, Maryland, USA). For gene expression assay, 4 housekeeping genes (B2M, GAPDH, HPRT1 and ACTB, Supplementary panel 1) were used and 17 target genes were analyzed (CHRNA3, CHRNA4, CHRNA5, CHRNA6, CHRNA7, CHRNB2, CHRNB3, CHRNB4, CHRNG, DRD1, DRD2, DRD3, DRD4, HTR3A, HTR3B, COMT, SLC6A3; Supplementary panel 2). Real-time PCR was programmed for PCR cycling under the following conditions: 1 cycle at 95 °C lasting 10 min; 40 cycles at 95 °C for 15 s and 60 °C for 30 s. Using the real-time cycler software (Qiagen Frederick, Maryland, USA), the threshold cycle was adjusted and the CT data were spread out for statistical analysis.
2.3. Statistical Analysis
Regarding demographic characteristics, continuous variables were presented as mean and standard deviation and categorical variables as frequencies. For the analysis of expression data, the 2−∆∆CT method was used [28]. Values of CT were normalized by housekeeping gene expression using the following formula: ∆CT = (CTgene target - the mean of CThousekeeping genes); ∆CT values are shown in Supplementary Table S1. Shapiro–Wilk and Kolmogorov–Smirnov tests were performed to verify the normality of gene expression levels (2−∆CT values). The 2−∆CT median values of each group were used for Mann–Whitney hypothesis testing (because the distribution of values is not normal). The values of ∆∆CT (median of ∆CTtest group - median of ∆CTreference group) were obtained for the calculation of fold change. The fold change values were generated using the following formula: 2−∆∆CT. The heat map graph was constructed using Gene-globe software (Qiagen, Frederick, Maryland, USA) to verify the differences in the expression profile of the studied genes according to the times (T0, T2 and T4) and according to the effectiveness of varenicline treatment. All statistical analysis was performed using SPSS software (version 20, IBM corp, Armonk, NewYork, USA), considering a significance level of P < 0.05.
3. Results
From the 74 patients treated with varenicline, we selected samples from 14 patients from the success group and 13 from the resistant group. Table 1 shows the clinical and demographic characteristics of the patients selected for the gene expression assay.

Table 1.
Clinical and demographic characteristics of patients treated with varenicline according to the treatment outcome.
Table 2 shows fold change and the association analysis between time periods and outcome groups. Of the 17 genes chosen to test treatment response, four showed amplification values which were more acceptable for the quality of the interpretation, more specifically CT values <33. We observed significant differences for CHRNA7 gene expression: in the resistant group, samples from T2 and T4 had lower expression compared with samples from T0 (fold change: 0.38, P = 0.007; fold change: 0.67, P = 0.004; respectively). For CHRNG gene expression, samples from T2 of the success group had lower expression compared with samples from T2 of the resistant group (fold change = 0.77, P = 0.006). We performed statistical correction for age, cigarettes per day and gender. These variables do not impact the identified significant data.

Table 2.
Fold change and association analysis between time periods and outcome groups.
Supplementary Figure S1 shows a heat map of gene expression magnitude. The heat map shows that there was no differentiation in gene expression profiles in a joint gene analysis between outcome and treatment times.
4. Discussion
The present clinical and exploratory study shows the results of CHRNA7 and CHRNG gene expression according to the collection times and the treatment outcome. Samples of the resistant group at times T2 and T4, that is, with varenicline, show lower levels of CHRNA7 gene expression compared to T0 samples (before starting pharmacological treatment). The CHRNA7 gene encodes the nAChR alpha7 subunit. These results suggest that the downregulation of α7 may have been responsible for the inability to stop smoking in individuals of the resistant group, which was not observed in samples of the success group.
The α7 subunit has great importance, is present in several tissues and has had a consistent association in several studies with the process of nicotine dependence (ND) cognitive and immunological processes [29]. Studies have also shown that there is a locus containing a truncated CHRNA7 gene duplication, composed of 5–10 CHRNA7 gene exons, preceded by four unique exons and a two-base-pair deletion in exon 6, which they named CHRNFAN7. Most individuals have one or two copies of this gene and it is rare for this copy to be absent [30].
In the brain of Sprague Dawley rats, Ryan and Loiacono [31] detected transcribed α7 levels at various locations, such as the substantia nigra pars compacta (SNpc), substantia nigra pars reticular (SNpr), ventral tegmental area (VTA), cortex and hippocampus. The rats submitted to chronic nicotine treatment showed higher levels of α7 subunit mRNA in SNpc, SNpr and VTA compared to the control group. VTA is part of the mesolimbic reward system, which is widely studied in ND processes [5,6]. In the present study (in peripheral blood mononuclear cell—PBMC samples), the resistant patient group also showed higher α7 expression when only nicotine was present (T0). By introducing varenicline, expressions were decreased at the T2 and T4 times, probably due to the competitive antagonism of varenicline with nicotine at the receptor.
Benhammou et al. [32] conducted a study with post-mortem brain samples from smokers and blood samples from smokers and non-smoker volunteers. Through the use of radiolabelled nicotine in brain samples, they showed that nicotine binding sites increased in proportion to the number of cigarettes per day. The same study analyzed the gene expression and protein in lymphocytes and polymorphonuclear cells for a subset of nicotinic receptor subunits; these cells, as well as brain tissue, exhibited higher numbers of high-affinity nicotine binding sites in smokers compared with non-smokers.
The varenicline in functional studies showed full agonist activity at the α7 receptors [11]. In one study, chronic treatment was performed with varenicline and/or nicotine in mice (10 days). Brain and plasma drug levels, and tolerance and expression of four nAChR subtypes after an acute dose of nicotine, were checked using autoradiography. Upregulation of α4β2 receptors, due to chronic varenicline treatment, was similar to that promoted by nicotine. Both varenicline and nicotine promoted downregulation of α6β2 nAChR. Varenicline significantly increased α3β4 and α7 levels, while nicotine had a minor effect on these sites. The combination of nicotine and varenicline was similar to varenicline alone for the α3β4 sites, while for the α7 site the combination promotes upregulation in fewer brain regions compared to varenicline monotherapy [33]. In the present study, resistant group patients showed similar effects associated with the combined use of varenicline and nicotine (due to smoking). CHRNA7 expression levels were not maintained as they were in patients who were successful in treatment. However, the success group was only using varenicline in the absence of nicotine between times T2 and T4, which suggests that this downregulation may occur in the response subgroup.
Several studies showed differences in α7 gene expression in sample patients with clinical psychiatric diagnosis compared to healthy individuals. Kunii et al. [34] showed a difference in α7 expression in dorsolateral prefrontal cortex postmortem tests in patients with depression, anxiety and bipolar disorder, and several studies showed lower α7 expression levels in patients with schizophrenia [27,35,36]. Studies of individuals with schizophrenia are great models for understanding the importance of the α7 receptor in cognitive processes in ND and how these mechanisms are closely related. Several studies showed that there is a higher prevalence of smoking in individuals with schizophrenia compared to the general population [37]. Mexal et al. [27] performed a study with post-mortem brain samples from four distinct groups: control, smoker control, non-smoker schizophrenia patients and smoker schizophrenia patients. The study performed mRNA and protein expression assays by real-time quantitative PCR and western blot, respectively. The PCR probes were designed to amplify the complete CHRNA7 gene and its duplicate copy, the CHRFAM7A gene. Non-smokers with schizophrenia present reduced mRNA and protein expression compared to smoker schizophrenic patients. Non-smokers with schizophrenia present lower expression than non-smoker controls, but this difference was statistically significant only at the mRNA level. Other brain post-mortem studies showed lower nicotinic receptor expression in the cortex and hippocampus of individuals with schizophrenia compared to healthy controls [35,36,38,39]. CHRFAM7A, like CHRNA7, is polymorphic, and variants in this gene were associated with auditory pathology in cases of schizophrenia. Differentiated processes in transcription mechanisms may contribute to abnormal α7 functioning, such as promoter variations, alternative splicing and/or linkage disequilibrium with SNPs (single-nucleotide polymorphisms) located at the neuroregulin locus (NRG1) on chr8 (the gene encoding the cell adhesion molecule involved in human synaptic neuroplasticity). These processes may be involved in schizophrenia and bipolar disorder endophenotypes [30,40,41,42,43,44,45]. Nicotine seems to improve hearing in individuals with α7-associated hearing deficit in schizophrenia, allowing better filtering of external auditory stimuli. Nicotine improves cognition in schizophrenia, and alternative agents that activate the nicotinic receptor have been tested and these compounds improved attention, working memory, and negative symptoms in a complementary study of non-smoking patients with schizophrenia [46].
The studies cited show us strong evidence of the association of α7 with ND, cognitive processes and varenicline response. These findings may explain our results regarding decreased expression of α7 in the group resistant to treatment. The success group patients maintained gene expression levels in response to varenicline, while, in the resistant group, there was a downregulation. We suggest that, possibly, this decrease may have been responsible, at least in part, for patients failing to quit smoking. Nicotine may be compensating for an α7 deficit that resistant individuals may have. However, we do not know what the α7 expression would be in these individuals in the absence of smoking. Another possibility is that the association occurred by chance: the response to varenicline in the resistant group could have resulted in the downregulation of CHRNA7, due to the genetic or epigenetic polymorphism at CHRNA7 or a regulator of CHRNA7, but without influencing lapse/relapse likelihood.
For the CHNRG gene, there are a paucity of studies in the literature. This encodes the γ subunit of nicotinic receptors. Most studies have indicated an association of the subunit with skeletal muscle disorders [47,48,49,50]. However, King et al. [16] found the association of polymorphisms in this gene with nausea in varenicline treatment. Keskitalo-Vuokko et al. [51] found the association of SNPs in the CHRNG-CHRND gene cluster loci (adjacent genes, the last encoding the nAChR delta subunit) with cotinine level. Saccone et al. [52] found the association of four loci in the CHRND-CHRNG cluster with ND. In another study, variants in CHRND-CHRNG genes showed a modest association with the risk of ND in African American samples [53]. In a study in which a rat model of schizophrenia was used, in the clozapine-treated animal group Chrng downregulation was found in the nucleus accumbens. In this same study, Santoro et al. failed to formulate a hypothesis for the finding due to the lack of information in the literature focusing on ND or cognitive performance [54].
There are limitations to the study. First, the gene expression assay was performed with peripheral blood samples rather than using central nervous system (CNS) samples, which could show us a clearer and more direct relationship of the drug to the response pathway in the brain. However, obtaining brain biopsy samples is not feasible. Cerebrospinal fluid could be used, but it is a painful procedure for the patient. In contrast, studies have already shown that there is similarity between the gene expressions of CNS cells and peripheral blood cells for some of these nicotinic receptor genes [27]. Second, the sample size of the success and resistant patient groups is small, but this patient cohort is well phenotyped for antismoking therapy. Third, no gene expression assay was performed for samples from non-smokers, which could provide more parameters for comparison with resistant and success groups. Fourth, we did not achieve the required quality (measurable amplification) in potentially important gene analysis.
5. Conclusions
In this exploratory clinical study searching for a possible predictor of the effectiveness of varenicline, we reaffirmed the association of the α7 nAChR subunit with nicotine dependence and treatment effect with varenicline. This data could contribute to the development of personalized therapy with varenicline. However, additional studies are needed to further understand the role of the α7 and γ nAChR subunits in smoking cessation therapy using varenicline.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/7/746/s1. Table S1: Median values of ∆CT genes according to time periods and outcome groups. Figure S1: Heat map of gene expression. Supplementary panel 1: Housekeeping genes. Supplementary panel 2: Genes chosen for expression assay.
Author Contributions
J.R.S. (Juliana Rocha Santos); P.R.X.T. and P.C.J.d.L.S. carried out the molecular genetics and statistical analysis and drafted the manuscript. P.C.J.d.L.S., J.R.S. (Juliana Rocha Santos); J.R.S. (Jaqueline Ribeiro Scholz); T.O.A.; P.V.G.; J.E.K. and A.C.P. participated in the design of the study. J.R.S. (Jaqueline Ribeiro Scholz); T.O.A. and P.V.G. selected the patients. J.E.K. and A.C.P. ceded the facilities. All authors contributed critically to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo, Brazil)-2013/09295-3 and 2019/08338-7. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001 (JR Santos PRX and Tomaz are recipients of fellowships from CAPES, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Proc. 470410/2013-2, Brazil.
Acknowledgments
We thank the patients who participated in the study, the technical assistance of the Laboratory of Genetics and Molecular Cardiology group and FAPESP, CAPES and CNPq for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oliveira, A.F.; Valente, J.G.; Leite, I.C. Aspects of tobacco attributable mortality: Systematic review. Rev. Saude Publica 2008, 42, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Koshiaris, C.; Aveyard, P.; Oke, J.; Ryan, R.; Szatkowski, L.; Stevens, R.; Farley, A. Smoking cessation and survival in lung, upper aero-digestive tract and bladder cancer: Cohort study. Br. J. Cancer 2017, 117, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, T.; Toyras, J.; Mervaala, E.; Penzel, T.; Kulkas, A. Severity of individual obstruction events increases with age in patients with obstructive sleep apnea. Sleep Med. 2017, 37, 32–37. [Google Scholar] [CrossRef]
- Cerda, J.; Bambs, C.; Vera, C. Infant morbidity and mortality attributable to prenatal smoking in Chile. Rev. Panam. Salud Publica 2017, 41, e106. [Google Scholar] [PubMed]
- Schultz, W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000, 1, 199–207. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef]
- Martin-Soelch, C. Neuroadaptive changes associated with smoking: Structural and functional neural changes in nicotine dependence. Brain Sci. 2013, 3, 159–176. [Google Scholar] [CrossRef]
- Boening, J.A. Neurobiology of an addiction memory. J. Neural Transm. 2001, 108, 755–765. [Google Scholar] [CrossRef]
- Cahill, K.; Stead, L.F.; Lancaster, T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Kassem, M.G.; Al Hossaini, A.M. Varenicline. Profiles Drug Subst. Excip. Relat. Methodol. 2012, 37, 389–411. [Google Scholar]
- Mihalak, K.B.; Carroll, F.I.; Luetje, C.W. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol. Pharmacol. 2006, 70, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, P.R.X.; Kajita, M.S.; Santos, J.R.; Scholz, J.; Abe, T.O.; Gaya, P.V.; Krieger, J.E.; Pereira, A.C.; Santos, P. Cytochrome P450 2A6 and 2B6 polymorphisms and smoking cessation success in patients treated with varenicline. Eur. J. Clin. Pharmacol. 2019, 75, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, P.R.X.; Santos, J.R.; Scholz, J.; Abe, T.O.; Gaya, P.V.; Negrao, A.B.; Krieger, J.E.; Pereira, A.C.; Santos, P. Cholinergic receptor nicotinic α 5 subunit polymorphisms are associated with smoking cessation success in women. BMC Med. Genet. 2018, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Santos, P.C.; Buzo, C.G.; Lopes, N.H.; Abe, T.O.; Gaya, P.V.; Pierri, H.; Amorim, C.; Pereira, A.C. Effects of aging on the effectiveness of smoking cessation medication. Oncotarget 2016, 7, 30032–30036. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.S.; Santos, P.C.; Vieira, L.P.; Abe, T.O.; Kuperszmidt, C.S.; Nakasato, M.; Cardoso, E.; Amorim, C.; Pereira, A.C. Smoking cessation and weight gain in patients with cardiovascular disease or risk factor. Int. J. Cardiol. 2014, 172, 485–487. [Google Scholar] [CrossRef] [PubMed]
- King, D.P.; Paciga, S.; Pickering, E.; Benowitz, N.L.; Bierut, L.J.; Conti, D.V.; Kaprio, J.; Lerman, C.; Park, P.W. Smoking cessation pharmacogenetics: Analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology 2012, 37, 641–650. [Google Scholar] [CrossRef]
- Saccone, N.L.; Baurley, J.W.; Bergen, A.W.; David, S.P.; Elliott, H.R.; Foreman, M.G.; Kaprio, J.; Piasecki, T.M.; Relton, C.L.; Zawertailo, L.; et al. The Value of Biosamples in Smoking Cessation Trials: A Review of Genetic, Metabolomic, and Epigenetic Findings. Nicotine Tob. Res. 2018, 20, 403–413. [Google Scholar] [CrossRef]
- Rocha Santos, J.; Tomaz, P.R.; Issa, J.S.; Abe, T.O.; Krieger, J.E.; Pereira, A.C.; Santos, P.C. CHRNA4 rs1044396 is associated with smoking cessation in varenicline therapy. Front. Genet. 2015, 6, 46. [Google Scholar] [CrossRef]
- Tomaz, P.R.; Santos, J.R.; Issa, J.S.; Abe, T.O.; Gaya, P.V.; Krieger, J.E.; Pereira, A.C.; Santos, P.C. CYP2B6 rs2279343 polymorphism is associated with smoking cessation success in bupropion therapy. Eur. J. Clin. Pharmacol. 2015, 71, 1067–1073. [Google Scholar] [CrossRef]
- Middleton, F.A.; Pato, C.N.; Gentile, K.L.; McGann, L.; Brown, A.M.; Trauzzi, M.; Diab, H.; Morley, C.P.; Medeiros, H.; Macedo, A.; et al. Gene expression analysis of peripheral blood leukocytes from discordant sib-pairs with schizophrenia and bipolar disorder reveals points of convergence between genetic and functional genomic approaches. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 136, 12–25. [Google Scholar] [CrossRef][Green Version]
- Takahashi, M.; Hayashi, H.; Watanabe, Y.; Sawamura, K.; Fukui, N.; Watanabe, J.; Kitajima, T.; Yamanouchi, Y.; Iwata, N.; Mizukami, K.; et al. Diagnostic classification of schizophrenia by neural network analysis of blood-based gene expression signatures. Schizophr. Res. 2010, 119, 210–218. [Google Scholar] [CrossRef]
- Lee, J.; Goh, L.K.; Chen, G.; Verma, S.; Tan, C.H.; Lee, T.S. Analysis of blood-based gene expression signature in first-episode psychosis. Psychiatry Res. 2012, 200, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Fan, C.; Perou, C.M. Evaluating the comparability of gene expression in blood and brain. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Swan, G.E.; Javitz, H.S.; Jack, L.M.; Wessel, J.; Michel, M.; Hinds, D.A.; Stokowksi, R.P.; McClure, J.B.; Catz, S.L.; Richards, J.; et al. Varenicline for smoking cessation: Nausea severity and variation in nicotinic receptor genes. Pharm. J. 2012, 12, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Kordi-Tamandani, D.M.; Tajoddini, S.; Salimi, F. Promoter Methylation and BDNF and DAT1 Gene Expression Profiles in Patients with Drug Addiction. Pathobiology 2015, 82, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ota, V.K.; Noto, C.; Gadelha, A.; Santoro, M.L.; Silva, P.N.; Melaragno, M.I.; Smith Mde, A.; Cordeiro, Q.; Bressan, R.A.; Belangero, S.I. Neurotransmitter receptor and regulatory gene expression in peripheral blood of Brazilian drug-naive first-episode psychosis patients before and after antipsychotic treatment. Psychiatry Res. 2013, 210, 1290–1292. [Google Scholar] [CrossRef] [PubMed]
- Mexal, S.; Berger, R.; Logel, J.; Ross, R.G.; Freedman, R.; Leonard, S. Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. J. Mol. Neurosci. 2010, 40, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sinkus, M.L.; Graw, S.; Freedman, R.; Ross, R.G.; Lester, H.A.; Leonard, S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology 2015, 96, 274–288. [Google Scholar] [CrossRef]
- Riley, B.; Williamson, M.; Collier, D.; Wilkie, H.; Makoff, A. A 3-Mb map of a large Segmental duplication overlapping the alpha7-nicotinic acetylcholine receptor gene (CHRNA7) at human 15q13-q14. Genomics 2002, 79, 197–209. [Google Scholar] [CrossRef]
- Ryan, R.E.; Loiacono, R.E. Nicotine regulates alpha7 nicotinic receptor subunit mRNA: Implications for nicotine dependence. Neuroreport 2001, 12, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Benhammou, K.; Lee, M.; Strook, M.; Sullivan, B.; Logel, J.; Raschen, K.; Gotti, C.; Leonard, S. [(3)H]Nicotine binding in peripheral blood cells of smokers is correlated with the number of cigarettes smoked per day. Neuropharmacology 2000, 39, 2818–2829. [Google Scholar] [CrossRef]
- Marks, M.J.; O’Neill, H.C.; Wynalda-Camozzi, K.M.; Ortiz, N.C.; Simmons, E.E.; Short, C.A.; Butt, C.M.; McIntosh, J.M.; Grady, S.R. Chronic treatment with varenicline changes expression of four nAChR binding sites in mice. Neuropharmacology 2015, 99, 142–155. [Google Scholar] [CrossRef]
- Kunii, Y.; Zhang, W.; Xu, Q.; Hyde, T.M.; McFadden, W.; Shin, J.H.; Deep-Soboslay, A.; Ye, T.; Li, C.; Kleinman, J.E.; et al. CHRNA7 and CHRFAM7A mRNAs: Co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am. J. Psychiatry 2015, 172, 1122–1130. [Google Scholar] [CrossRef]
- Martin-Ruiz, C.M.; Haroutunian, V.H.; Long, P.; Young, A.H.; Davis, K.L.; Perry, E.K.; Court, J.A. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol. Psychiatry 2003, 54, 1222–1233. [Google Scholar] [CrossRef]
- Marutle, A.; Zhang, X.; Court, J.; Piggott, M.; Johnson, M.; Perry, R.; Perry, E.; Nordberg, A. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J. Chem. Neuroanat. 2001, 22, 115–126. [Google Scholar] [CrossRef]
- De Leon, J.; Diaz, F.J. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005, 76, 135–157. [Google Scholar] [CrossRef]
- Breese, C.R.; Lee, M.J.; Adams, C.E.; Sullivan, B.; Logel, J.; Gillen, K.M.; Marks, M.J.; Collins, A.C.; Leonard, S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology 2000, 23, 351–364. [Google Scholar] [CrossRef]
- Leonard, S.; Breese, C.; Adams, C.; Benhammou, K.; Gault, J.; Stevens, K.; Lee, M.; Adler, L.; Olincy, A.; Ross, R.; et al. Smoking and schizophrenia: Abnormal nicotinic receptor expression. Eur. J. Pharmacol. 2000, 393, 237–242. [Google Scholar] [CrossRef]
- Dempster, E.L.; Toulopoulou, T.; McDonald, C.; Bramon, E.; Walshe, M.; Wickham, H.; Sham, P.C.; Murray, R.M.; Collier, D.A. Episodic memory performance predicted by the 2bp deletion in exon 6 of the “α 7-like” nicotinic receptor subunit gene. Am. J. Psychiatry 2006, 163, 1832–1834. [Google Scholar] [CrossRef] [PubMed]
- Flomen, R.H.; Collier, D.A.; Osborne, S.; Munro, J.; Breen, G.; St Clair, D.; Makoff, A.J. Association study of CHRFAM7A copy number and 2 bp deletion polymorphisms with schizophrenia and bipolar affective disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Gault, J.; Hopkins, J.; Berger, R.; Drebing, C.; Logel, J.; Walton, C.; Short, M.; Vianzon, R.; Olincy, A.; Ross, R.G.; et al. Comparison of polymorphisms in the alpha7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003, 123, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Perl, O.; Strous, R.D.; Dranikov, A.; Chen, R.; Fuchs, S. Low levels of alpha7-nicotinic acetylcholine receptor mRNA on peripheral blood lymphocytes in schizophrenia and its association with illness severity. Neuropsychobiology 2006, 53, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.V.; Law, A.J.; Lipska, B.K.; Davila-Garcia, M.I.; Zamora, E.D.; Mitkus, S.N.; Vakkalanka, R.; Straub, R.E.; Weinberger, D.R.; Kleinman, J.E.; et al. Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum. Mol. Genet. 2007, 16, 2921–2932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Severance, E.G.; Dickerson, F.B.; Stallings, C.R.; Origoni, A.E.; Sullens, A.; Monson, E.T.; Yolken, R.H. Differentiating nicotine- versus schizophrenia-associated decreases of the alpha7 nicotinic acetylcholine receptor transcript, CHRFAM7A, in peripheral blood lymphocytes. J. Neural Transm. 2009, 116, 213–220. [Google Scholar] [CrossRef]
- Olincy, A.; Freedman, R. Nicotinic mechanisms in the treatment of psychotic disorders: A focus on the alpha7 nicotinic receptor. Handb. Exp. Pharmacol 2012, 211–232. [Google Scholar] [CrossRef]
- Kariminejad, A.; Almadani, N.; Khoshaeen, A.; Olsson, B.; Moslemi, A.R.; Tajsharghi, H. Truncating CHRNG mutations associated with interfamilial variability of the severity of the Escobar variant of multiple pterygium syndrome. BMC Genet. 2016, 17, 71. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Yang, Y.K.; Liang, Y.; Zhang, T.J.; Liang, N.; Yang, L.M.; Li, S.J.; Shan, D.; Wu, Q.Q. Prenatal diagnosis of fetal skeletal dysplasia using targeted next-generation sequencing: An analysis of 30 cases. Diagn. Pathol. 2019, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Natera-de Benito, D.; Topf, A.; Vilchez, J.J.; Gonzalez-Quereda, L.; Dominguez-Carral, J.; Diaz-Manera, J.; Ortez, C.; Bestue, M.; Gallano, P.; Dusl, M.; et al. Molecular characterization of congenital myasthenic syndromes in Spain. Neuromuscul. Disord. 2017, 27, 1087–1098. [Google Scholar] [CrossRef]
- Seo, J.; Choi, I.H.; Lee, J.S.; Yoo, Y.; Kim, N.K.; Choi, M.; Ko, J.M.; Shin, Y.B. Rare cases of congenital arthrogryposis multiplex caused by novel recurrent CHRNG mutations. J. Hum. Genet. 2015, 60, 213–215. [Google Scholar] [CrossRef]
- Keskitalo-Vuokko, K.; Pitkaniemi, J.; Broms, U.; Heliovaara, M.; Aromaa, A.; Perola, M.; Ripatti, S.; Salminen, O.; Salomaa, V.; Loukola, A.; et al. Associations of nicotine intake measures with CHRN genes in Finnish smokers. Nicotine Tob. Res. 2011, 13, 686–690. [Google Scholar] [CrossRef]
- Saccone, N.L.; Saccone, S.F.; Hinrichs, A.L.; Stitzel, J.A.; Duan, W.; Pergadia, M.L.; Agrawal, A.; Breslau, N.; Grucza, R.A.; Hatsukami, D.; et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Saccone, N.L.; Schwantes-An, T.H.; Wang, J.C.; Grucza, R.A.; Breslau, N.; Hatsukami, D.; Johnson, E.O.; Rice, J.P.; Goate, A.M.; Bierut, L.J. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010, 9, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.L.; Ota, V.K.; Stilhano, R.S.; Silva, P.N.; Santos, C.M.; Diana, M.C.; Gadelha, A.; Bressan, R.A.; Melaragno, M.I.; Han, S.W.; et al. Effect of antipsychotic drugs on gene expression in the prefrontal cortex and nucleus accumbens in the spontaneously hypertensive rat (SHR). Schizophr. Res. 2014, 157, 163–168. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).