Autosomal Dominantly Inherited GREB1L Variants in Individuals with Profound Sensorineural Hearing Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Clinical Assessment
2.2. Exome Sequencing
3. Results
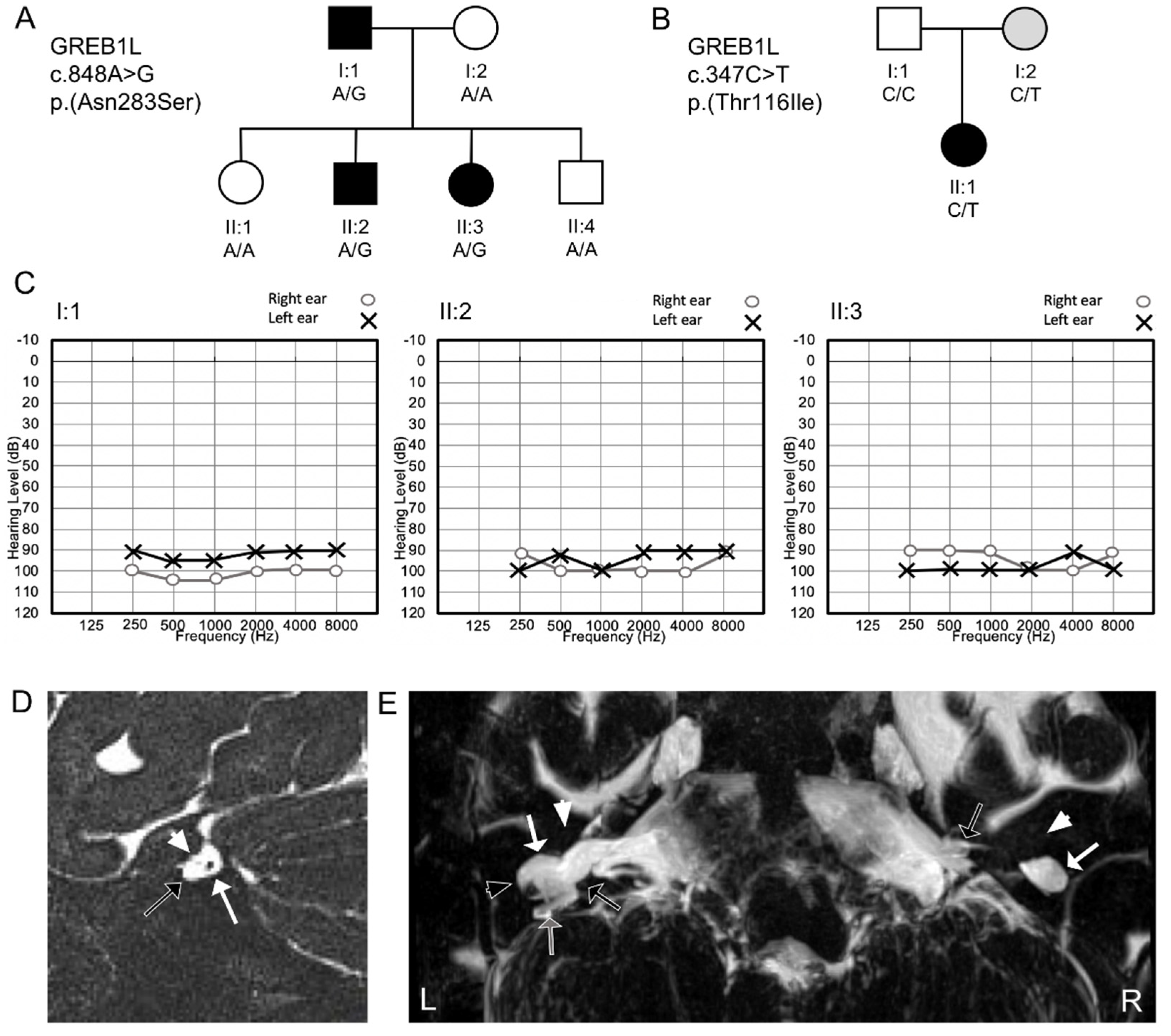
3.1. Clinical Findings
3.2. Exome Sequencing
3.3. Phenotypic Spectrum of GREB1L Variation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Web Resources
| ANNOVAR | http://annovar.openbioinformatics.org/ |
| Burrows-Wheeler aligner | http://bio-bwa.sourceforge.net/ |
| ClinVar | https://www.ncbi.nlm.nih.gov/clinvar/ |
| combined annotation dependent depletion (CADD) | http://cadd.gs.washington.edu/ |
| dbSNFP | https://sites.google.com/site/jpopgen/dbNSFP |
| dbSNP | https://www.ncbi.nlm.nih.gov/projects/SNP/ |
| gEAR | https://umgear.org/ |
| genome aggregation database (gnomAD) | http://gnomad.broadinstitute.org/ |
| genome analysis toolkit (GATK) | https://software.broadinstitute.org/gatk/ |
| genomic evolutionary rate profiling (GERP) | http://mendel.stanford.edu/SidowLab/downloads/gerp/ |
| Greater Middle East (GME) variome project | http://igm.ucsd.edu/gme |
| Hereditary hearing loss homepage | https://hereditaryhearingloss.org |
| Online Mendelian inheritance of man (OMIM) | https://www.omim.org/ |
| PhastCons and PhyloP | http://compgen.cshl.edu/phast/ |
| Picard | http://broadinstitute.github.io/picard/ |
| SHIELD | https://shield.hms.harvard.edu/ |
| World Health Organization | https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss |
References
- Shearer, A.E.; Hildebrand, M.S.; Smith, R.J. Hereditary hearing loss and deafness overview. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Chakchouk, I.; Zhang, D.; Zhang, Z.; Francioli, L.C.; Santos-Cortez, R.L.P.; Schrauwen, I.; Leal, S.M. Disparities in discovery of pathogenic variants for autosomal recessive non-syndromic hearing impairment by ancestry. Eur. J. Hum. Genet. 2019, 27, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.B.; Kozin, E.D.; Puram, S.V.; Owoc, M.S.; Shah, P.V.; Hight, A.E.; Sethi, R.K.V.; Remenschneider, A.K.; Lee, D.J. Auditory brainstem implant candidacy in the United States in children 0–17 years old. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Sennaroglu, L.; Saatci, I. A new classification for cochleovestibular malformations. Laryngoscope 2002, 112, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Kari, E.; Llaci, L.; Go, J.L.; Naymik, M.; Knowles, J.A.; Leal, S.M.; Rangasamy, S.; Huentelman, M.J.; Liang, W.; Friedman, R.A.; et al. Genes Implicated in Rare Congenital Inner Ear and Cochleovestibular Nerve Malformations. Ear Hear. 2020. [Google Scholar] [CrossRef]
- Schrauwen, I.; Kari, E.; Mattox, J.; Llaci, L.; Smeeton, J.; Naymik, M.; Raible, D.W.; Knowles, J.A.; Crump, J.G.; Huentelman, M.J.; et al. De novo variants in GREB1L are associated with non-syndromic inner ear malformations and deafness. Hum. Genet. 2018, 137, 459–470. [Google Scholar] [CrossRef]
- Brunskill, E.W.; Potter, A.S.; Distasio, A.; Dexheimer, P.; Plassard, A.; Aronow, B.J.; Potter, S.S. A gene expression atlas of early craniofacial development. Dev. Biol. 2014, 391, 133–146. [Google Scholar] [CrossRef]
- De Tomasi, L.; David, P.; Humbert, C.; Silbermann, F.; Arrondel, C.; Tores, F.; Fouquet, S.; Desgrange, A.; Niel, O.; Bole-Feysot, C.; et al. Mutations in GREB1L cause bilateral kidney agenesis in humans and mice. Am. J. Hum. Genet. 2017, 101, 803–814. [Google Scholar] [CrossRef]
- Cacheiro, P.; Haendel, M.A.; Smedley, D. International Mouse Phenotyping Consortium and the Monarch Initiative New models for human disease from the International Mouse Phenotyping Consortium. Mamm. Genome 2019, 30, 143–150. [Google Scholar] [CrossRef]
- Cartegni, L.; Wang, J.; Zhu, Z.; Zhang, M.Q.; Krainer, A.R. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003, 31, 3568–3571. [Google Scholar] [CrossRef]
- Desmet, F.-O.; Hamroun, D.; Lalande, M.; Collod-Béroud, G.; Claustres, M.; Béroud, C. Human splicing finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Boissel, S.; Fallet-Bianco, C.; Chitayat, D.; Kremer, V.; Nassif, C.; Rypens, F.; Delrue, M.-A.; Soglio, D.D.; Oligny, L.L.; Patey, N.; et al. Genomic study of severe fetal anomalies and discovery of GREB1L mutations in renal agenesis. Genet. Med. 2018, 20, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Sanna-Cherchi, S.; Khan, K.; Westland, R.; Krithivasan, P.; Fievet, L.; Rasouly, H.M.; Ionita-Laza, I.; Capone, V.P.; Fasel, D.A.; Kiryluk, K.; et al. Exome-wide Association Study Identifies GREB1L Mutations in Congenital Kidney Malformations. Am. J. Hum. Genet. 2017, 101, 789–802. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Purification of nucleic acids by extraction with phenol: Chloroform. CSH Protoc. 2006, 2006. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.M.; Khan, S.N.; Ahmed, Z.M.; Riazuddin, S.; Waryah, A.M.; Chhatre, D.; Starost, M.F.; Ploplis, B.; Buckley, S.; Velásquez, D.; et al. Noncoding mutations of HGF are associated with nonsyndromic hearing loss, DFNB39. Am. J. Hum. Genet. 2009, 85, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Riazuddin, S.; Belyantseva, I.A.; Giese, A.P.J.; Lee, K.; Indzhykulian, A.A.; Nandamuri, S.P.; Yousaf, R.; Sinha, G.P.; Lee, S.; Terrell, D.; et al. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat. Genet. 2012, 44, 1265–1271. [Google Scholar] [CrossRef]
- Shahzad, M.; Sivakumaran, T.A.; Qaiser, T.A.; Schultz, J.M.; Hussain, Z.; Flanagan, M.; Bhinder, M.A.; Kissell, D.; Greinwald, J.H.; Khan, S.N.; et al. Genetic Analysis through OtoSeq of Pakistani Families Segregating Prelingual Hearing Loss. Otolaryngol. -Head Neck Surg. 2013, 149, 478–487. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Yang, H.; Wang, K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat. Protoc. 2015, 10, 1556–1566. [Google Scholar] [CrossRef]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Halees, A.; Itan, Y.; Spencer, E.G.; He, Y.; Azab, M.A.; Gabriel, S.B.; Belkadi, A.; Boisson, B.; Abel, L.; et al. Characterization of Greater Middle Eastern genetic variation for enhanced disease gene discovery. Nat. Genet. 2016, 48, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, C.; Li, C.; Boerwinkle, E. dbNSFP v3.0: A one-stop database of functional predictions and annotations for human Nonsynonymous and splice-site SNVs. Hum. Mutat. 2016, 37, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Boerwinkle, E.; Liu, X. In silico prediction of splice-altering single nucleotide variants in the human genome. Nucleic Acids Res. 2014, 42, 13534–13544. [Google Scholar] [CrossRef]
- Davydov, E.V.; Goode, D.L.; Sirota, M.; Cooper, G.M.; Sidow, A.; Batzoglou, S. Identifying a High Fraction of the Human Genome to be under Selective Constraint Using GERP++. PLoS Comput. Biol. 2010, 6, e1001025. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Scheffer, D.I.; Kwan, K.Y.; Corey, D.P. SHIELD: An integrative gene expression database for inner ear research. Database (Oxford) 2015, 2015. [Google Scholar] [CrossRef]
- Schrauwen, I.; Hasin-Brumshtein, Y.; Corneveaux, J.J.; Ohmen, J.; White, C.; Allen, A.N.; Lusis, A.J.; Van Camp, G.; Huentelman, M.J.; Friedman, R.A. A comprehensive catalogue of the coding and non-coding transcripts of the human inner ear. Hear. Res. 2016, 333, 266–274. [Google Scholar] [CrossRef]
- Krumm, N.; Sudmant, P.H.; Ko, A.; O’Roak, B.J.; Malig, M.; Coe, B.P.; Quinlan, A.R.; Nickerson, D.A.; Eichler, E.E. Copy number variation detection and genotyping from exome sequence data. Genome Res. 2012, 22, 1525–1532. [Google Scholar] [CrossRef]
- Zhang, J.; Haider, S.; Baran, J.; Cros, A.; Guberman, J.M.; Hsu, J.; Liang, Y.; Yao, L.; Kasprzyk, A. BioMart: A data federation framework for large collaborative projects. Database (Oxford) 2011, 2011. [Google Scholar] [CrossRef]
- MacDonald, J.R.; Ziman, R.; Yuen, R.K.C.; Feuk, L.; Scherer, S.W. The Database of Genomic Variants: A curated collection of structural variation in the human genome. Nucleic Acids Res. 2014, 42, D986–D992. [Google Scholar] [CrossRef] [PubMed]
- Optimizing Genomic Medicine in Epilepsy Through a Gene-Customized Approach to Missense Variant Interpretation. Available online: https://genome.cshlp.org/content/27/10/1715/F1.expansion.html (accessed on 6 March 2020).
- Jarvik, G.P.; Browning, B.L. Consideration of Cosegregation in the Pathogenicity Classification of Genomic Variants. Am. J. Hum. Genet. 2016, 98, 1077–1081. [Google Scholar] [CrossRef]
- Rasmussen, M.; Sunde, L.; Nielsen, M.L.; Ramsing, M.; Petersen, A.; Hjortshøj, T.D.; Olsen, T.E.; Tabor, A.; Hertz, J.M.; Johnsen, I.; et al. Targeted gene sequencing and whole-exome sequencing in autopsied fetuses with prenatally diagnosed kidney anomalies. Clin. Genet. 2018, 93, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Herlin, M.K.; Le, V.Q.; Højland, A.T.; Ernst, A.; Okkels, H.; Petersen, A.C.; Petersen, M.B.; Pedersen, I.S. Whole-exome sequencing identifies a GREB1L variant in a three-generation family with Müllerian and renal agenesis: A novel candidate gene in Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. A case report. Hum. Reprod. 2019, 34, 1838–1846. [Google Scholar] [CrossRef]
- Adeline, J.; Bouchra, B.; Corinne, F.; Jerôme, T.; Claire, J.; Aimé, L.; Elise, B.-B.; Christèle, D.; Véronique, D.; Laurent, P.; et al. GREB1L variants in familial and sporadic hereditary urogenital adysplasia and Mayer-Rokitansky-Kuster-Hauser syndrome. Clin. Genet. 2020. [Google Scholar] [CrossRef]
- Brophy, P.D.; Rasmussen, M.; Parida, M.; Bonde, G.; Darbro, B.W.; Hong, X.; Clarke, J.C.; Peterson, K.A.; Denegre, J.; Schneider, M.; et al. A Gene Implicated in Activation of Retinoic Acid Receptor Targets Is a Novel Renal Agenesis Gene in Humans. Genetics 2017, 207, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Zhang, D.; Burroughs, A.M.; Aravind, L. Computational identification of novel biochemical systems involved in oxidation, glycosylation and other complex modifications of bases in DNA. Nucleic Acids Res. 2013, 41, 7635–7655. [Google Scholar] [CrossRef] [PubMed]
- Plouhinec, J.-L.; Roche, D.D.; Pegoraro, C.; Figueiredo, A.-L.; Maczkowiak, F.; Brunet, L.J.; Milet, C.; Vert, J.-P.; Pollet, N.; Harland, R.M.; et al. Pax3 and Zic1 trigger the early neural crest gene regulatory network by the direct activation of multiple key neural crest specifiers. Dev. Biol. 2014, 386, 461–472. [Google Scholar] [CrossRef]
- Shakhova, O.; Sommer, L. Neural crest-derived stem cells. In StemBook; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008. [Google Scholar]
- Whitfield, T.T. Development of the inner ear. Curr. Opin. Genet. Dev. 2015, 32, 112–118. [Google Scholar] [CrossRef]
- Webb, B.D.; Shaaban, S.; Gaspar, H.; Cunha, L.F.; Schubert, C.R.; Hao, K.; Robson, C.D.; Chan, W.-M.; Andrews, C.; MacKinnon, S.; et al. HOXB1 founder mutation in humans recapitulates the phenotype of Hoxb1-/- mice. Am. J. Hum. Genet. 2012, 91, 171–179. [Google Scholar] [CrossRef]
- Vogel, M.; Velleuer, E.; Schmidt-Jiménez, L.F.; Mayatepek, E.; Borkhardt, A.; Alawi, M.; Kutsche, K.; Kortüm, F. Homozygous HOXB1 loss-of-function mutation in a large family with hereditary congenital facial paresis. Am. J. Med. Genet. A 2016, 170, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Epstein, D.J. Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development 2011, 138, 3967–3976. [Google Scholar] [CrossRef] [PubMed]
- Orten, D.J.; Fischer, S.M.; Sorensen, J.L.; Radhakrishna, U.; Cremers, C.W.R.J.; Marres, H.A.M.; Van Camp, G.; Welch, K.O.; Smith, R.J.H.; Kimberling, W.J. Branchio-oto-renal syndrome (BOR): Novel mutations in the EYA1 gene, and a review of the mutational genetics of BOR. Hum. Mutat. 2008, 29, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Vega-Lopez, G.A.; Cerrizuela, S.; Tribulo, C.; Aybar, M.J. Neurocristopathies: New insights 150 years after the neural crest discovery. Dev. Biol. 2018, 444 (Suppl 1), S110–S143. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.H.; O’Connor, T.G.; Roth, C.; Susser, E.; Bjørke-Monsen, A.-L. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front. Neurosci. 2013, 7, 120. [Google Scholar] [CrossRef]
- Cable, J.; Barkway, C.; Steel, K.P. Characteristics of stria vascularis melanocytes of viable dominant spotting (Wv/Wv) mouse mutants. Hear. Res. 1992, 64, 6–20. [Google Scholar] [CrossRef]
- Mochizuki, T.; Lemmink, H.H.; Mariyama, M.; Antignac, C.; Gubler, M.C.; Pirson, Y.; Verellen-Dumoulin, C.; Chan, B.; Schröder, C.H.; Smeets, H.J. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat. Genet. 1994, 8, 77–81. [Google Scholar] [CrossRef]
- Birkenhäger, R.; Otto, E.; Schürmann, M.J.; Vollmer, M.; Ruf, E.M.; Maier-Lutz, I.; Beekmann, F.; Fekete, A.; Omran, H.; Feldmann, D.; et al. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat. Genet. 2001, 29, 310–314. [Google Scholar] [CrossRef]

| Family Type | Variant Segregation | Inheritance Model | Predicted Variant Effect | cDNA Change 1 | AA Change | gnomAD | CADD Score (v1.3) | GERP++RS | Splicing Effect Prediction 2 | Phenotype | ACMG 3 | Study |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trio | de novo | AD | splicing | c.4368G>T | p.(Glu1410fs) | absent | 26 | 5.17 | splice site loss | profound bilateral SNHI; UCA (right); UIP-I (left); BVES + SCC; BCNA | P | [6] |
| Trio | de novo | AD | nonsense | c.982C>T | p.(Arg328*) | absent | 38 | 4.52 | NA | profound bilateral SNHI; BCA; BVES + SCC; BCNA | P | [6] |
| Family | inherited | AD | missense/splicing | c.848A>G | p.(Asn283Ser) | absent | 10 | 3.44 | ESE site loss | profound bilateral SNHI 4 | LP | This study (Family 1) |
| Trio | inherited 5 | AD | missense | c.347C>T | p.(Thr116Ile) | absent | 30 | 5.25 | NA | profound bilateral SNHI; BCA; BVES + SCC; BCNA | VUS | This study (Family 2) |
| cDNA Variant 1 | Amino Acid Variant | Urinary Phenotype | Genital Phenotype | Ear Phenotype 2 | Other Phenotypes | Inheritance | Reduced Penetrance | Reference |
|---|---|---|---|---|---|---|---|---|
| c.37C>T | p.(Arg13*) | unilateral MCD, congenital megaureter | – | – | – | NA | NA | [14] |
| c.293C>G | p.(Ser98*) | BKA | – | – | – | de novo | no | [13] |
| c.347C>T | p.(Thr116Ile) | – | – | profound bilateral SNHI, BCA; BVES+SCC; BCNA | – | mat | yes (mat) | This study |
| c.371G>T | p.(Gly124Val) | BKA, UKA, bladder hypoplasia | – | – | Potter sequence | suspected pat 4 | NA | [35] |
| c.383G>A | p.(Arg128His) | UKA | unicornate uterus, agenesis of left ovary | – | – | NA | NA | [14] |
| c.575G>T | p.(Arg192Leu) | BKA, UKA | unique fallopian trump and ovary | – | insulin-dependent diabetes | mat | no | [8] |
| c.705G>T | p.(Trp235Cys) | BKA, UKA, renal cysts, clear cell renal carcinoma | MRKH, arcuate uterus | – | – | AD family (2 mat, 2 pat) | yes (2 mat, 1 unaffected female sib) | [36] |
| c.818G>T | p.(Gly273Val) | UKA | – | – | – | NA | NA | [14] |
| c.848A>G | p.(Asn283Ser) + splicing | – | – | profound bilateral SNHI 3 | – | AD family (1 pat) | no | This study |
| c.982C>T | p.(Arg328*) | – | – | profound bilateral SNHI, BCA; BVES+SCC; BCNA | – | de novo | no | [6] |
| c.983G>A | p.(Arg328Gln) | pelvic kidney, MCD, VUR | – | – | mat | no | [8] | |
| c.1490C>G | p.(Ala497Gly) | UKA | – | – | – | NA | NA | [14] |
| c.1582delC | p.(Gln528Argfs*12) | BKA, UKA | UA, unicornuated uterus | – | clinodactyly | mat | yes (mat) | [8] |
| c.1780G>T | p.(Glu594*) | BKA, UKA, VUR | UA, fallopian trumps absence, ovarian hernia, uterine left artery absent | – | – | mat | no | [8] |
| c.1813A>C | p.(Ser605Arg) | UKA, multilocular cyst | blind ending hemi-vagina and bicornuated uterus | – | – | pat | yes (pat) | [8] |
| c.1852G>A | p.(Asp618Asn) | Ectopic kidney, VUR, duplicated ureter | MRKH type 2 | – | unilateral polydactyly, facial asymmetry | NA | NA | [37] |
| c.2148G>T | p.(Leu716Phe) | VUR | – | – | iris anomaly | NA | NA | [8] |
| c.2227del | p.(Gln743Argfs*10) | UKA, MCD | MRKH type 2, UA | – | scoliosis | AD family (1 mat) | yes (mat) | [37] |
| c.2251C>T | p.(Arg751Cys) | BKA, unilateral hypoplasia | unicornuated uterus | – | – | mat | no | [8] |
| c.2252G>A | p.(Arg751His) | UKA, MCD, megaurethra | – | – | hepatic portal fibrosis | mat 5 | NA | [8] |
| c.2281G>C | p.(Glu761Gln) | UKA, duplication of the ureter, unilateral MCD, congenital megaureter | – | – | – | pat | yes (pat) | [14] |
| c.2312C>T | p.(Pro771Leu) | UKA, MCD | UA, streak ovaries, rudimentary follopian tubes | – | Right unique umbilical artery, 11 pairs of ribs, 6 cervical hemivertebrae with 1 hemivertebrae | NA | NA | [37] |
| c.2787_2788del | p.(Asp930Profs*12) | BKA, UKA, MCD | UA | – | – | AD family (1 mat; 1pat) | yes (mat) | [37] |
| c.2903C>T | p.(Ala968Val) | BKA, with agenesis of ureters, bladder hypoplasia | – | – | – | de novo | no | [13] |
| c.2926C>T | p.(Gln976*) | BKA, UKA | – | – | – | mat | no | [8] |
| c.3197G>C | p.(Arg1066Pro) | UKA | – | – | – | NA | NA | [14] |
| c.3295C>T | p.(Gln1099*) | unilateral MCD | – | – | – | mat | no | [14] |
| c.3970-20A>G | splicing | BKA, ureter and bladder aplasia | UA | – | Unilateral hexadactyly | pat | yes (pat) | [37] |
| c.3983G>A | p.(Gly1328Asp) | UKA | MRKH type 2 | – | – | NA | NA | [37] |
| c.3998_3999insC | p.(Leu1334Profs*18) | UKA | – | – | – | mat | no | [14] |
| c.4368G>T | splicing | – | – | profound bilateral SNHI, UCA (right); UIP-I (left); BVES+SCC; BCNA | – | de novo | no | [37] |
| c.4369−1G>C | splicing | BKA | UA | – | thickened left ventricular wall, 10 pairs of ribs | mat | yes (mat) | [8] |
| c.4505T>C | p.(Met1502Thr) | BKA | – | – | retro-esophageal subclavian artery, adrenal gland hypoplasia, enlarged thymus, one pair of cervical ribs | mat 5 | NA | [8] |
| c.4526A>T | p.(Asp1509Val) | BKA | – | – | adrenal cytomegaly | NA | NA | [8] |
| c.4607A>G | p.(His1536Arg) | BKA, UKA, MCD, horseshoe kidney | UA | – | 11 pairs of ribs | mat | no | [8] |
| c.4646T>C | p.(Val1549Ala) | UKA | UA | – | Henoch Schönlein Purpura | NA | NA | [14] |
| c.4672C>A | p.(Arg1558Ser) | BKA | – | – | – | mat | yes (mat) | [8] |
| c.4680C>A | p.(Tyr1560*) | BKA, bladder agenesis, VUR | uterus anomaly | – | Potter sequence | AD family (1 mat) | no | [14] |
| c.4700T>C | p.(Leu1567Pro) | UKA | – | – | – | de novo | no | [14] |
| c.4727C>T | p.(Ala1576Val) | BKA | – | auricular tag | hypertrophic left ventricle, aortic stenosis | NA | NA | [8] |
| c.4843G>A | p.(Val1615Ile) | BRHD, congenital hydronephrosis | – | – | – | mat | yes (mat) | [14] |
| c.4964T>C | p.(Ile1655Thr) | UKA | – | unilateral SNHI | genu valgum, flat feet | pat | yes (pat) | [14] |
| c.4991A>C | p.(Tyr1664Cys) | UKA | – | – | – | NA | NA | [14] |
| c.5068G>A | p.(Val1690Met) | UKA, VUR | – | – | – | pat 5 | NA | [14] |
| c.5198A>G | p.(Asn1733Ser) | BKA, UKA, ureter and bladder aplasia | UA, hemi-uterus, streak ovaries | – | – | AD family (1 mat, 1 pat) | yes (mat; unaffected female sib) | [37] |
| c.5323G>A | p.(Asp1775Asn) | BKA | – | preauricular tag, lop ear | – | NA | NA | [8] |
| c.5378T>G | p.(Leu1793Arg) | BKA, RKA, hypertrophy of the kidney | – | – | – | AD family (2 mat) | yes (1 mat) | [38] |
| c.5608+1delG | splicing | BKA, UKA, bladder agenesis | undifferentiated external female genitalia | – | Potter sequence | de novo, mat | yes (2 male siblings) | [35,38] |
| c.5651G>A | p.(Arg1884His) | URHD | – | – | – | NA | NA | [14] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schrauwen, I.; Liaqat, K.; Schatteman, I.; Bharadwaj, T.; Nasir, A.; Acharya, A.; Ahmad, W.; Van Camp, G.; Leal, S.M. Autosomal Dominantly Inherited GREB1L Variants in Individuals with Profound Sensorineural Hearing Impairment. Genes 2020, 11, 687. https://doi.org/10.3390/genes11060687
Schrauwen I, Liaqat K, Schatteman I, Bharadwaj T, Nasir A, Acharya A, Ahmad W, Van Camp G, Leal SM. Autosomal Dominantly Inherited GREB1L Variants in Individuals with Profound Sensorineural Hearing Impairment. Genes. 2020; 11(6):687. https://doi.org/10.3390/genes11060687
Chicago/Turabian StyleSchrauwen, Isabelle, Khurram Liaqat, Isabelle Schatteman, Thashi Bharadwaj, Abdul Nasir, Anushree Acharya, Wasim Ahmad, Guy Van Camp, and Suzanne M. Leal. 2020. "Autosomal Dominantly Inherited GREB1L Variants in Individuals with Profound Sensorineural Hearing Impairment" Genes 11, no. 6: 687. https://doi.org/10.3390/genes11060687
APA StyleSchrauwen, I., Liaqat, K., Schatteman, I., Bharadwaj, T., Nasir, A., Acharya, A., Ahmad, W., Van Camp, G., & Leal, S. M. (2020). Autosomal Dominantly Inherited GREB1L Variants in Individuals with Profound Sensorineural Hearing Impairment. Genes, 11(6), 687. https://doi.org/10.3390/genes11060687






