Abstract
We identified the known c.1_9del mutation in the PLEC gene in four unrelated females from consanguineous families of Turkish origin. All individuals presented with slowly progressive limb-girdle weakness without any dermatological findings, and dystrophic changes observed in their muscle biopsies. Additionally, the neurological examination revealed ptosis, facial weakness, fatigability, and muscle cramps in all four cases. In two patients, repetitive nerve stimulation showed a borderline decrement and a high jitter was detected in all patients by single-fiber electromyography. Clinical improvement was observed after treatment with pyridostigmine and salbutamol was started. We further characterize the phenotype of patients with limb-girdle muscular dystrophy R17 clinically, by muscle magnetic resonance imaging (MRI) features and by describing a common 3.8 Mb haplotype in three individuals from the same geographical region. In addition, we review the neuromuscular symptoms associated with PLEC mutations and the role of plectin in the neuromuscular junction.
1. Introduction
The PLEC gene consists of 32 exons and is ubiquitously expressed, including in skeletal muscle, skin and heart [1]. Plectin is a large protein ranging from 4684 amino acids in the canonical form to 3447 amino acids in the smallest stable isoform. It has a unique molecular structure: the N-terminal domain is encoded by multiple short exons, whereas the central-rod domain and the C-terminal domain are encoded by single large exons. Exons 2-32 are constant, but eight different-sized forms of exon 1 exist, which undergo alternative splicing into exon 2. At least eight different plectin isoforms (1, 1a, 1b, 1c, 1d, 1e, 1f, and 1g) resulting from the different splicing of exon 1 have been identified [2]. These isoforms have various lengths, localizations, interacting partners, and specific functions. For instance, plectin 1a is localized to the outer nuclear/endoplasmic reticulum membrane system, plectin 1b to the mitochondria and plectin 1d at the Z-disks [3]. Plectin 1f, in particular in striated muscle, has been shown to interact with components of the dystrophin-glycoprotein complex [4] but also to play an important role in the neuromuscular junction (NMJ) where it interacts directly with rapsyn [5]. Plectin stabilizes the desmin cytoskeleton and interacts with its three main components: actin microfilaments, microtubules, and intermediate filaments. Other tissue specific roles depend on the specific splicing isoform and the numerous binding partners, such as α-actinin, vimentin, keratin, and desmin, which interact with different regions of the plectin protein [3,6].
Mutations in PLEC are associated with multiple phenotypes depending on the location of the mutation and isoform affected. Epidermolysis bullosa simplex with muscular dystrophy [EBS-MD (MIM #226670)] is caused by recessive mutations [7,8], mostly nonsense, out-of-frame insertions or deletions within exon 31 and 32, leading to premature protein termination. Individuals with EBS-MD show severe skin blistering, sometimes accompanied by skin atrophy, alopecia, and nail dystrophy, with first symptoms usually starting in infancy and even leading to premature death in childhood. The EBS-MD phenotype shows high clinical variability with a few individuals also presenting with myasthenic symptoms and additional features, such as anemia and cardiomyopathy [9]. Additionally, a phenotype of recessive limb-girdle muscular dystrophy, LGMD R17 plectin-related (MIM #613723, previously known as LGMD 2Q), was reported [10]. LGMD R17 is associated so far with only recessive truncating mutations located in exon 1f, and manifests with muscle weakness without any skin involvement. This phenotype was first described in three consanguineous Turkish families carrying a c.1_9del mutation and presenting with young adult/childhood onset muscle weakness and dystrophic changes in the muscle biopsy, without skin involvement or myasthenic features [10].
The association of plectin deficiency with muscle weakness is already well characterized. However, the role of plectin deficiency in patients with features of a congenital myasthenic syndrome is still not well understood and is only based on a few case reports. Herein, we describe four individuals with LGMD, easy fatigability, ptosis, and clinical responsiveness to acetylcholinesterase inhibitors and salbutamol, features commonly associated with myasthenic syndromes. They all carry the known c.1_9del PLEC mutation in homozygosity. All four patients included in this study originate from the same region near the Black Sea, in Turkey. We also identified a shared haplotype in three individuals suggesting a common origin of the mutation. Patients with myasthenic symptoms of unknown origin should be tested for mutations in the PLEC gene.
2. Materials and Methods
2.1. Clinical Assessment
Four patients with LGMD from unrelated families born to consanguineous parents were evaluated at the Department of Neurology, Istanbul Faculty of Medicine, Istanbul University, Turkey. The efficacy of oral salbutamol administration was assessed by muscle strength measurements, the 6-min walk test (6MWT) and spirometry (forced vital capacity; FVC). Muscle magnetic resonance imaging (MRI) was performed on a 1.5-Tesla MR scanner (1.5 T Philips Achieva, Philips Medical Systems, Best, Netherlands), using conventional T1-weighted and T2-weighted SPIR (Spectral Presaturation with Inversion Recovery) axial images of the thigh. Eight-micrometer sections from shock-frozen tissue samples were stained with hematoxylin and eosin, modified Gomori trichrome, acid phosphatase, periodic acid–Schiff, NADH dehydrogenase, succinate dehydrogenase, cytochrome c oxidase, and oil red O using standard procedures.
2.2. Standard Protocol Approvals, Registrations, and Patient Consents
Photographs and blood samples from patients and family members were obtained upon written informed consent according to the Declaration of Helsinki. The study protocol was approved by the Istanbul University Institutional Review Board for Research with Human Participants. DNA samples were submitted to the Newcastle Medical Research Council (MRC) Center Biobank for Neuromuscular Diseases for which ethical approval was granted by the NRES (National Research Ethics Service) Committee North-East—Newcastle & North Tyneside 1 (reference 08/H0906/28). Informed written consent was given by the patients. Phenotype data collection was performed using the PhenoTips online software tool (https://phenotips.com).
2.3. Genetic Analysis
All families characterized in this study were consanguineous and showed a recessive inheritance pattern. Three individuals (family A, B and C) were included in the MYO–SEQ project [11]. For these patients, whole exome sequencing (WES) was performed at the Broad Institute’s Genomics Platform, using Illumina exome capture on a cohort of >1800 patients with limb-girdle muscle weakness. Exome data was analyzed using standard filtering criteria (i.e., VEP moderate to high and MAF <1% and a gene list of 429 genes associated with muscle disease). The patient from family D was diagnosed by exome sequencing analysis performed at the Intergen Genetic Laboratory in Ankara, Turkey and the PLEC mutation was confirmed by Sanger sequencing. The size of the haplotype for three patients (A, B and C) was estimated using unfiltered WES data. Annotation is based on gene accession number NM_201378, ENST00000356346 and NP_958780.1.
3. Results
3.1. Clinical Presentation
All four female patients were born to consanguineous parents (Figure 1), but with no family history of a neuromuscular disorder. All presented with slowly progressive proximal muscle weakness, easy fatigability, and muscle cramps. Two of them (C and D) had delayed motor milestones; three were slower than their peers during childhood, only patient B presented at 26 years old. Their neurological examination revealed fatigable ptosis (Figure 2), nasal speech, tongue weakness, neck flexor weakness, proximal weakness and paraspinal muscle weakness (Table 1). A high arched palate, mild scoliosis, and bilateral pseudohypertrophy in the gastrocnemius muscle were noted in all. Two patients (A and C) were wheelchair-bound at age 35 and 39 years. Serum creatine kinase (CK) levels were elevated (Table 1) and electromyography (EMG) showed myopathic changes. Patients A and B were negative for anti-AChR and anti-MUSK antibodies. In retrospective, the myasthenic symptoms, such as mild ptosis, were present since childhood, in patients A, C, D, and since her early twenties in patient B. All patients showed easy fatigability since childhood.
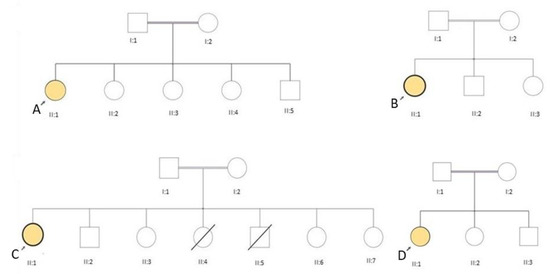
Figure 1.
Pedigrees of our four LGMD R17 patients carrying the c.1_9del mutation in homozygosis. Patients A, B, C and D are indicated.
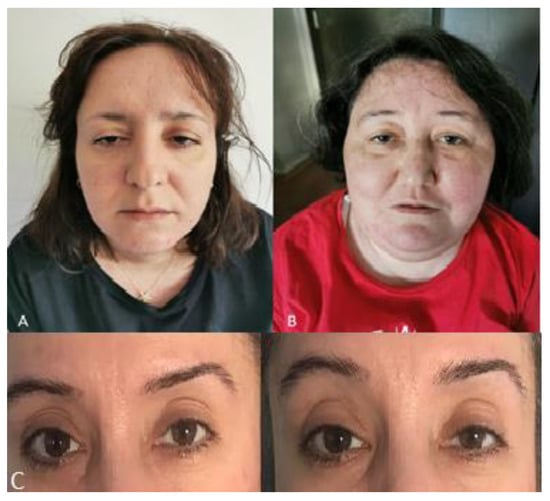
Figure 2.
(A) Patient B showing ptosis and facial weakness at age 39 years old; (B) Patient A showing ptosis and facial weakness at age 38 years old; (C) Patient D with fatigable ptosis.

Table 1.
Comparison of clinical phenotypes between patients with c.1_9del PLEC deletion.
All patients had high jitter in single-fiber EMG. Two patients who had milder weakness had over 10% decremental response in repetitive nerve stimulation (RNS). Muscle biopsy analysis showed dystrophic changes in three patients and myopathic changes in one, with increased number of internal nuclei (over 10%) and a few angular atrophic fibers in all (Figure 3A,B). Muscle MRI showed relative sparing of the rectus femoris, gracilis and sartorius muscles in three patients (Figure 3C–G). Cardiac examinations including echocardiogram (ECHO) and 24 h Holter monitoring were normal. Treatment with pyridostigmine 180 mg/day and salbutamol 8 mg/day showed benefit in all patients. Salbutamol was well tolerated with no noted side effects. After six months of therapy, patient A was functionally better and noticed that she could use her arms for much longer without fatigue. Her baseline and 6-month scores according to the MRC scale were similar. Baseline respiratory function tests of patient B revealed a vital capacity (VC) of 53% and an forced vital capacity (FVC) of 54%. After 6 months of salbutamol treatment, her FVC increased to 66%. She also performed better in the 6MWT, with an increase from 274 m to 326 m. She only needed unilateral support for walking. Patient C’s baseline and 3-month MRC scores were similar, but her FVC in sitting increased from 57% to 62%. She was able to use her arms and hands more effectively and felt less tired after six months of salbutamol treatment. Salbutamol was well tolerated except for mild tremor in the hands. Patient D started treatment only recently and already reported that she felt less fatigued and was able to climb stairs more easily. Her 6MWT rose from 150 m before treatment to 180 m after treatment, while her VC increased respectively from 59% to 74%.
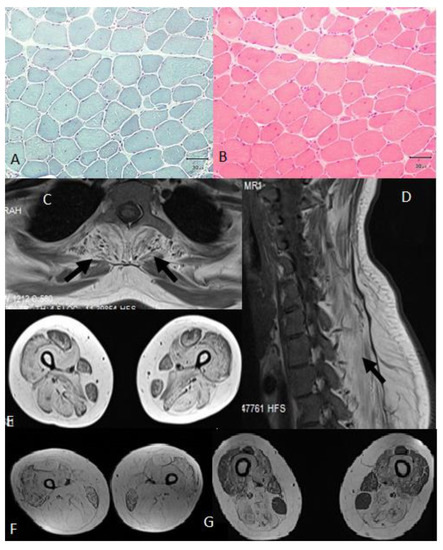
Figure 3.
(A) and (B) A muscle biopsy from the deltoid muscle of patient D showed myopathic changes with increased numbers of internal nuclei (over 10%) and with a few angular atrophic fibers; (C), (D) and (E) A muscle MRI of patient D showed paraspinal muscle involvement and good preservation of the sartorius and gracilis muscles in the lower limbs. (F) The muscle MRI of patient A showed advanced fatty transformation, but fairly good preservation of the gracilis muscles. (G) The muscle MRI of patient B showed clear pathology of the adductor magnus, adductor longus, biceps femoris, semitendinosus, and semimembranosus muscles and sparing of the gracilis muscles with good preservation of the rectus femoris and sartorius muscles.
3.2. Variant Characterization
Whole exome sequencing identified a homozygous PLEC variant (hg19: chr8:145047630_1450476308delGCCGGCCAT; c.1_9delATGGCCGGC; p.[Met1_Gly3del]) in four unrelated patients; its segregation with the disease was ascertained by Sanger sequencing and revealed a heterozygous variant in a healthy sister and the unaffected parents. This variant is absent in the control population (https://gnomad.broadinstitute.org/region/8-145047531-145047731) and has already been associated with LGMD R17 in three consanguineous families of Turkish origin [10]. No other likely candidate gene was identified in the targeted analysis of the exomes; in particular no disease-causing variant in any of the known congenital myasthenic syndrome (CMS) genes (RAPSN, CHRNE, AGRN, ALG2, CHAT, CHRNA1, CHRNB1, CHRND, CHRNE, COLQ, DOK7, GFPT1, GMPPB, MUSK, PREPL and SLC5A7) was found.
In addition, based on the WES data for three of the patients (A, B and C), a shared 0.485 Mb (hg38:chr8:143930378-144415811) homozygous haplotype defined by 95 single nucleotide and small in/del variants, and surrounding the PLEC mutation was identified, suggesting a founder event for this variant. A larger shared haplotype of 3.4 Mb (hg38:chr8:140457861-143608716) defined by an additional ~350 variants can be seen for only two of the patients (A and B), indicating that a recombination event took place in patient C (Figure 4). The 0.485 Mb and the larger 3.4 Mb regions encompass 17 and 45 genes, respectively. According to the medical history, the families were not related (previous 4–5 generations were analyzed); however, their ancestors all came from Giresun, a city by the Black Sea in Turkey.
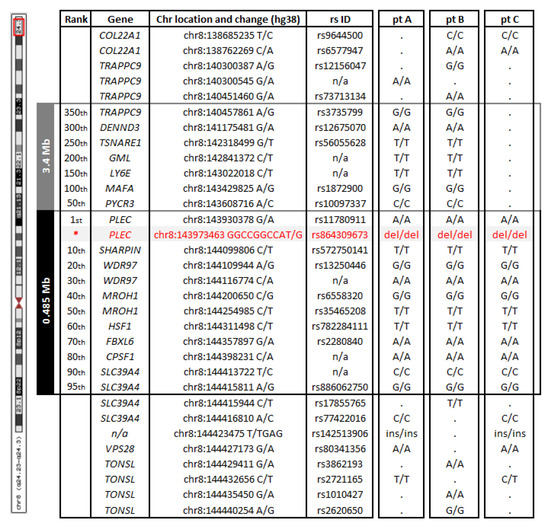
Figure 4.
Common haplotype based on WES data. Patients A, B, and C shared a 0.485 Mb haplotype (defined by 95 variants and encompassing 17 genes; shown in black) from chr8:143930378 to chr8:144415811. Patients A and B shared a larger haplotype (3.4 Mb, in grey), defined by ~350 variants from chr8:140457861 to chr8:143608716 and encompassing 45 genes. Selected variants and their genotypes are indicated both within and outside the haplotype. A dot indicates that the variant was not present in that patient. The PLEC mutation identified in the four Turkish patients is shown in red.
4. Discussion
We present four patients from unrelated families carrying the same PLEC mutation and presenting with limb-girdle weakness and myasthenic symptoms. All four patients had shoulder and pelvic girdle weakness with variable degree of fatigable ptosis, nasal speech, and swallowing difficulties. Paraspinal muscle weakness was also very prominent in our patients. All complained of easy fatigability and muscle cramps. The age of onset of symptoms varied from the infantile period with delayed motor milestones to early adulthood. They all had elevated serum CK levels and a high jitter on RNS. All patients benefited from pyridostigmine and salbutamol treatment.
The most common neuromuscular phenotype associated with mutations in PLEC is epidermolysis bullosa simplex with muscular dystrophy (EBS-MD; MIM #226670). Symptoms include skin blistering and ocular and neuromuscular involvement later in life [9]. The disease is inherited in an autosomal recessive manner and is associated with a dissociation of desmin filaments and, consequently, protein aggregates positive for desmin, syncoilin, and synemin accumulate in muscle fibers. Myofibrillar changes can be observed histologically and secondary mitochondrial pathology has been described [12]. Additionally, some patients present with systemic involvement (e.g., gastroenterological, dental, urogenital and cardiac) [13,14,15]. A few individuals with EBS-MD show myasthenic features, such as fatigability, ptosis, dysphagia or nasal speech [7,16,17]. However, these features were not investigated systematically and additional tests, such as SF-EMG had previously only been performed in a single case. The data from our four patients showed that the myasthenic phenotype may be more common in patients with PLEC mutations but may be overlooked when the clinical presentation is only mild. All EBS-MD mutations identified so far, apart from one, were in exons common to all plectin isoforms, and in the majority of cases lead to premature termination of protein translation. Other phenotypes associated with PLEC mutations are autosomal recessively inherited EBS with pyloric atresia (EBS-PA; MIM #612138) [18] and autosomal dominantly inherited EB simplex Ogna type (EBS-OG; MIM #131950) [19]. These diseases are associated mainly with C-domain PLEC mutations.
In addition, patients with recessive PLEC mutations and a LGMD phenotype, but without any skin involvement (LGMD R17, previously known as LGMD 2Q), have also been reported. The phenotype was first described in three consanguineous Turkish families (six affected individuals in total) carrying the same homozygous c.1_9del mutation identified in the patients described here. This 9 bp deletion contains the ATG initiation codon located in exon 1f, and results in impaired translation of the plectin 1f isoform exclusively [10]. More recently, another truncating variant in exon 1f (p.Glu20Ter), also resulting in a LGMD R17 phenotype, has been reported [20].
The four families homozygous for the c.1_9del mutation that are characterized in this manuscript show many common features with the previously described families with LGMD R17, and none of the patients showed skin or nail involvement. However, there were some important differences between the patients described in this manuscript and those reported by Gundesli et al. [10] (Table 1). All patients displayed clear myasthenic symptoms, such as ptosis, fatigability, nasal speech, and a positive clinical response to the treatment with pyridostigmine and salbutamol. Additionally, one patient’s onset was in adulthood with a completely asymptomatic childhood period. All four patients described in this manuscript had paraspinal muscle weakness and calf hypertrophy. It is possible, however, that the myasthenic features might indeed not have been manifested in patients that were even younger than the ones we report. Alternatively, the more severe muscular dystrophy phenotype seen in the c.1_9del patients reported by Gundesli et al. might have masked the myasthenic symptoms.
Our patients come from unrelated consanguineous families from the Black Sea region in Turkey, as were the individuals described by Gundesli et al. who identified –using polymorphic DNA markers—a shared haplotype of ~2.3 Mb upstream of the PLEC variant in five of their patients [10]. Our haplotype analysis using WES data for three of our patients confirms the founder effect for the PLEC variant in the Turkish population but also delineates the breakpoints and size of the haplotype more clearly at 3.8 Mb.
Up until now, myasthenic phenotypes were frequently associated with mutations in the C-domain of PLEC. However, the cases described here carry the c.1_9del mutation, located in exon 1 of the plectin 1f isoform, and no other rare damaging variants in the PLEC actin binding domain have been detected (Figure 5). Plectin 1f plays an important role in the NMJ and interacts directly with rapsyn [5]. Our findings support the hypothesis of a crucial role of plectin 1f isoform in the NMJ and in the pathogenesis of myasthenic features. Although we only found a decremental response of 10% or more on RNS in two of our patients, the other two patients had severe weakness due to the muscular dystrophy, which might have masked the dysfunction of the NMJ. The patients who had less severe weakness and more prominent decremental response on RNS, had the most benefit from treatment. Early diagnosis and treatment of these patients may be more effective and lead to an improved quality of life.
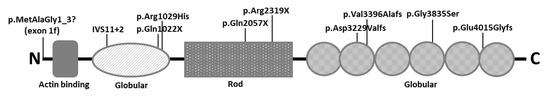
Figure 5.
Location of PLEC mutations associated with myasthenic phenotypes. Most cases are compound heterozygous for null mutations located in the globular domains; the only homozygous mutation with myasthenic phenotype described so far, and in this article, is in exon 1f [21,22,23,24].
To the best of our knowledge, only a few cases of EBS-MD with myasthenia have been described so far (Table 2). However, myasthenic features in EBS-MD patients may be missed if they are not investigated systematically and no additional tests such as SF-EMG are performed.

Table 2.
Review summary of cases with EBS-MD-MyS or EBS-MD with myasthenic features.
Plectin is a structural scaffolding protein concentrated at sites of mechanical stress and in the Z-disc. In PLEC patients with myasthenic features, neuromuscular junctions showed abnormal morphology with disrupted structures and degeneration [5,26]. At the molecular level, it was shown that plectin 1f plays an important role in the stability of the NMJ [4]. Electron microscopy analysis of muscle biopsies of patients with start codon loss in 1f isoform showed empty spaces between the sarcolemma and the contractile elements [10]. It was suggested that PLEC 1f interacts with desmin intermediate filaments (IFs) in the postsynaptic domain of the NMJ and plays a central role in their stability by directly interacting with rapsyn. Staining performed specifically for the PLEC 1f isoform in the NMJ showed co-localization with acetylcholine receptors (AChRs) [5] and in plectin-deficient myoblasts, AChRs are highly mobile and cannot form stable clusters. PLEC 1f also interacts with desmin and studies in PLEC 1f deficient myoblasts showed that without such a linkage the structural network of NMJ collapses [5,17]. Conditional plectin knockout mice (Pax7-Cre/cKO) with postsynaptic absence of plectin, resulted in a phenotype similar to EBS-MD-MyS, with severe lack of coordination, reduced motility, and shortened life span. In this model, uncoupling of AChRs from IFs resulted in severe disorganization and fragmentation of the NMJ and depletion of AChRs. Instead, presynaptic lack of plectin did not cause reduced muscle strength in mice lacking P1c plectin isoform [5].
Phenotypes associated with PLEC mutations are highly variable, which may reflect the multiple binding partners of the plectin protein. The cause of this clinical variability is not yet fully understood but could be explained by the location of the mutations in different domains and various isoforms [27]. As many patients with plectin deficiency have not been thoroughly investigated for NMJ defects, it may be that myasthenic features have been underestimated. Our cases carrying the c.1_9del PLEC mutation broaden the LGMD R17 phenotype with onset in adulthood along with extraocular, facial, and tongue involvement. Individuals with PLEC mutations should be carefully examined for myasthenic features and SF-EMG is crucial to confirm the diagnosis.
Author Contributions
Conceptualization, M.M. and H.D.; data curation, M.M. and A.T.; writing—original draft preparation, M.M. and H.D.; writing—review and editing, Y.P., A.T. and V.S.; writing—resubmission, A.T. and V.S.; funding acquisition, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
The MYO–SEQ project and was funded by Sanofi Genzyme, Ultragenyx, LGMD2I Research Fund, Samantha J Brazzo Foundation, LGMD2D Foundation and Kurt + Peter Foundation, Muscular Dystrophy UK, and Coalition to Cure Calpain 3. Analysis was provided by the Broad Institute of MIT and Harvard Center for Mendelian Genomics (Broad CMG) and was funded by the National Human Genome Research Institute, the National Eye Institute, and the National Heart, Lung and Blood Institute grant UM1 HG008900 and in part by National Human Genome Research Institute grant R01 HG009141. We thank Eleina M. England for her support with data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, C.G.; Maercker, C.; Castañon, M.J.; Hauptmann, R.; Wiche, G. Human plectin: Organization of the gene, sequence analysis, and chromosome localization (8q24). Proc. Natl. Acad. Sci. USA 1996, 93, 4278–4283. [Google Scholar] [CrossRef]
- Elliott, C.E.; Becker, B.; Oehler, S.; Castañón, M.J.; Hauptmann, R.; Wiche, G. Plectin transcript diversity: Identification and tissue distribution of variants with distinct first coding exons and rodless isoforms. Genomics 1997, 42, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wiche, G.; Osmanagic-Myers, S.; Castanon, M.J. Networking and anchoring through plectin: A key to IF functionality and mechanotransduction. Curr. Opin. Cell Biol. 2014, 32, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Rezniczek, G.A.; Konieczny, P.; Nikolic, B.; Reipert, S.; Schneller, D.; Abrahamsberg, C.; Davies, K.E.; Winder, S.J.; Wiche, G. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J. Cell Biol. 2007, 176, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Mihailovska, E.; Raith, M.; Valencia, R.G.; Fischer, I.; Al Banchaabouchi, M.; Herbst, R.; Wiche, G. Neuromuscular synapse integrity requires linkage of acetylcholine receptors to postsynaptic intermediate filament networks via rapsyn-plectin 1f complexes. Mol. Biol. Cell 2014, 25, 4130–4149. [Google Scholar] [CrossRef]
- Rezniczek, G.A.; Winter, L.; Walko, G.; Wiche, G. Chapter thirteen—Functional and genetic analysis of plectin in skin and muscle. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2016; Volume 569, pp. 235–259. [Google Scholar]
- Pulkkinen, L.; Smith, F.J.D.; Shimizu, H.; Murata, S.; Yaoita, H.; Hachisuka, H.; Nishikawa, T.; McLean, W.H.I.; Uitto, J. Homozygous deletion mutations in the plectin gene (PLEC1) in patients with epidermolysis bullosa simplex associated with late-onset muscular dystrophy. Hum. Mol. Genet. 1996, 5, 1539–1546. [Google Scholar] [CrossRef]
- Fine, J.D.; Stenn, J.; Johnson, L.; Wright, T.; Bock, H.G.O.; Horiguchi, Y. Autosomal recessive epidermolysis bullosa simplex: Generalized phenotypic features suggestive of junctional or dystrophic epidermolysis bullosa, and association with neuromuscular diseases. Arch. Dermatol. 1989, 125, 931–938. [Google Scholar] [CrossRef]
- Charlesworth, A.; Chiaverini, C.; Chevrant-Breton, J.; DelRio, M.; Diociaiuti, A.; Dupuis, R.P.; El Hachem, M.; Le Fiblec, B.; Sankari-Ho, A.M.; Valhquist, A.; et al. Epidermolysis bullosa simplex with PLEC mutations: New phenotypes and new mutations. Br. J. Dermatol. 2013, 168, 808–814. [Google Scholar] [CrossRef]
- Gundesli, H.; Talim, B.; Korkusuz, P.; Balci-Hayta, B.; Cirak, S.; Akarsu, N.A.; Topaloglu, H.; Dincer, P. Mutation in exon 1f of PLEC, leading to disruption of plectin isoform 1f, causes autosomal-recessive limb-girdle muscular dystrophy. Am. J. Hum. Genet. 2010, 87, 834–841. [Google Scholar] [CrossRef]
- Töpf, A.; Johnson, K.; Bates, A.; Phillips, L.; Chao, K.R.; England, E.M.; Laricchia, K.M.; Mullen, T.; Valkanas, E.; Xu, L.; et al. MYO-SEQ consortium, Straub, V. Sequential targeted exome sequencing of 1001 patients affected by unexplained limb-girdle weakness. Genet. Med. 2020. [Google Scholar] [CrossRef]
- Konieczny, P.; Fuchs, P.; Reipert, S.; Kunz, W.S.; Zeöld, A.; Fischer, I.; Paulin, D.; Schröder, R.; Wiche, G. Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J. Cell Biol. 2008, 181, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Bolling, M.C.; Pas, H.H.; De Visser, M.; Aronica, E.; Pfendner, E.G.; Van den Berg, M.P.; Diercks, G.F.H.; Suurmeijer, A.J.H.; Jonkman, M.F. PLEC1 mutations underlie adult-onset dilated cardiomyopathy in epidermolysis bullosa simplex with muscular dystrophy. J. Investig. Dermatol. 2010, 130, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Koss-Harnes, D.; Høyheim, B.; Jonkman, M.F.; De Groot, W.P.; De Weerdt, C.J.; Nikolic, B.; Wiche, G.; Gedde-Dahl, T., Jr. Life-long course and molecular characterization of the original Dutch family with epidermolysis bullosa simplex with muscular dystrophy due to a homozygous novel plectin point mutation. Acta Derm. Venereol. 2004, 84, 124–131. [Google Scholar] [PubMed]
- Kunz, M.; Rouan, F.; Pulkkinen, L.; Hamm, H.; Jeschke, R.; Bruckner-Tuderman, L.; Bröcker, E.B.; Wiche, G.; Uitto, J.; Zillikens, D. Mutation reports: Epidermolysis bullosa simplex associated with severe mucous membrane involvement and novel mutations in the plectin gene. J. Investig. Dermatol. 2000, 114, 376–380. [Google Scholar] [CrossRef]
- Doriguzzi, C.; Palmucci, L.; Mongini, T.; Bertolotto, A.; Maniscalco, M.; Chiadò-Piat, L.; Zina, A.M.; Bundino, S. Congenital muscular dystrophy associated with familial junctional epidermolysis bullosa letalis. Eur. Neurol. 1993, 33, 454–460. [Google Scholar] [CrossRef]
- Schröder, R.; Fürst, D.O.; Klasen, C.; Reimann, J.; Herrmann, H.; Van der Ven, P.F. Association of plectin with Z-discs is a prerequisite for the formation of the intermyofibrillar desmin cytoskeleton. Lab. Investig. 80 2000, 80, 455–464. [Google Scholar] [CrossRef][Green Version]
- Cowton, J.A.; Beattie, T.J.; Gibson, A.A.; Mackie, R.; Skerrow, C.J.; Cockburn, F. Epidermolysis bullosa in association with aplasia cutis congenita and pyloric atresia. Acta Paediatr. Scand. 1982, 71, 155–160. [Google Scholar] [CrossRef]
- Gedde-Dahl, T., Jr. Epidermolysis Bullosa: A Clinical, Genetic and Epidemiological Study; Johns Hopkins Press (Pub.): Baltimore, MD, USA, 1971. [Google Scholar]
- Deev, R.V.; Bardakov, S.N.; Mavlikeev, M.O.; Yakovlev, I.A.; Umakhanova, Z.R.U.; Akhmedova, P.G.; Magomedova, R.M.; Chekmaryeva, I.A.; Dalgatov, G.D.; Isaev, A.A. Glu20Ter variant in PLEC 1f isoform causes limb-girdle muscle dystrophy with lung injury. Front. Neurol. 2017, 8, 367. [Google Scholar] [CrossRef]
- Selcen, D.; Juel, V.C.; Hobson-Webb, L.D.; Smith, E.C.; Stickler, D.E.; Bite, A.V.; Ohno, K.; Engel, A.G. Myasthenic syndrome caused by plectinopathy. Neurology 2011, 76, 327–336. [Google Scholar] [CrossRef]
- Fattahi, Z.; Kahrizi, K.; Nafissi, S.; Fadaee, M.; Abedini, S.S.; Kariminejad, A.; Akbari, M.R.; Najmabadi, H. Report of a patient with limb-girdle muscular dystrophy, ptosis and ophthalmoparesis caused by plectinopathy. Arch. Iran. Med. 2015, 18, 60–64. [Google Scholar]
- Gonzalez Garcia, A.; Tutmaher, M.; Upadhyayula, S.; Sanchez Russo, R.; Verma, S. Novel PLEC gene variants causing congenital myasthenic syndrome. Muscle Nerve 2019, 60, E40–E43. [Google Scholar] [CrossRef] [PubMed]
- Forrest, K.; Mellerio, J.E.; Robb, S.; Dopping-Hepenstal, P.J.; McGrath, J.A.; Liu, L.; Buk, S.J.; Al-Sarraj, S.; Wraige, E.; Jungbluth, H. Congenital muscular dystrophy, myasthenic symptoms and epidermolysis bullosa simplex (EBS) associated with mutations in the PLEC1 gene encoding plectin. Neuromuscul. Disord. 2010, 20, 709–711. [Google Scholar] [CrossRef]
- Banwell, B.L.; Russel, J.; Fukudome, T.; Shen, X.M.; Stilling, G.; Engel, A.G. Myopathy, myasthenic syndrome, and epidermolysis bullosa simplex due to plectin deficiency. J. Neuropathol. Exp. Neurol. 1999, 58, 832–846. [Google Scholar] [CrossRef]
- Gache, Y.; Chavanas, S.; Lacour, J.P.; Wiche, G.; Owaribe, K.; Meneguzzi, G.; Ortonne, J.P. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J. Clin. Investig. 1996, 97, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Wiche, G. The many faces of plectin and plectinopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 77–93. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).