Abstract
This study aims to determine whether genetic variants that influence CYP3A4 expression are associated with platelet reactivity in clopidogrel-treated patients undergoing elective percutaneous coronary intervention (PCI), and to evaluate the influence of statin/fibrate co-medication on these associations. A study cohort was used containing 1124 consecutive elective PCI patients in whom CYP3A4*22 and PPAR-α (G209A and A208G) SNPs were genotyped and the VerifyNow P2Y12 platelet reactivity test was performed. Minor allele frequencies were 0.4% for CYP3A4*22/*22, 6.8% for PPAR-α G209A AA, and 7.0% for PPAR-α A208G GG. CYP3A4*22 was not associated with platelet reactivity. The PPAR-α genetic variants were significantly associated with platelet reactivity (G209A AA: −24.6 PRU [−44.7, −4.6], p = 0.016; A208G GG: −24.6 PRU [−44.3, −4.8], p = 0.015). Validation of these PPAR-α results in two external cohorts, containing 716 and 882 patients, respectively, showed the same direction of effect, although not statistically significant. Subsequently, meta-analysis of all three cohorts showed statistical significance of both variants in statin/fibrate users (p = 0.04 for PPAR-a G209A and p = 0.03 for A208G), with no difference in statin/fibrate non-users. In conclusion, PPAR-α G209A and A208G were associated with lower platelet reactivity in patients undergoing elective PCI who were treated with clopidogrel and statin/fibrate co-medication. Further research is necessary to confirm these findings.
Keywords:
clopidogrel; PPAR; statin; fibrate; CYP3A4; genotyping; pharmacogenomics; PCI; personalized medicine 1. Introduction
Dual antiplatelet therapy (DAPT), i.e., the combination of clopidogrel and aspirin, significantly reduces the risk of cardiovascular death, myocardial infarction, or urgent target vessel revascularization, compared to aspirin monotherapy in patients undergoing percutaneous coronary intervention (PCI) [1]. However, the response to clopidogrel varies between patients. This variability is caused by several factors, including polymorphisms in genes encoding for enzymes involved in the metabolism of clopidogrel, drug interactions, a history of diabetes, smoking status, body mass index (BMI), and age [2,3,4,5,6,7,8,9].
Clopidogrel is a prodrug activated by cytochrome P450 enzymes through two oxidative steps into an active metabolite that inhibits ADP-induced platelet aggregation. CYP2C19 contributes to both steps and CYP3A4 contributes to the second step [10]. Factors affecting CYP2C19 and CYP3A4 expression and activity may influence the blood concentration of clopidogrel’s active metabolite, and eventually platelet aggregation. CYP2C19*2 and *3 have been shown to affect platelet reactivity and cardiovascular outcome in patients treated with clopidogrel, while the data about the clinical relevance of the CYP2C19*17 gain-of-function allele is conflicting [11,12,13]. A GWAS study in a large Amish population indicated that approximately 70% of the variability in clopidogrel response may be due to genetic factors, while CYP2C19*2 being responsible for approximately 12% of the overall variation in platelet reactivity [14]. This suggests that other genetic factors influencing clopidogrel efficacy remain to be identified.
The CYP3A4*22 genetic variant has been shown to decrease the expression of CYP3A4 [15]. Although the minor allele frequency in the European population is only about 5%, it may serve as a marker to predict the response to drugs metabolized by CYP3A4 [15,16]. Two linked peroxisome proliferator-activated receptor alpha (PPAR-α) genetic variants (G209A and A208G) have also been identified as genetic determinants that affect CYP3A4 expression, explaining ~5% and ~9% of the variation in CYP3A4 protein and activity level, respectively, which might influence clopidogrel active metabolite level and thereby platelet reactivity [17]. With a minor allele frequency of 27% and 28%, respectively, in the European population, those SNPs are more common [15]. Statins and fibrates are both ligands of PPAR-α, and the resulting activation of PPAR-α will therefore reduce platelet aggregation [18]. Furthermore, CYP3A4*22 and the two variants of the PPAR-α gene were associated with a lower required dose of CYP3A4-metabolized drugs such as simvastatin and atorvastatin [16,17].
So far, the available data describing the influence of CYP3A4 genetic variations on platelet reactivity in patients treated with clopidogrel is limited, and as far as the authors are aware the influence of PPAR-α on platelet reactivity in the presence of statin co-medication has not been studied.
Since platelet reactivity in clopidogrel-treated patients who underwent PCI has been associated with cardiovascular outcomes [19,20], the influence of genetic factors and co-medications on this on-treatment platelet reactivity is relevant for clinical practice. In this study, we aimed to investigate the association between the genetic variations of the CYP3A4 and PPAR-α genes and platelet reactivity in clopidogrel-treated patients undergoing elective PCI, and to evaluate the influence of statin/fibrate co-medication on these associations.
2. Materials and Methods
2.1. Study Population
The study population included all consecutive patients who underwent non-urgent PCI with stent implantation, in whom genotyping for the CYP2C19*2 and *3 alleles was performed between July 2010 and May 2013 in the St. Antonius Hospital, Nieuwegein, The Netherlands. CYP2C19 genotyping, and subsequent adjustment of antiplatelet therapy based on a combination of platelet function testing, genotyping results and clinical risk factors, was performed as part of routine patient care in those patients [21]. According to hospital protocols, the remaining blood from the genotyping samples could be used for further research, in an anonymized manner, if the patient did not object. For the current analysis, CYP3A4*22 and PPAR-α genotyping was performed.
Patients were excluded from the analysis if they had a history of stroke or transient ischemic attack, were treated with oral anticoagulants, had a platelet count below 100 × 109/L, or received a glycoprotein IIb/IIIa-inhibitor before a blood sample was collected. All patients were adequately pre-treated with clopidogrel (defined as 75 mg daily for ≥5 days, a loading dose of 300 mg ≥ 6 h or 600 mg ≥ 2 h before testing) and aspirin (80–100 mg daily).
The study complies with the Declaration of Helsinki and received approval from the hospital’s medical research ethics committee (Verenigde Commissies Mensgebonden Onderzoek/VCMO). Approval for this registry included a waiver of informed consent.
2.2. Exposure and Outcome
The exposures in this study were the genetic variations of CYP3A4 (CYP3A4*22 or rs35599367) and PPAR-α (G209A or rs4253728, and A208G or rs4823613) genes. Blood samples for DNA analysis were obtained with K3-EDTA tubes as part of routine patient care for CYP2C19 genotyping. Coded blood samples and DNA isolates were stored at −70 °C. The samples were genotyped for CYP3A4*22, PPAR-α G209A, PPAR-α A208G, and CYP2C19*2 and *3 using the StepOnePlus® Real-Time PCR system. The TaqMan® SNP Genotyping Assay, which includes two allele-specific probes and PCR primer pairs to detect specific genetic polymorphism targets, was used. The StepOnePlus® software was used to determine the genotype of individual patients. The potential effect modifier in this study was statins/fibrates co-medication.
The outcome was on-treatment platelet reactivity, defined as the absolute level of platelet reactivity during treatment with clopidogrel. Platelet reactivity was measured with the VerifyNow® P2Y12-assay (Werfen, Barcelona, Spain). All platelet reactivity measurements were performed within 2 h after blood sample collection. Platelet reactivity was expressed in P2Y12 reaction unit (PRU). High on-clopidogrel platelet reactivity (HPR) was defined as the platelet reactivity of ≥236 PRU [19], but the same analysis was performed for the >208 PRU cut off value [22].
Several potential confounding variables were a priori considered based on previous publications, namely age, sex, current smoking, body mass index (BMI), diabetes, prior myocardial infarction, impaired renal function, clopidogrel loading before PCI (as compared to patients who were already using a maintenance dose), co-medication (proton pump inhibitors, statin/fibrate use, calcium channel blockers), and CYP2C19 metabolizer status. Impaired renal function was defined as serum creatinine level ≥ 200 µmol/L. The CYP2C19 metabolizer status was categorized into three groups: normal metabolizer (*1/*1 genotype), intermediate metabolizer (*1/*2 or *1/*3 genotype), and poor metabolizer (*2/*2, *3/*3, or *2/*3 genotype). Information on these potential confounders was obtained from hospital records.
2.3. Validation Cohorts
To validate our findings regarding the PPAR-α G209A and PPAR-α A208G SNPs, two different external databases were used. The first validation cohort consisted of the patients described in the POPular study. This observational single center study analyzed the predictive value of different platelet function tests on clinical outcome in patients using clopidogrel and was performed in the same hospital as our current analysis [19]. All patients undergoing elective PCI in whom platelet function testing was performed using the VerifyNow P2Y12 assay and from whom a DNA sample was available for the genotyping of both PPAR SNPs were selected. The technique used for the genotyping was the same as described for the main analysis.
The second validation cohort consisted of all patients in the genome wide association study (GWAS) subgroup of the International Clopidogrel Pharmacogenomics Consortium (ICPC) database. The aim of the ICPC is to find novel genetic markers which influence clopidogrel efficacy using GWAS and candidate gene approaches, combined with pharmacodynamic and clinical outcome data [23,24]. All clopidogrel-treated patients undergoing elective PCI were selected in whom ADP-stimulated platelet function testing was performed. Because different platelet function tests were used among different cohorts, a standardized ADP-induced platelet reactivity measure was used (z-score). Therefore, HPR status could not be determined in this validation cohort. Calculation of the standardized antiplatelet measure has been described in the ICPC design paper [23]. For GWAS the Illumina Omni Express with Exome chip was used. The PPAR SNPs were available in all patients as part of the GWAS analysis. Due to missing baseline variables, no multivariate analysis was performed in the ICPC cohort analysis.
2.4. Statistical Analysis
Continuous variables are presented as mean ± SD, and categorical variables are presented as proportions. The on-treatment platelet reactivity was normally distributed. Chi-square tables were used to compare the observed number of each genotype with the expected number for a population in Hardy–Weinberg equilibrium (p > 0.05). The pairwise linkage disequilibrium between PPAR-α G209A and PPAR-α A208G was calculated. Multivariate linear regression analysis was used to test the association between the genetic variants and on-treatment platelet reactivity as continuous variable, adjusted for confounders. Multivariate logistic regression analysis was conducted to test the association between the genetic variants and HPR, adjusted for confounders. Stratified analyses were conducted for patients with statin/fibrate co-medication users versus non-users. A recessive model was used in all the analyses. To compare the main analysis to the validation cohorts, a meta-analysis was performed with an inverse variance method, using a random effects model. Statistical analyses were conducted with SPSS 24 and R 3.1.3.
3. Results
3.1. Study Cohort
This study included 1124 patients who underwent elective PCI. Most of the patients were male (75.4%), mean 63.9 years of age (±10.8 years), with some overweight (BMI 27.5 ± 4.2 kg/m2), and with a history of hypertension (83.3%). At the time of PCI, 88.6% of patients were using statin and/or fibrate therapy, mostly simvastatin (67.7%) or atorvastatin (19.4%), while 3 patients (0.3%) were using a fibrate. Of all patients, 0.4% were homozygous carriers of the CYP3A4*22 allele, 6.9% were homozygous for the PPAR-α G209A minor allele, and 7.0% were homozygous for the PPAR-α A208G minor allele. Poor CYP2C19 metabolizer status was present in 2.6% of patients (Table 1). All genetic variants were in Hardy–Weinberg equilibrium (p > 0.05). Strong linkage disequilibrium was observed between PPAR-α G209A and PPAR-α A208G (r2 = 0.96).

Table 1.
Baseline characteristics.
There was no difference in the on-treatment platelet reactivity between the homozygous CYP3A4*22 allele carriers versus the heterozygous or non-carriers (*1/*22 or *1/*1) (p = 0.88) (Figure 1a).
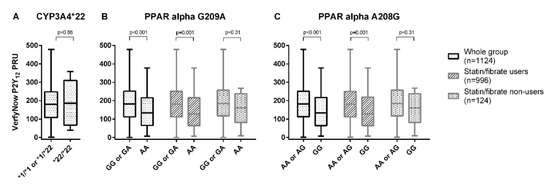
Figure 1.
The on-treatment platelet reactivity, as measured with the VerifyNow® P2Y12 assay for CYP3A4*22 (A), PPAR-α G209A (B), and PPAR-α A208G (C).
A significantly lower on-treatment platelet reactivity was found in those with the PPAR-α G209A AA genotype compared to the GG or GA genotype (non-adjusted mean difference −40.1 PRU [95%CI −62.1, −18.0], p < 0.001) (Figure 1b, Table 2).

Table 2.
On-treatment platelet reactivity in carriers of recessive alleles of CYP3A4*22, PPAR-α G209A, and PPAR-α A208G.
A significant difference was also found between the patients with the PPAR-α A208G GG genotype and the patients with the AA or AG genotypes (non-adjusted mean difference = −39.1 PRU [95%CI −60.9, −17.3], p < 0.001) (Figure 1c, Table 2). After adjustment for possible confounders, the effect for both PPAR SNPs was still significant (Table 2). The subgroup analysis for statin/fibrate co-medication showed a comparable effect for both the statin/fibrate users and non-users, although not statistically significant in the subgroup of statin/fibrate non-users, due to the lower number of patients in each subgroup (Table 2). Results for PPAR-α G209A analyzed in an additive or dominant model are shown in Online Supplemental Figure S1, while the results for each individual statin are shown in Online Supplemental Figure S2.
A lower incidence of HPR > 208 PRU was observed in those with the PPAR-α G209A AA genotype compared to the heterozygous and non-carriers of the minor allele (28.6 vs. 39.5%, Odds Ratio 0.61 [95%CI 0.37, 1.02], p = 0.06) and in those with PPAR-α A208G GG genotype (29.1 vs. 39.5%, OR 0.63 [95%CI 0.38, 1.04], p = 0.07) (Table 3).

Table 3.
Odds ratio for high platelet reactivity in carriers of recessive alleles of CYP3A4*22, PPAR-α G209A, and PPAR-α A208G for the >208 PRU cut-off value.
A subgroup analysis for the association with HPR in statin/fibrate users and non-users showed a similar trend in results as with the continuous platelet reactivity outcome. However, the associations were not significant, both for univariate and multivariate analysis. Results for the HPR ≥ 236 PRU cut off value are presented in Supplemental Table S1.
3.2. Validation Cohorts
Baseline characteristics of the POPular validation cohort (n = 729) and the ICPC validation cohort (n = 882) are shown in Supplemental Table S2. In general, clinical characteristics and gene frequencies were comparable between the three cohorts. The lower platelet reactivity found in the main analysis for the PPAR-α G209A AA and the PPAR-α A208G GG genotype was also found in the POPular validation cohort, but the effect was less pronounced and not statistically significant (G209A AA: −10.5 PRU [95%CI −33.8, 12.7], p = 0.35; A208G GG: −7.0 [95%CI −29.5, 15.6], p = 0.51) (Supplemental Figure S3 and Table S3). Still, the odds ratio for HPR > 208 PRU was comparable to the results found in the main analysis (G209A AA: OR 0.67 [95%CI 0.36; 1.23], p = 0.22; A208G GG: OR 0.75 [95%CI 0.41, 1.34], p = 0.37) (Supplemental Table S4). When stratified to statin/fibrate users versus non-users there was a lower platelet reactivity in statin/fibrate users (G209A AA: −18.1 PRU [95%CI −44.6, 8.5], p = 0.18; A208G GG: −14.5 [95%CI −39.8, 10.7], p = 0.18), but a higher platelet reactivity in the statin/fibrate non-users (G209A AA: +13.6 [95%CI −33.7, 60.9], p = 0.57; A208G GG: +24.6 [95%CI −25.0, 74.2], p = 0.33) (Supplemental Figure S3 and Table S3). In the ICPC validation cohort both PPAR-α SNPs were also associated with numerically lower ADP-induced platelet reactivity, but without statistically significant differences (Supplemental Table S5). This held true for both the whole group and for the statin/fibrate user or non-user subgroups. When all three databases where combined using a random effects meta-analysis model, platelet reactivity was significantly lower for both the PPAR-α G209A and A208G homozygous minor allele carriers in the subgroup of statin users (p = 0.04 for G209A and p = 0.03 for A208G) (Figure 2), although the effect is driven by the results of the main analysis. There was no significant effect found in the combined analysis for the subgroup of statin/fibrate non-users.
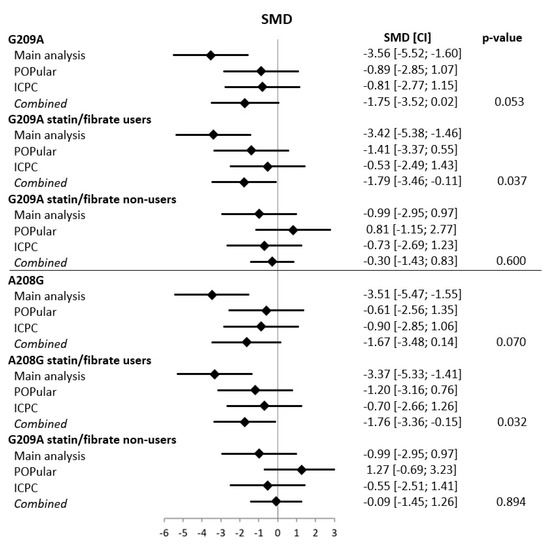
Figure 2.
Meta-analysis for G209A and A208G variants in the main analysis and the POPular and ICPC validation cohorts. CI = confidence interval, SMD = standardized mean difference. For the meta-analysis, a random effects model was used.
4. Discussion
In this analysis, clopidogrel-treated patients undergoing elective PCI who were homozygous for the PPAR-α G209A and A208G minor allele had a significantly lower platelet reactivity when measured with the VerifyNow P2Y12 platelet function test compared to heterozygous or wild-type patients. In two external validation cohorts, we also found a lower platelet reactivity associated with those SNPs, although this difference was not statistically significant. The effect seems to be driven by the patients using statin or fibrate co-medication. CYP3A4*22 was not found to be associated with a difference in platelet reactivity, but this analysis is hampered by a very low number of patients homozygous for the minor allele.
A previous study published by Kreutz et al. did not find an association between PPAR-α and platelet reactivity [25]. This could have been due to the lower sample size, and as a consequence, the additive model that was used in their analysis. In our study, a recessive model was used, in accordance with the results of the study by Klein et al., which demonstrated that only homozygous carriers showed a decrease in PPAR-α protein and activity levels [17].
PPAR-α is one of the three PPARs found in the cell nucleus that modulate the transcription of various genes associated with lipid metabolism and inflammation. Recent studies have shown that all three types of PPAR proteins (α, β, and γ) are also expressed in the human bone marrow megakaryocytes and anucleate platelets. The proteins are susceptible to their specific endogenous and exogenous ligands, which affect platelet reactivity through a non-genomic mechanism [18,26,27]. A previous study showed that the two linked genetic variants of the PPAR-α gene (G209A and A208G) were associated with a reduced expression of PPAR-α, and they also directly or indirectly modulated CYP3A4, as proven by the decrease in expression and activity of CYP3A4 in the liver [17]. Based on those findings, subjects who are homozygous for the PPAR-α G209A or A208G minor allele are expected to have a lower expression of CYP3A4, leading to a lower blood level of the active metabolite of clopidogrel and eventually higher on-treatment platelet reactivity, compared to heterozygous or wild-type patients.
However, there is an additional effect of statin and fibrate drugs on platelet reactivity in the other direction, leading to a net effect of lower platelet reactivity. PPAR-α has a direct effect on platelet reactivity. Ligand-activated PPAR-α will rapidly inhibit PKC-α and will suppress platelet activation and aggregation. Aside from that, ligand-activated PPAR-α increases the level of cAMP that leads to the inhibition of platelet activation [18]. It also inhibits cyclooxygenase-1, leading to inhibition of arachidonic acid-related platelet aggregation. In addition, ligand-induced PPAR-α increases the activity of nitric oxide synthase and guanylyl cyclase, leading to the inhibition of collagen-induced platelet aggregation [28]. Statins and fibrates are strong ligands for PPAR-α [29,30] and PPAR-γ [18,29]. Both types of PPARs will inhibit platelet aggregation when they are activated by statins or fibrates. Furthermore, patients who are homozygous for the PPAR-α G209A or A208G minor allele will have reduced expression of CYP3A4, which acts as a metabolizing enzyme for statins [17]. This results in higher blood concentration of statins and most likely fibrates. Therefore, since 88.6% of our patients were users of a statin or fibrate, the on-treatment platelet reactivity in this study was the result of the net effect of clopidogrel and statin/fibrate. In clopidogrel users who were homozygous carriers of the PPAR-α minor allele and were also using a statin or fibrate, the on-treatment platelet reactivity reflected a balance between the increased platelet reactivity caused by reduced expression of CYP3A4, the reduced platelet reactivity caused by the higher blood concentration of statin/fibrate and statin/fibrate-induced PPAR-α-mediated and PPAR-γ-mediated antiplatelet activity.
CYP3A4*22 has been reported to be associated with the reduction in CYP3A4 activity [16]. This genetic variant was shown to affect the blood level or dose requirement of CYP3A4-metabolized drugs such as tacrolimus, simvastatin, and atorvastatin [16,17,30]. Our results on the effect of CYP3A4*22 on the on-treatment platelet reactivity showed a slightly increased platelet reactivity, but the association was not significant and limited by the small proportion of homozygous carriers of the minor allele in this study (n = 5). A previous study on the same genetic variant on the effect of clopidogrel did not include any subjects with the *22/*22 genotype, so that an additive model was used, in which no association between CYP3A4*22 and on-treatment platelet reactivity was found [25].
Based on previous publications, there is a strong correlation between HPR, defined as > 208 or ≥ 236 PRU, and major adverse cardiovascular events (MACE) [19,31]. In our analysis we found a non-significant correlation between both PPAR-α genetic variants and HPR, both for the > 208 PRU and ≥ 236 PRU cut off level (Table 3 and Supplemental Table S4). A meta-analysis by Brarr et al. showed that, on a continuous scale, every 10 unit increase in PRU was significantly associated with a 4% increased risk of the composite of death, myocardial infarction, or stent thrombosis (HR 1.04; 95% CI 1.03–1.06) [20]. In our results, clopidogrel users who were homozygous carriers of a PPAR-α minor allele and were statin/fibrate users had a significant 26 unit decrease in PRU (Table 2), implicating that this group of patients may have a 10% decreased risk of the composite of death, myocardial infarction, or stent thrombosis compared to the patients with heterozygous and wild-type PPAR-α genotypes.
The strength of our analysis is that we can evaluate the association between the genetic variants in PPAR-α genes and the on-treatment platelet reactivity in a patient cohort with a large sample size, and we could validate our findings in two patient cohorts with comparable patient characteristics. We took into account the biological mechanism of ligand-activated PPAR proteins-associated platelet reactivity when evaluating the association. Since the genetic polymorphisms in CYP2C19 have a considerable contribution in the platelet reactivity during treatment with clopidogrel, our study included the CYP2C19*2 and *3 genetic variants for the statistical analysis. Other drugs that might influence clopidogrel’s response as a result of a pharmacokinetic interaction were also included, such as the use of calcium channel blockers and proton pump inhibitors. Platelet reactivity was measured while the patients were adequately treated with clopidogrel. The VerifyNow P2Y12 assay, a reliable and sensitive tool to measure platelet response to clopidogrel therapy, was used to measure platelet reactivity.
This study also has its limitations. First, the frequency of CYP3A4*22/*22 was too small to draw any definite conclusions regarding its association with on-treatment platelet reactivity or HPR. Also, the subgroup of patients without statin/fibrate use was small, which results in a very wide confidence interval for the calculated odds ratios in this subgroup, and a valid multivariate analysis could not be performed. A possible difference between statin/fibrate users and non-users could have been missed. Second, our analysis was limited to elective PCI patients only, while the correlation between platelet reactivity and CYP2C19 genotype with clinical outcome is stronger in high risk patients, for example after acute coronary syndrome (ACS) [32]. Therefore, the effect of PPAR SNPs on platelet reactivity and clinical outcome might also be stronger in ACS-patients. Third, multiple platelet function testing methods were used in the ICPC validation cohort, making it necessary to calculate a standardized ADP induced antiplatelet measure. This makes it difficult to compare the ICPC results to the results of the main analysis and the POPular validation cohort, in which a single platelet function test has been used in all patients. A meta-analysis approach was used to account for this difference. Finally, although both validation cohorts showed an effect on platelet reactivity for the PPAR-α genetic variants in the same direction as the main analysis, the effect size was smaller and the results were not statistically significant. Also, active metabolite levels were not available, but would have been useful to support our hypothesis about the mechanism of effect on platelet reactivity.
Nevertheless, the trend towards lower platelet reactivity warrant further analysis in other cohorts, focusing on patients with a higher ischemic risk and using clinical endpoint data.
5. Conclusions
In our analysis, linked G209A and A208G genetic variants in the PPAR-α gene were associated with lower platelet reactivity in elective PCI patients treated with clopidogrel and statin/fibrate co-medication. Replication in two external validation cohorts showed the same direction of effect, but without statistical significance. However, meta-analysis of the three cohorts shows that both variants are statistically significant.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/9/1068/s1. Table S1: On-treatment platelet reactivity in carriers of recessive alleles of CYP3A4*22, PPAR-α G209A, and PPAR-α A208G for the HPR ≥ 236 PRU cut off in the main analysis cohort. Table S2: Baseline characteristics for the main analysis cohort and both validation cohorts. Table S3: Estimates for the on-treatment platelet reactivity outcome in carriers of recessive alleles of PPAR-α G209A, and PPAR-α A208G in the POPular validation cohort. Table S4: Odds ratio for high platelet reactivity outcome in carriers of recessive alleles of PPAR-α G209A, and PPAR-α A208G in the POPular validation cohort. Table S5: Estimates for the on-treatment platelet reactivity outcome in carriers of recessive alleles of PPAR-α G209A, and PPAR-α A208G in the ICPC validation cohort. Figure S1: The on-treatment platelet reactivity in the main analysis cohort, as measured with the VerifyNow® assay for PPAR alpha G209A (rs4253728), analyzed in a (a) additive model, (b) dominant model. Figure S2: The on-treatment platelet reactivity in the main analysis cohort, as measured with the VerifyNow® assay for PPAR alpha G209A (rs4253728), stratified according to different statin and fibrate use. Figure S3: The on-treatment platelet reactivity, as measured with the VerifyNow® assay: (a) PPAR-α G209A (rs4253728), (b) PPAR-α A208G (rs4823613) in the POPular validation cohort. Online Supplementary Data: International Clopidogrel Pharmacogenomic Consortium (ICPC) author list.
Author Contributions
Conceptualization, A.Y., A.d.B., and V.H.M.D.; Data curation, T.O.B., A.Y., G.J.A.V., P.W.A.J., and ICPC investigators; Formal analysis, T.O.B., A.Y., J.C.K., and V.H.M.D.; Funding acquisition, C.M.H., A.d.B., J.M.t.B., and V.H.M.D.; Investigation, T.O.B., G.J.A.V., P.W.A.J., and V.H.M.D.; Methodology, T.O.B., A.Y., J.C.K., A.d.B., J.M.t.B., and V.H.M.D.; Project administration, A.Y.; Resources, C.M.H., J.M.t.B., and V.H.M.D.; Supervision, A.d.B., J.M.t.B., and V.H.M.D.; Validation, T.O.B.; Visualization, T.O.B.; Writing—original draft, T.O.B., A.Y. and V.H.M.D.; Writing—review and editing, T.O.B., A.Y., G.J.A.V., P.W.A.J., C.M.H., J.C.K., S.S.V., M.D.R., L.G., T.E.K., A.d.B., O.H.K., J.M.t.B., and V.H.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the St Antonius Innovation fund and a ZonMw TopZorg grant. ZonMw is a Dutch organization funded by the government promoting health care research and the implementation of study results in daily practice. The International Clopidogrel Pharmacogenomics Consortium was supported by NIH [grant numbers U01 HL105198, R24 GM61374], and the Robert Bosch Stiftung Stuttgart, Germany
Acknowledgments
We thank Richard van de Heide, Remko Harms, Sylvia van der Steen van Vuren, Tamimount el Dahri, and Kees de Bruijn from the Pharmacogenetics, Pharmaceutical and Toxicological Laboratory of the Department of Clinical Pharmacy for their work in genotyping. We thank the participating centers of the ICPC for their contribution to this analysis in sharing data for the validation cohort and evaluation of the manuscript (full author list included in the supplementary data).
Conflicts of Interest
J.B. and P.J. received funding from ZonMW TopZorg grant for a part of the submitted study. The Division of Pharmacoepidemiology and Clinical Pharmacology employing authors A.B. and O.K. has received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, the private-public funded Top Institute Pharma, and the EU Innovative medicines Initiate (IMI). A.Y. received a scholarship for her doctorate degree from the Dikti-Neso Scholarship Award from the Directorate General of Higher Education, Ministry of Education and Culture, Indonesia. M.R. received travel/consulting/speaker fees from Cipherome, Goldfinch, DNAnexus, and the American Society of Health System Pharmacists. The other co-authors do not have any conflicts of interest to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, H.-J.; Zhao, F.; Chrolavicius, S.; et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Fernández-Ortiz, A.; Bernardo, E.; Ramírez, C.; Cavallari, U.; Trabetti, E.; Sabaté, M.; Hernández, R.; Moreno, R.; Escaned, J.; et al. Contribution of gene sequence variations of the hepatic cytochrome P450 3A4 enzyme to variability in individual responsiveness to clopidogrel. Arter. Thromb. Vasc. Biol. 2006, 26, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Bhindi, R.; Ormerod, O.; Newton, J.; Banning, A.P.; Testa, L. Interaction between statins and clopidogrel: Is there anything clinically relevant? QJM Int. J. Med. 2008, 101, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Bliden, K.P.; DiChiara, J.; Lawal, L.; Singla, A.; Antonino, M.J.; Baker, B.A.; Bailey, W.L.; Tantry, U.S.; Gurbel, P.A. The association of cigarette smoking with enhanced platelet inhibition by clopidogrel. J. Am. Coll. Cardiol. 2008, 52, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Kopp, C.W. Calcium-channel blockers decrease clopidogrel-mediated platelet inhibition. Heart 2009, 96, 186–189. [Google Scholar] [CrossRef]
- Harmsze, A.M.; Van Werkum, J.W.; Berg, J.M.T.; Zwart, B.; Bouman, H.J.; Breet, N.J.; Hof, A.W.V.; Ruven, H.J.T.; Hackeng, C.M.; Klungel, O.H.; et al. CYP2C19*2 and CYP2C9*3 alleles are associated with stent thrombosis: A case-control study. Eur. Heart J. 2010, 31, 3046–3053. [Google Scholar] [CrossRef]
- Ho, P.M.; Maddox, T.M.; Wang, L.; Fihn, S.D.; Jesse, R.L.; Peterson, E.D.; Rumsfeld, J.S. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009, 301, 937. [Google Scholar] [CrossRef]
- Hochholzer, W.; Trenk, D.; Fromm, M.F.; Valina, C.M.; Stratz, C.; Bestehorn, H.-P.; Büttner, H.J.; Neumann, F.-J. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J. Am. Coll. Cardiol. 2010, 55, 2427–2434. [Google Scholar] [CrossRef]
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Shen, L.; Hockett, R.D.; Brandt, J.T.; Walker, J.R.; Antman, E.M.; Macias, W.; Braunwald, E.; et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009, 360, 354–362. [Google Scholar] [CrossRef]
- Kazui, M.; Nishiya, Y.; Ishizuka, T.; Hagihara, K.; Farid, N.A.; Okazaki, O.; Ikeda, T.; Kurihara, A. Identification of the human cytochrome p450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 2009, 38, 92–99. [Google Scholar] [CrossRef]
- Yasmina, A.; De Boer, A.; Klungel, O.H.; Deneer, V.H. Pharmacogenomics of oral antiplatelet drugs. Pharmacogenomics 2014, 15, 509–528. [Google Scholar] [CrossRef]
- Park, J.J.; Park, K.W.; Kang, J.; Jeon, K.-H.; Kang, S.-H.; Ahn, H.S.; Han, J.-K.; Koh, J.-S.; Lee, S.-E.; Yang, H.-M.; et al. Genetic determinants of clopidogrel responsiveness in Koreans treated with drug-eluting stents. Int. J. Cardiol. 2013, 163, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Simon, T.; Collet, J.P.; Anderson, J.L.; Antman, E.M.; Bliden, K.; Cannon, C.P.; Danchin, N.; Giusti, B.; Gurbel, P.; et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. JAMA 2010, 304, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Shuldiner, A.R.; O’Connell, J.R.; Bliden, K.P.; Gandhi, A.; Ryan, K.; Horenstein, R.B.; Damcott, C.M.; Pakyz, R.; Tantry, U.S.; Gibson, Q.; et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009, 302, 849. [Google Scholar] [CrossRef] [PubMed]
- NIH, National Library of Medicine, National Center for Biotechnology Information, dbSNP Database. Available online: https://ncbi.nlm.nih.gov/snp/ (accessed on 27 August 2020).
- Wang, D.; Guo, Y.; Wrighton, S.A.; Cooke, G.E.; Sadee, W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharm. J. 2010, 11, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Thomas, M.; Winter, S.; Nussler, A.K.; Niemi, M.; Schwab, M.; Zanger, U.M. PPARA: A novel genetic determinant of CYP3A4 in vitro and in vivo. Clin. Pharmacol. Ther. 2012, 91, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.Y.; Armstrong, P.C.; Dhanji, A.-R.A.; Tucker, A.T.; Paul-Clark, M.J.; Mitchell, J.A.; Warner, T.D. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arter. Thromb. Vasc. Biol. 2009, 29, 706–711. [Google Scholar] [CrossRef]
- Breet, N.J.; Van Werkum, J.W.; Bouman, H.J.; Kelder, J.C.; Ruven, H.J.T.; Bal, E.T.; Deneer, V.H.; Harmsze, A.M.; Van Der Heyden, J.A.S.; Rensing, B.J.W.M.; et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010, 303, 754. [Google Scholar] [CrossRef]
- Brar, S.S.; Berg, J.T.; Marcucci, R.; Price, M.J.; Valgimigli, M.; Kim, H.-S.; Patti, G.; Breet, N.J.; DiSciascio, G.; Cuisset, T.; et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2011, 58, 1945–1954. [Google Scholar] [CrossRef]
- Janssen, P.W.A.; Bergmeijer, T.O.; Vos, G.-J.A.; Kelder, J.C.; Qaderdan, K.; Godschalk, T.C.; Breet, N.J.; Deneer, V.H.M.; Hackeng, C.M.; Berg, J.M.T. Tailored P2Y12 inhibitor treatment in patients undergoing non-urgent PCI—The POPular Risk Score study. Eur. J. Clin. Pharmacol. 2019, 75, 1201–1210. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bonello, L.; Aradi, D.; Price, M.J.; Jeong, Y.-H.; Angiolillo, D.J.; Stone, G.W.; Curzen, N.; Geisler, T.; Berg, J.T.; et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 2013, 62, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Bergmeijer, T.O.; Reny, J.-L.; Pakyz, R.E.; Gong, L.; Lewis, J.P.; Kim, E.-Y.; Aradi, D.; Fernández-Cadenas, I.; Horenstein, R.B.; Lee, M.T.M.; et al. Genome-wide and candidate gene approaches of clopidogrel efficacy using pharmacodynamic and clinical end points—Rationale and design of the International Clopidogrel Pharmacogenomics Consortium (ICPC). Am. Heart J. 2018, 198, 152–159. [Google Scholar] [CrossRef]
- Verma, S.S.; Bergmeijer, T.O.; Gong, L.; Reny, J.; Lewis, J.P.; Mitchell, B.D.; Alexopoulos, D.; Aradi, D.; Altman, R.B.; Bliden, K.; et al. Genome-wide association study of platelet reactivity and cardiovascular response in patients treated with clopidogrel: A study by the International Clopidogrel Pharmacogenomics Consortium (ICPC). Clin. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Kreutz, R.P.; Owens, J.; Jin, Y.; Nystrom, P.; Desta, Z.; Kreutz, Y.; Breall, J.A.; Li, L.; Chiang, C.; Kovacs, R.J.; et al. Cytochrome P450 3A4*22, PPAR-α, and ARNT polymorphisms and clopidogrel response. Clin. Pharmacol. Adv. Appl. 2013, 5, 185–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akbiyik, F.; Ray, D.M.; Gettings, K.F.; Blumberg, N.; Francis, C.W.; Phipps, R.P. Human bone marrow megakaryocytes and platelets express PPARγ, and PPARγ agonists blunt platelet release of CD40 ligand and thromboxanes. Blood 2004, 104, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.Y.; Davidson, S.J.; Moraes, L.A.; Traves, S.L.; Paul-Clark, M.; Bishop-Bailey, D.; Warner, T.D.; Mitchell, J.A. Role of nuclear receptor signaling in platelets: Antithrombotic effects of PPARβ. FASEB J. 2005, 20, 326–328. [Google Scholar] [CrossRef]
- Fuentes, E.; Palomo, I. Mechanism of antiplatelet action of hypolipidemic, antidiabetic and antihypertensive drugs by PPAR activation. Vasc. Pharmacol. 2014, 62, 162–166. [Google Scholar] [CrossRef]
- Willson, T.M.; Brown, P.J.; Sternbach, D.D.; Henke, B.R. The PPARs: From Orphan Receptors to Drug Discovery†. J. Med. Chem. 2000, 43, 527–550. [Google Scholar] [CrossRef]
- Paumelle, R.; Blanquart, C.; Briand, O.; Barbier, O.; Duhem, C.; Woerly, G.; Percevault, F.; Fruchart, J.C.; Dombrowicz, D.; Glineur, C.; et al. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-alpha via inhibition of the protein kinase C signaling pathway. Circ. Res. 2006, 98, 361–369. [Google Scholar] [CrossRef]
- Elens, L.; Bouamar, R.; Hesselink, D.A.; Haufroid, V.; Van Der Heiden, I.P.; Van Gelder, T.; Van Schaik, R.H.N. A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin. Chem. 2011, 57, 1574–1583. [Google Scholar] [CrossRef]
- Stone, G.W.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Neumann, F.-J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; Duffy, P.L.; Mazzaferri, E.; et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013, 382, 614–623. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).