Sexual Differentiation Is Coordinately Regulated by Cryptococcus neoformans CRK1 and GAT1
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media and Growth Conditions
2.2. Construction of C. neoformans mat2 and znf2 Mutant Strains
2.3. Overexpression of C. neoformans CRK1 in the MATα crk1 Mutant
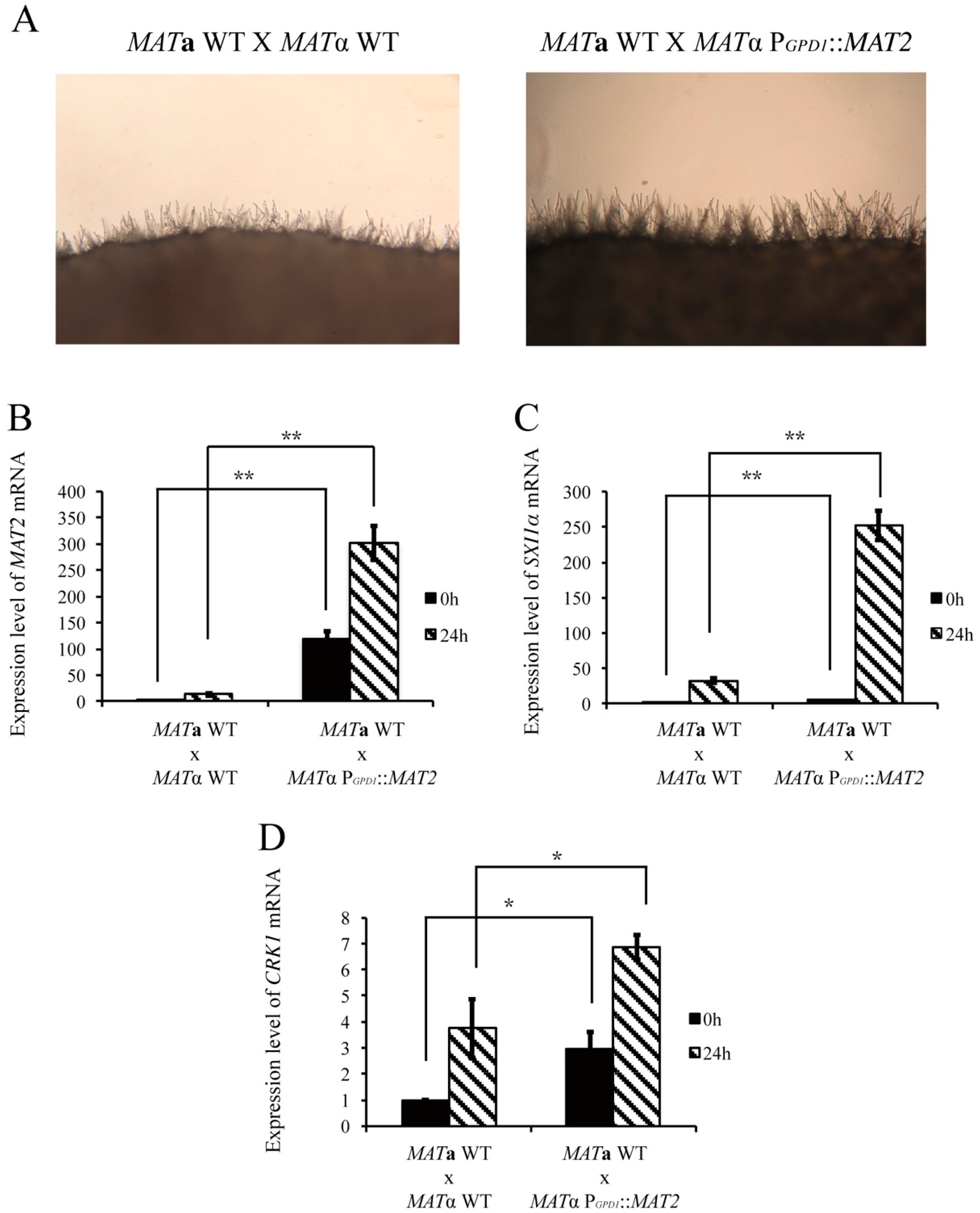
2.4. Overexpression of C. neoformans MAT2 in the MATα Wild-type Strain
2.5. Genetic Manipulation of C. neoformans GAT1 Gene
2.6. Sample Preparation for Gene Expression Analyses
2.7. Fluorescence Microscopy
3. Results
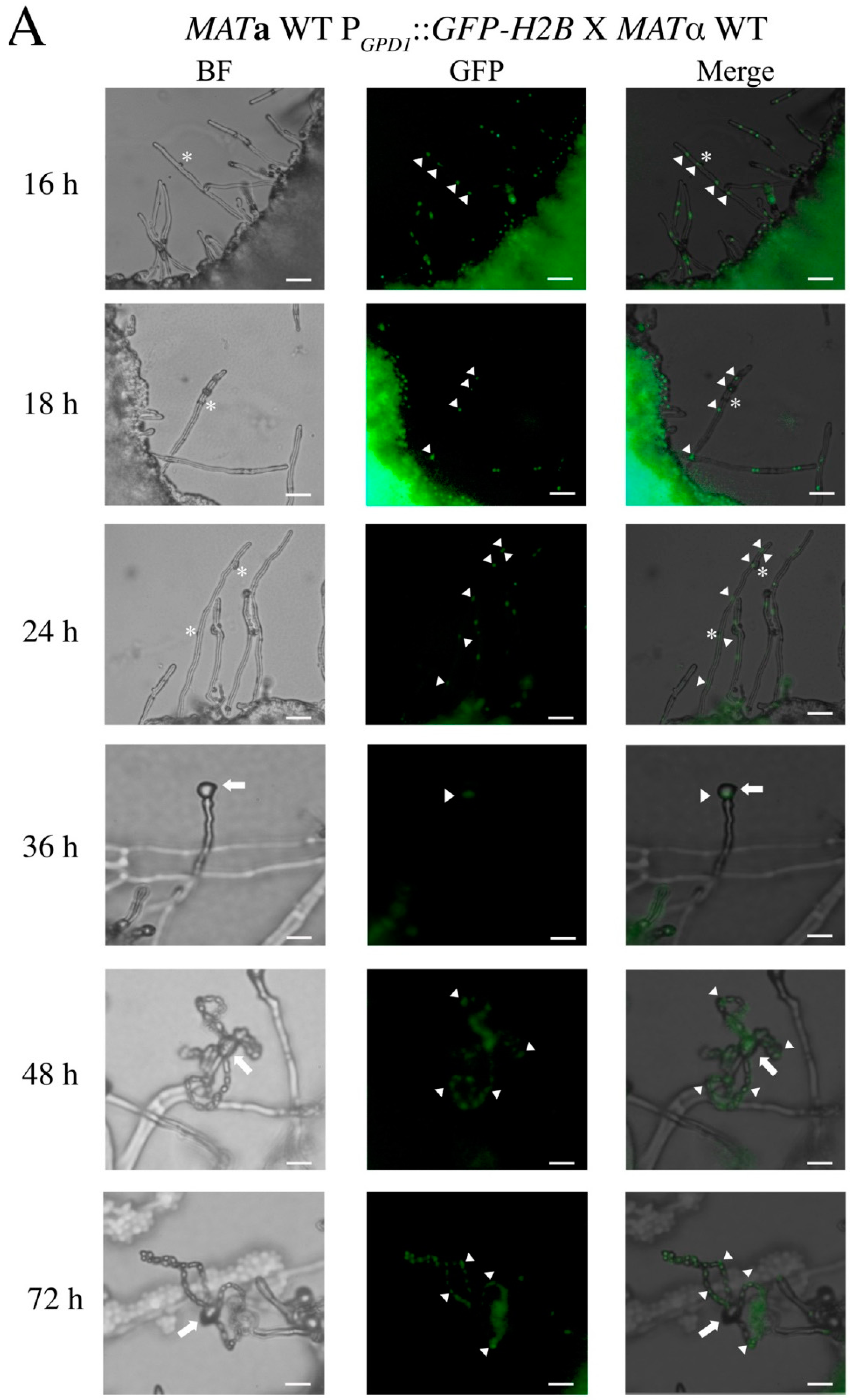
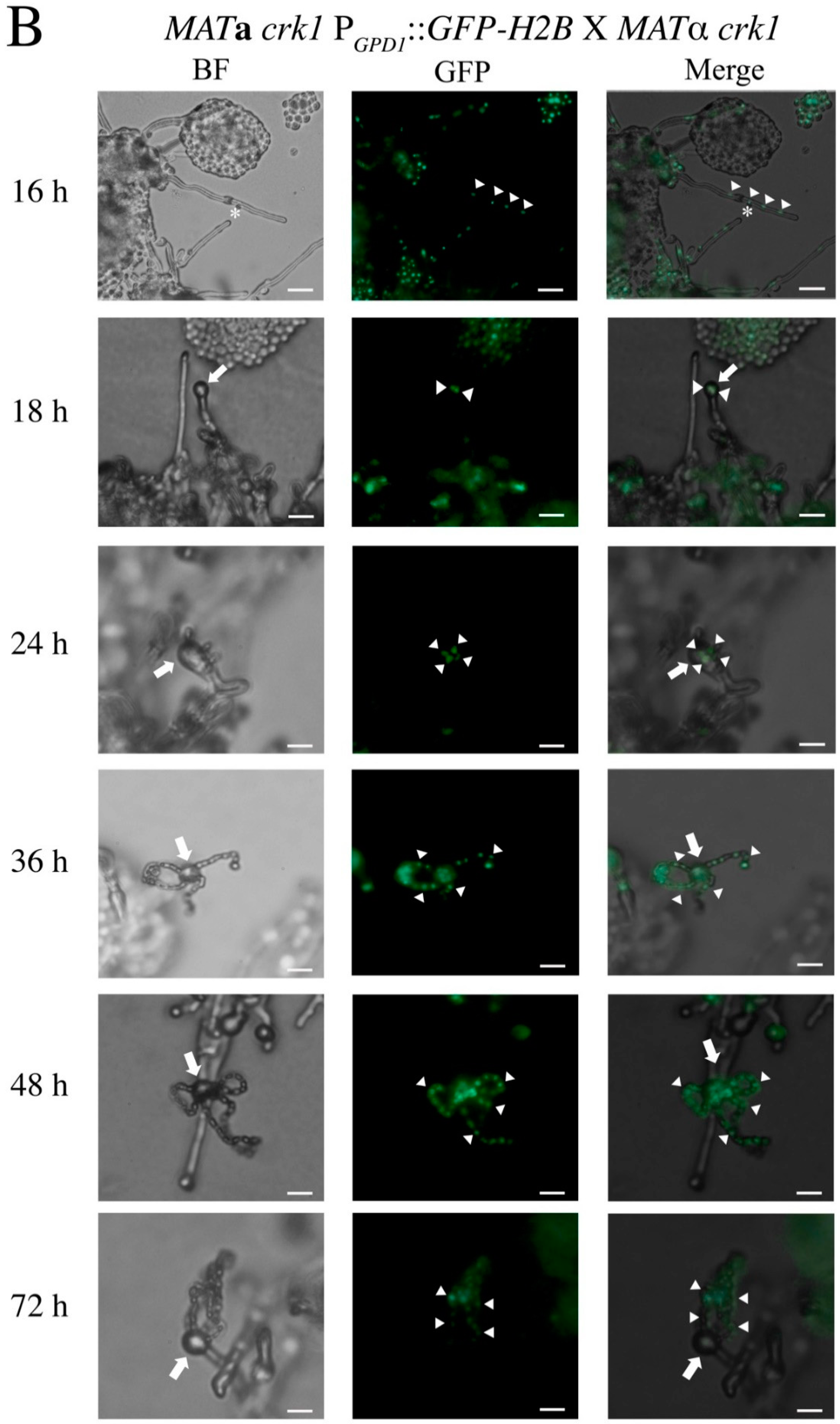
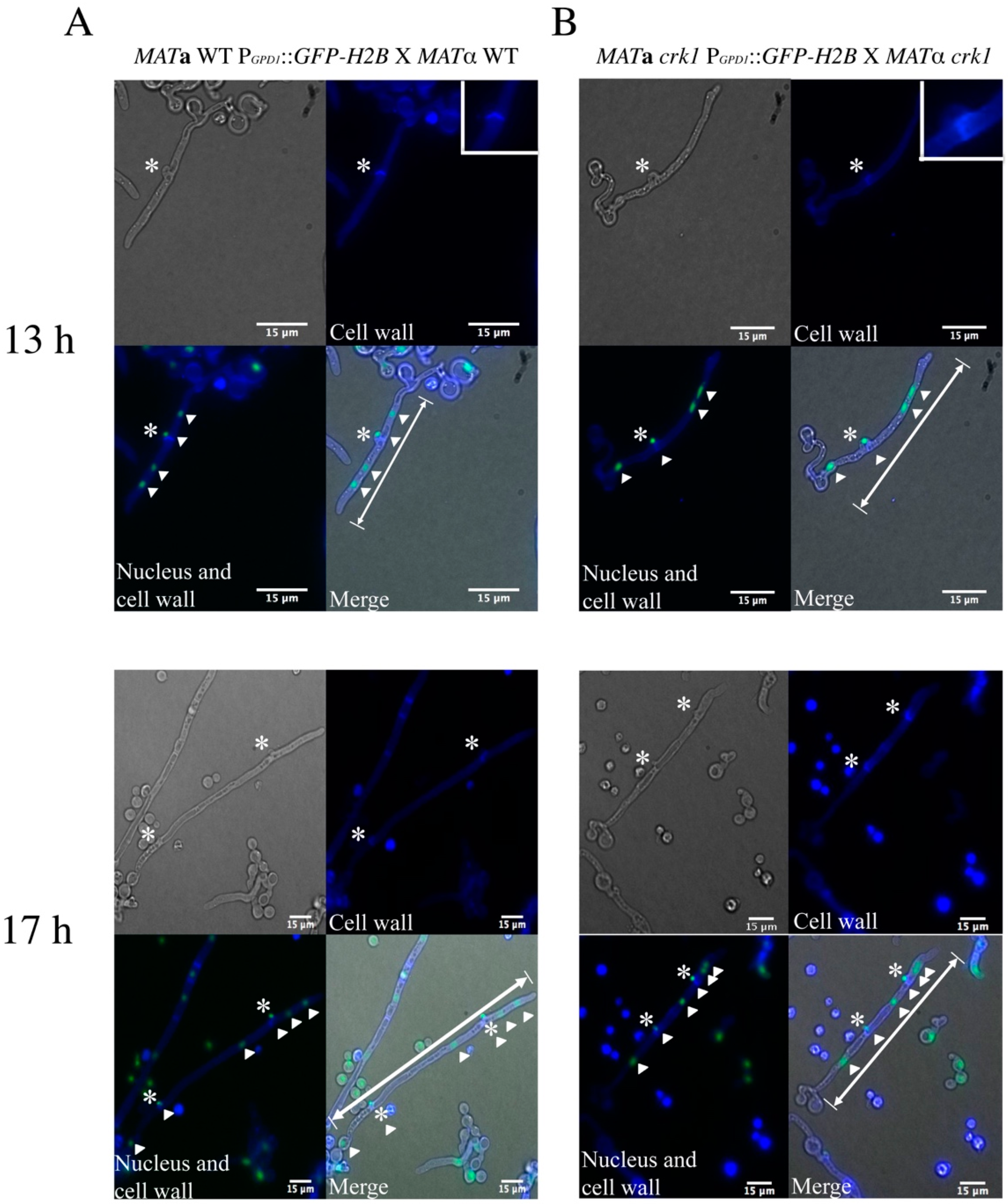
3.1. In the C. neoformans Bilateral crk1 Mutant Cross, Dikaryotic Filamentation was Altered but Nucleus Distribution Was Normal
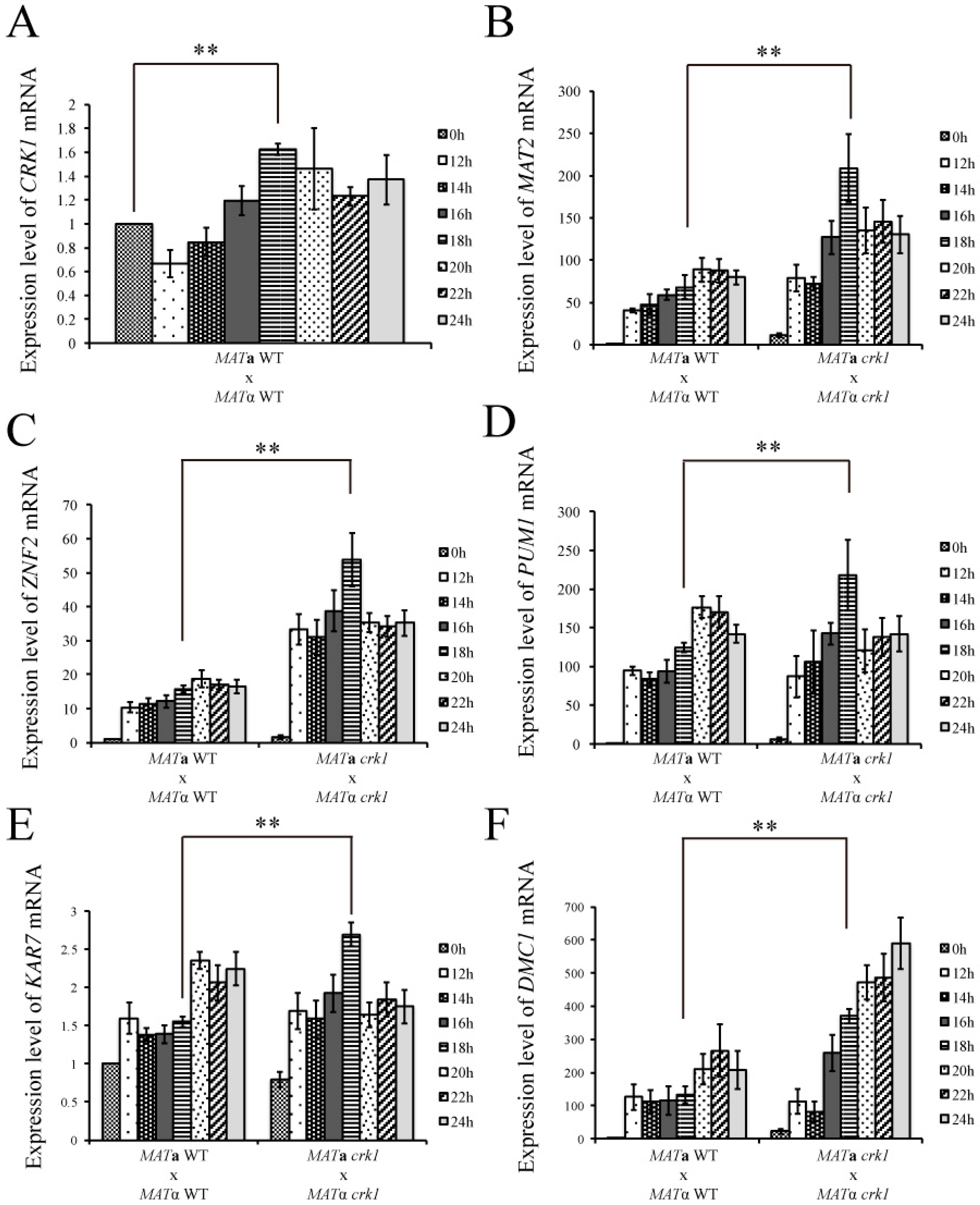
3.2. C. neoformans CRK1 Repressed Cell Fusion, Filamentation, Karyogamy and Meiotic Gene Expression during Bisexual Mating
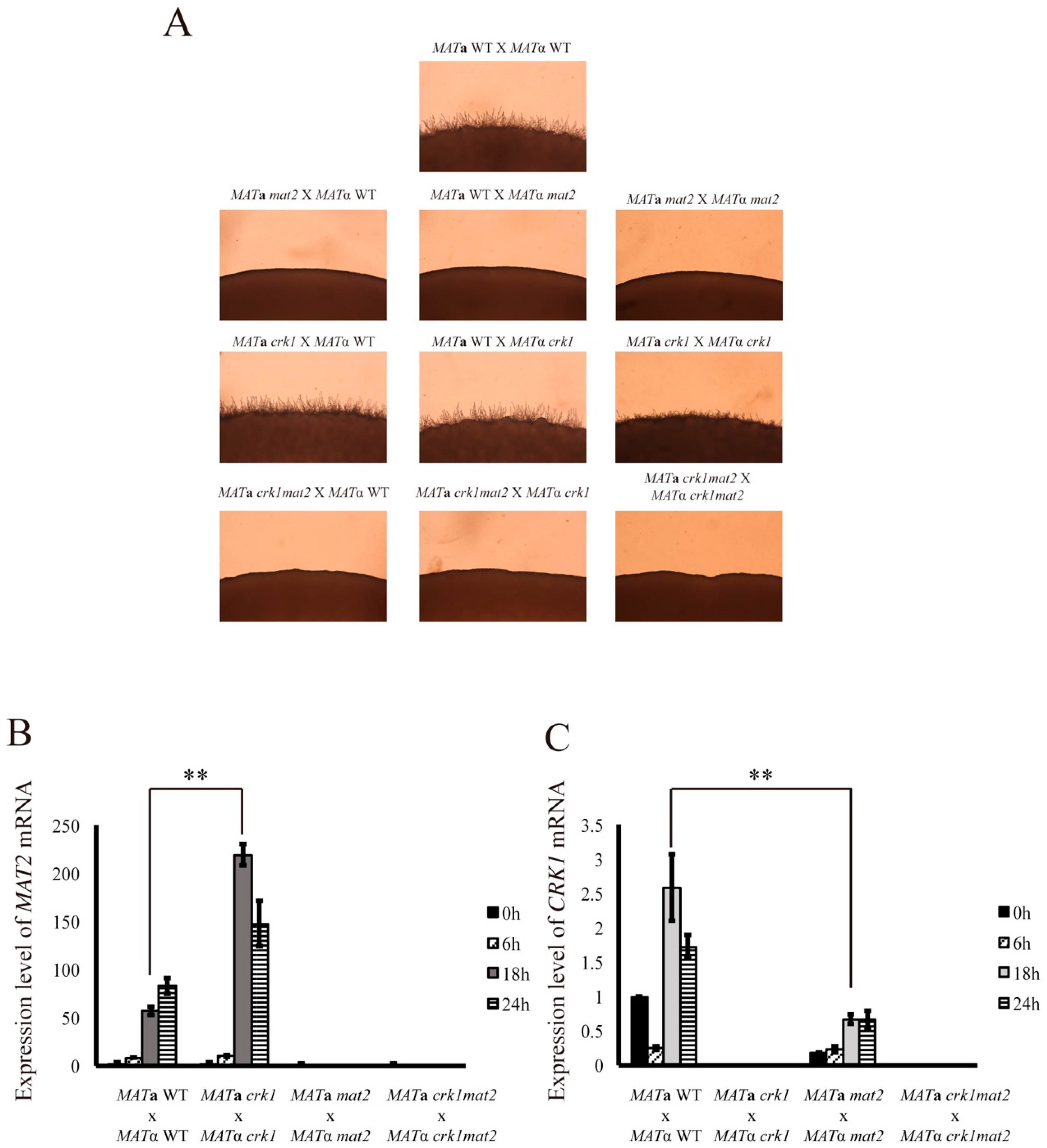
3.3. CRK1 Expression Level was Decreased in the Bilateral mat2 Mutant Cross
3.4. Deletion of C. neoformans ZNF2 Resulted in Non-Filamentation in the crk1 Mutant
3.5. Gat1 Contains the Predicted Crk1 Consensus Phosphorylation Site
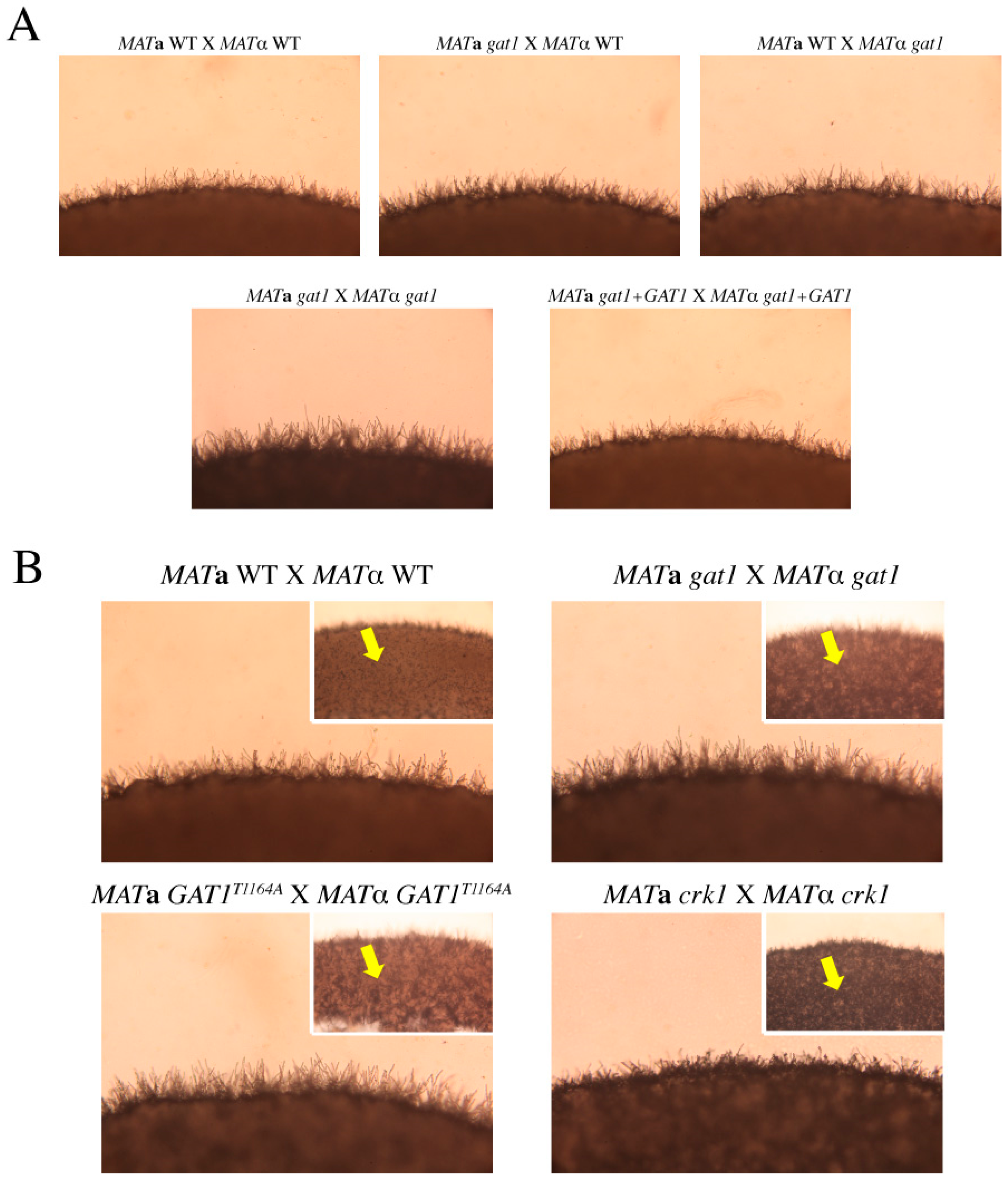
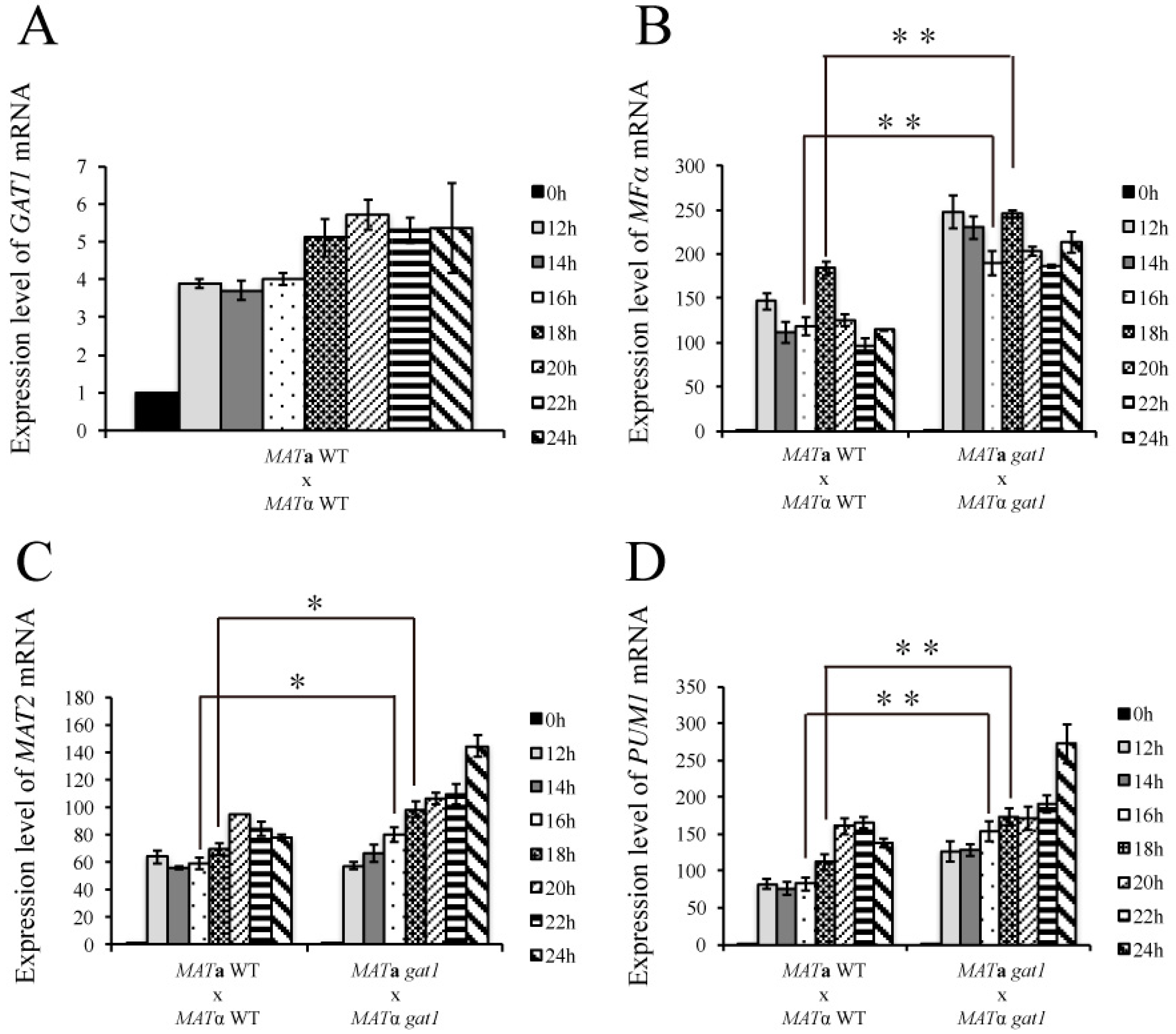
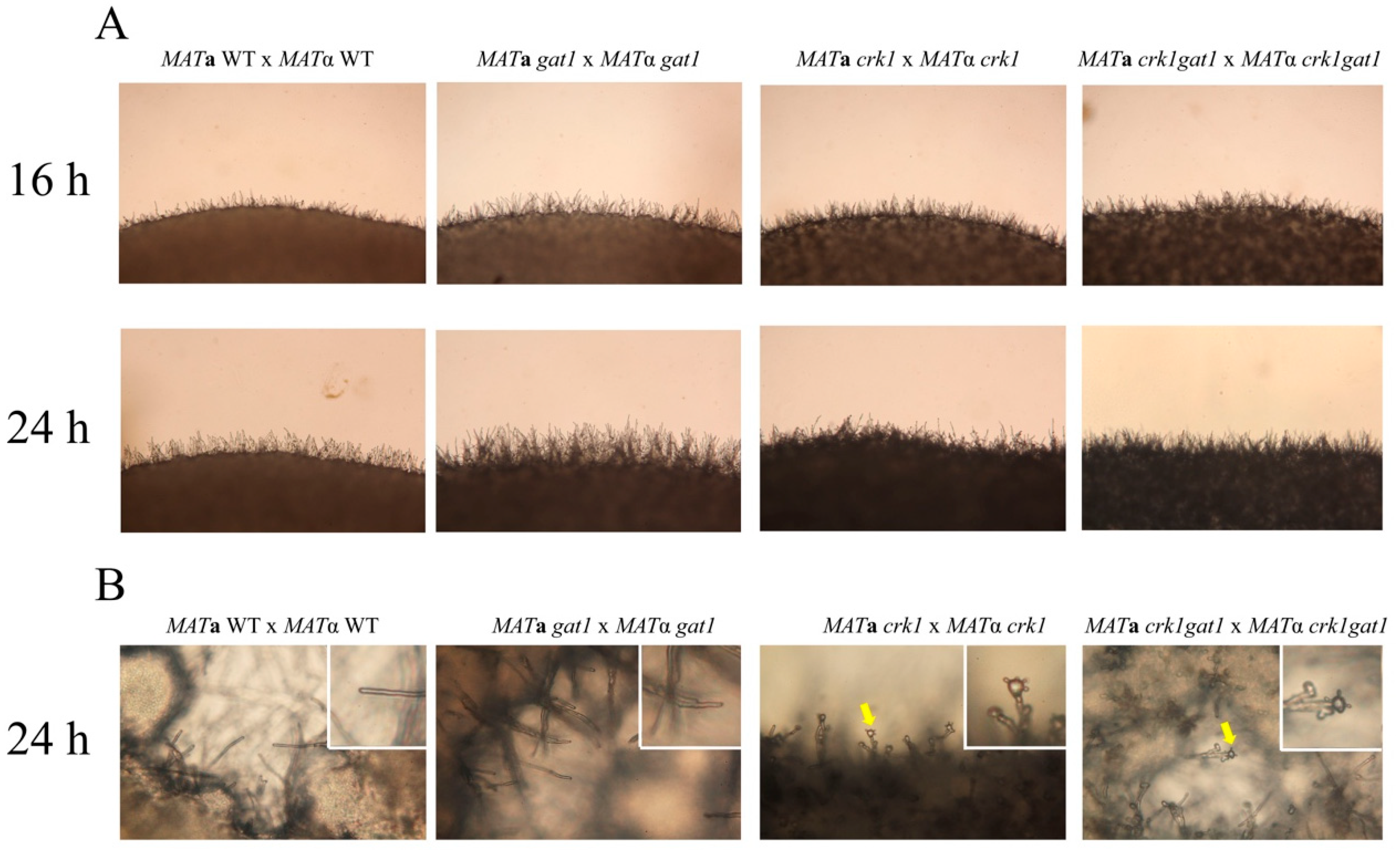
3.6. Deletion of GAT1 Enhanced Pheromone Expression and Increased Aerial Hyphae Formation
3.7. The C. neoformans crk1gat1 Mutants Exhibited Similar Phenotypes as the crk1 Mutant in Bisexual Mating
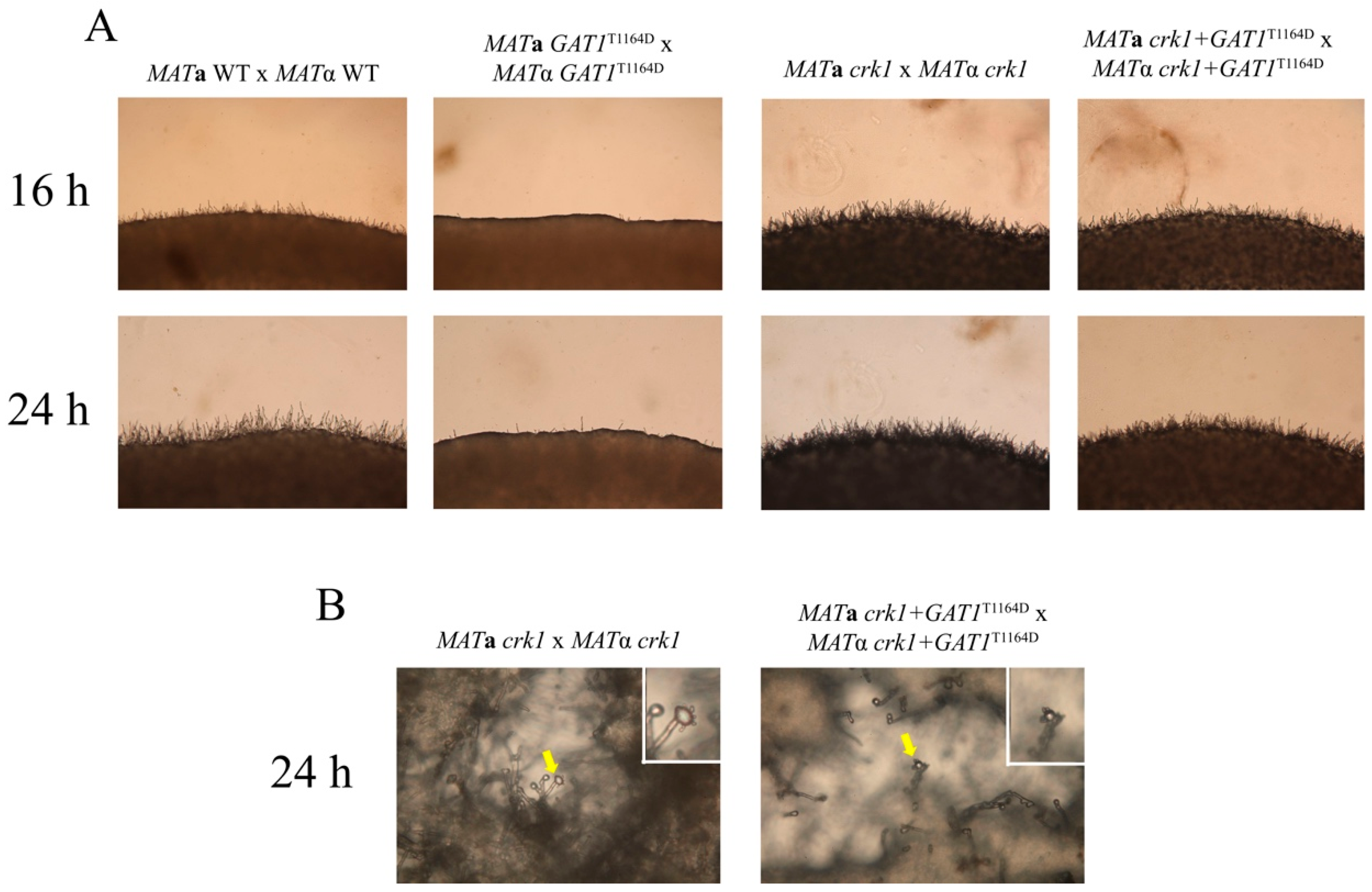
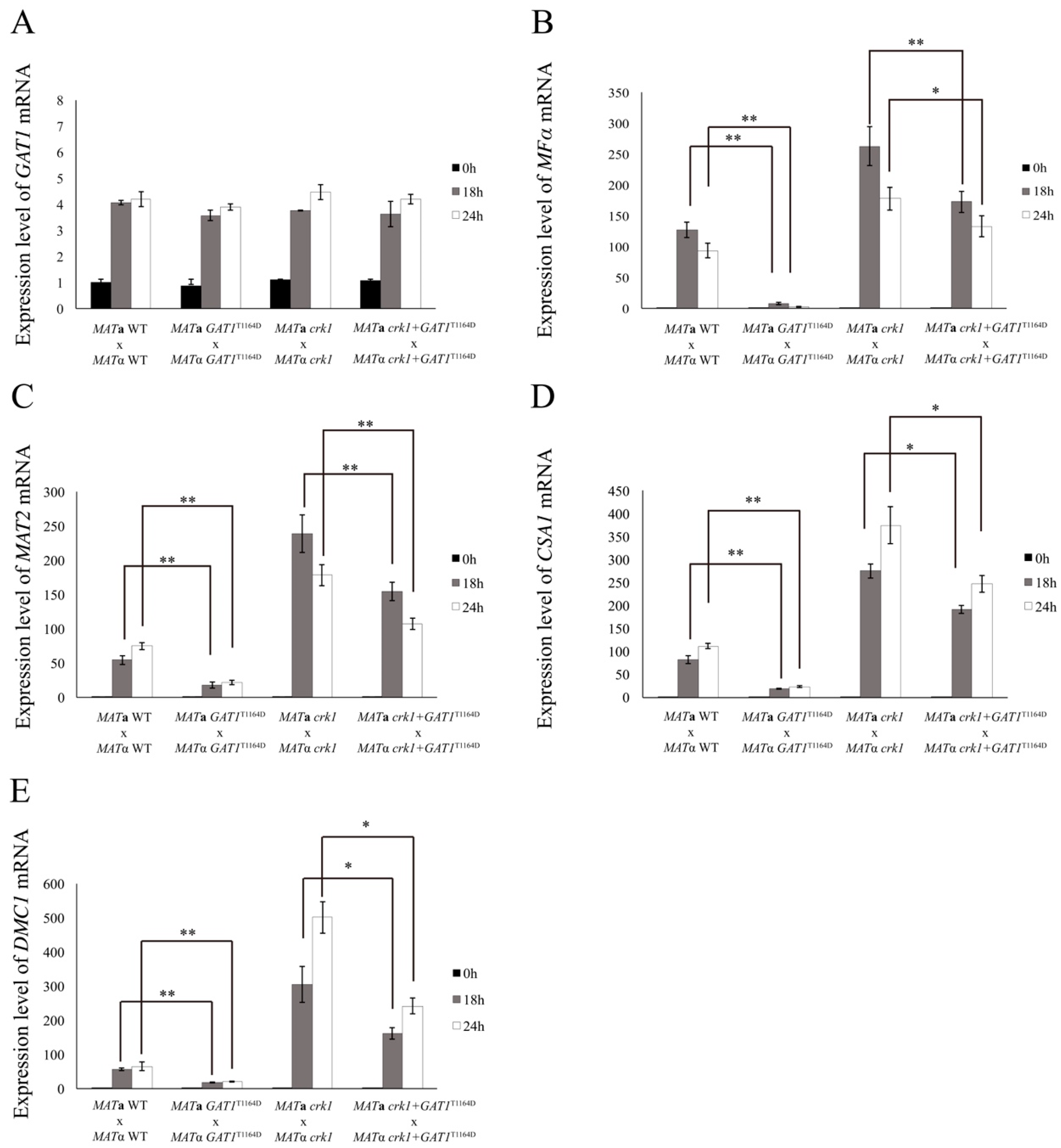
3.8. The Predicted IME2 Consensus Phosphorylation Site Was Important for the Function of Gat1 in Bisexual Mating
3.9. Deletion of GAT1 Partially Restored Dikaryotic Filamentation of CRK1 Overexpression Strain
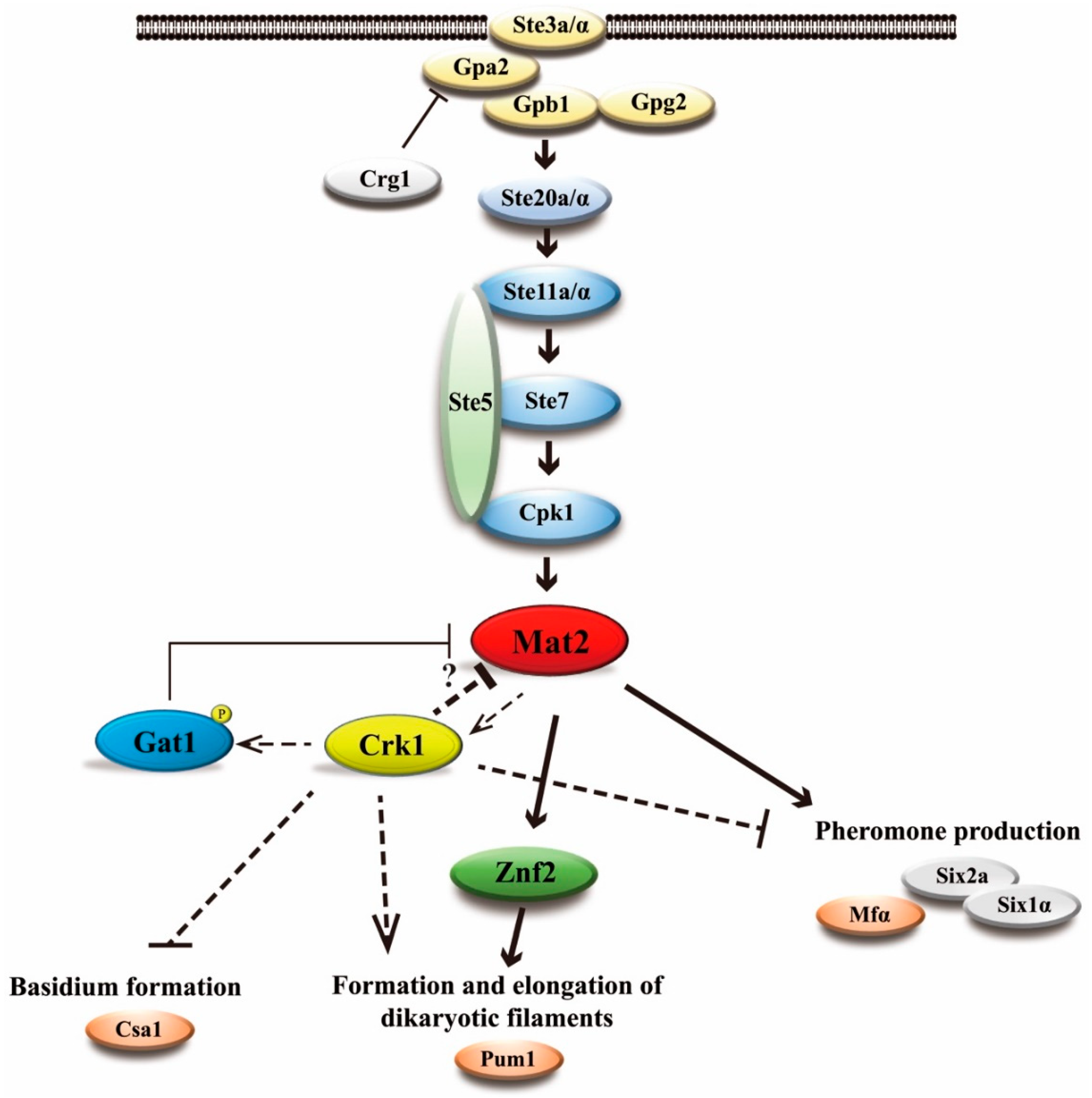
3.10. C. neoformans CRK1 Coordinated with GAT1 to Repress Bisexual Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hull, C.M.; Heitman, J. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 2002, 36, 557–615. [Google Scholar] [CrossRef] [PubMed]
- Idnurm, A.; Bahn, Y.S.; Nielsen, K.; Lin, X.; Fraser, J.A.; Heitman, J. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 2005, 3, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Bennett, J.E. High prevalence of Cryptococcus neoformans var. gattii in tropical and subtropical regions. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1984, 257, 213–218. [Google Scholar] [CrossRef]
- Kozubowski, L.; Heitman, J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol. Rev. 2012, 36, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, E.K.; Giles, S.S.; Hull, C.M. Analysis of Cryptococcus neoformans sexual development reveals rewiring of the pheromone-response network by a change in transcription factor identity. Genetics 2012, 191, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Wong, B.; Chang, Y.C.; Kwon-Chung, K.J.; Williamson, P.R. Cryptococcus neoformans: Virulence and host defences. Med. Mycol. 1998, 36 (Suppl. 1), 79–86. [Google Scholar] [PubMed]
- Zhao, Y.; Lin, J.; Fan, Y.; Lin, X. Life cycle of Cryptococcus neoformans. Annu. Rev. Microbiol. 2019, 73, 17–42. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Shen, W.C. A homolog of Ste6, the a-factor transporter in Saccharomyces cerevisiae, is required for mating but not for monokaryotic fruiting in Cryptococcus neoformans. Eukaryot. Cell 2005, 4, 147–155. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Xue, C.; Heitman, J. G protein signaling governing cell fate decisions involves opposing Gα subunits in Cryptococcus neoformans. Mol. Biol. Cell 2007, 18, 3237–3249. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.B.; Fraser, J.A.; Heitman, J. PAK kinases Ste20 and Pak1 govern cell polarity at different stages of mating in Cryptococcus neoformans. Mol. Biol. Cell 2004, 15, 4476–4489. [Google Scholar] [CrossRef]
- Shen, W.C.; Davidson, R.C.; Cox, G.M.; Heitman, J. Pheromones stimulate mating and differentiation via paracrine and autocrine signaling in Cryptococcus neoformans. Eukaryot. Cell 2002, 1, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Perfect, J.R.; Heitman, J. The G-protein beta subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 2000, 20, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.L.; Woodlee, G.L.; McClelland, C.M.; Seymour, T.S.; Wickes, B.L. The Cryptococcus neoformans STE11α gene is similar to other fungal mitogen-activated protein kinase kinase kinase (MAPKKK) genes but is mating type specific. Mol. Microbiol. 2001, 40, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.C.; Nichols, C.B.; Cox, G.M.; Perfect, J.R.; Heitman, J. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 2003, 49, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Feretzaki, M.; Heitman, J. Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLoS Genet. 2013, 9, e1003688. [Google Scholar] [CrossRef]
- Lin, X.; Jackson, J.C.; Feretzaki, M.; Xue, C.; Heitman, J. Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans. PLoS Genet. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Hull, C.M.; Boily, M.J.; Heitman, J. Sex-specific homeodomain proteins Sxi1α and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot. Cell 2005, 4, 526–535. [Google Scholar] [CrossRef]
- Ekena, J.L.; Stanton, B.C.; Schiebe-Owens, J.A.; Hull, C.M. Sexual development in Cryptococcus neoformans requires CLP1, a target of the homeodomain transcription factors Sxi1α and Sxi2a. Eukaryot. Cell 2008, 7, 49–57. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, B.; Lin, X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 2012, 8, e1002765. [Google Scholar] [CrossRef]
- Kaur, J.N.; Panepinto, J.C. Morphotype-specific effector functions of Cryptococcus neoformans PUM1. Sci. Rep. 2016, 6, 23638. [Google Scholar] [CrossRef]
- Wang, L.; Tian, X.; Gyawali, R.; Upadhyay, S.; Foyle, D.; Wang, G.; Cai, J.J.; Lin, X. Morphotype transition and sexual reproduction are genetically associated in a ubiquitous environmental pathogen. PLoS Pathog. 2014, 10, e1004185. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, Y.; Ferraro, A.R.; Yang, E.; Lewis, Z.A.; Lin, X. Transcription factor Znf2 coordinates with the chromatin remodeling SWI/SNF complex to regulate cryptococcal cellular differentiation. Commun. Biol. 2019, 2, 412. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Heitman, J. Function of Cryptococcus neoformans KAR7 (SEC66) in karyogamy during unisexual and opposite-sex mating. Eukaryot. Cell 2012, 11, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Heitman, J. PRM1 and KAR5 function in cell-cell fusion and karyogamy to drive distinct bisexual and unisexual cycles in the Cryptococcus pathogenic species complex. PLoS Genet. 2017, 13, e1007113. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, G.J.; Chen, L.; Zheng, J.; Chen, Y.; Shen, L.; Tian, X.; Li, E.; Yang, E.; Liao, G.; et al. Genetic basis for coordination of meiosis and sexual structure maturation in Cryptococcus neoformans. eLife 2018, 7, e38683. [Google Scholar] [CrossRef] [PubMed]
- Idnurm, A.; Heitman, J. Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol. 2005, 3, e95. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.K.; Sun, K.H.; Shen, W.C. Blue light negatively regulates the sexual filamentation via the Cwc1 and Cwc2 proteins in Cryptococcus neoformans. Mol. Microbiol. 2005, 56, 480–491. [Google Scholar] [CrossRef]
- Yeh, Y.L.; Lin, Y.S.; Su, B.J.; Shen, W.C. A screening for suppressor mutants reveals components involved in the blue light-inhibited sexual filamentation in Cryptococcus neoformans. Fungal Genet. Biol. 2009, 46, 42–54. [Google Scholar] [CrossRef]
- Liu, K.H.; Shen, W.C. Mating differentiation in Cryptococcus neoformans is negatively regulated by the Crk1 protein kinase. Fungal Genet. Biol. 2011, 48, 225–240. [Google Scholar] [CrossRef]
- Smith, H.E.; Mitchell, A.P. A transcriptional cascade governs entry into meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1989, 9, 2142–2152. [Google Scholar] [CrossRef]
- Honigberg, S.M.; Purnapatre, K. Signal pathway integration in the switch from the mitotic cell cycle to meiosis in yeast. J. Cell Sci. 2003, 116, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Kassir, Y.; Adir, N.; Boger-Nadjar, E.; Raviv, N.G.; Rubin-Bejerano, I.; Sagee, S.; Shenhar, G. Transcriptional regulation of meiosis in budding yeast. Int. Rev. Cytol. 2003, 224, 111–171. [Google Scholar] [PubMed]
- Clifford, D.M.; Marinco, S.M.; Brush, G.S. The meiosis-specific protein kinase Ime2 directs phosphorylation of replication protein A. J. Biol. Chem. 2004, 279, 6163–6170. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.M.; Stark, K.E.; Gardner, K.E.; Hoffmann-Benning, S.; Brush, G.S. Mechanistic insight into the Cdc28-related protein kinase Ime2 through analysis of replication protein A phosphorylation. Cell Cycle 2005, 4, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Holt, L.J.; Hutti, J.E.; Cantley, L.C.; Morgan, D.O. Evolution of Ime2 phosphorylation sites on Cdk1 substrates provides a mechanism to limit the effects of the phosphatase Cdc14 in meiosis. Mol. Cell 2007, 25, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J.; Hanson-Smith, V.; Kennedy, K.J.; Miller, C.J.; Lou, H.J.; Johnson, A.D.; Turk, B.E.; Holt, L.J. Ancestral resurrection reveals evolutionary mechanisms of kinase plasticity. eLife 2014, 3, e04126. [Google Scholar] [CrossRef]
- Moore, M.; Shin, M.E.; Bruning, A.; Schindler, K.; Vershon, A.; Winter, E. Arg-Pro-X-Ser/Thr is a consensus phosphoacceptor sequence for the meiosis-specific Ime2 protein kinase in Saccharomyces cerevisiae. Biochemistry 2007, 46, 271–278. [Google Scholar] [CrossRef]
- Sawarynski, K.E.; Kaplun, A.; Tzivion, G.; Brush, G.S. Distinct activities of the related protein kinases Cdk1 and Ime2. Biochim. Biophys. Acta 2007, 1773, 450–456. [Google Scholar] [CrossRef][Green Version]
- Sedgwick, C.; Rawluk, M.; Decesare, J.; Raithatha, S.; Wohlschlegel, J.; Semchuk, P.; Ellison, M.; Yates, J., 3rd; Stuart, D. Saccharomyces cerevisiae Ime2 phosphorylates Sic1 at multiple PXS/T sites but is insufficient to trigger Sic1 degradation. Biochem. J. 2006, 399, 151–160. [Google Scholar] [CrossRef][Green Version]
- Shin, M.E.; Skokotas, A.; Winter, E. The Cdk1 and Ime2 protein kinases trigger exit from meiotic prophase in Saccharomyces cerevisiae by inhibiting the Sum1 transcriptional repressor. Mol. Cell. Biol. 2010, 30, 2996–3003. [Google Scholar] [CrossRef][Green Version]
- Sopko, R.; Raithatha, S.; Stuart, D. Phosphorylation and maximal activity of Saccharomyces cerevisiae meiosis-specific transcription factor Ndt80 is dependent on Ime2. Mol. Cell. Biol. 2002, 22, 7024–7040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hutchison, E.A.; Glass, N.L. Meiotic regulators Ndt80 and ime2 have different roles in Saccharomyces and Neurospora. Genetics 2010, 185, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, E.A.; Bueche, J.A.; Glass, N.L. Diversification of a protein kinase cascade: IME-2 is involved in nonself recognition and programmed cell death in Neurospora crassa. Genetics 2012, 192, 467–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bielska, E.; Higuchi, Y.; Schuster, M.; Steinberg, N.; Kilaru, S.; Talbot, N.J.; Steinberg, G. Long-distance endosome trafficking drives fungal effector production during plant infection. Nat. Commun. 2014, 5, 5097. [Google Scholar] [CrossRef]
- Garrido, E.; Pérez-Martín, J. The crk1 gene encodes an Ime2-related protein that is required for morphogenesis in the plant pathogen Ustilago maydis. Mol. Microbiol. 2003, 47, 729–743. [Google Scholar] [CrossRef]
- Garrido, E.; Voß, U.; Müller, P.; Castillo-Lluva, S.; Kahmann, R.; Pérez-Martín, J. The induction of sexual development and virulence in the smut fungus Ustilago maydis depends on Crk1, a novel MAPK protein. Genes Dev. 2004, 18, 3117–3130. [Google Scholar] [CrossRef][Green Version]
- Higuchi, Y. Initial fungal effector production is mediated by early endosome motility. Commun. Integr. Biol. 2015, 8, e1025187. [Google Scholar] [CrossRef][Green Version]
- Sartorel, E.; Pérez-Martín, J. The distinct interaction between cell cycle regulation and the widely conserved morphogenesis-related (MOR) pathway in the fungus Ustilago maydis determines morphology. J. Cell Sci. 2012, 125, 4597–4608. [Google Scholar] [CrossRef]
- Alspaugh, J.A.; Perfect, J.R.; Heitman, J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 1997, 11, 3206–3217. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Edman, J.C.; Wickes, B.L. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 1992, 60, 602–605. [Google Scholar] [CrossRef]
- Moore, T.D.; Edman, J.C. The alpha-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol. Cell. Biol. 1993, 13, 1962–1970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fraser, J.A.; Subaran, R.L.; Nichols, C.B.; Heitman, J. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: Implications for an outbreak on Vancouver Island, Canada. Eukaryot. Cell 2003, 2, 1036–1045. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, S.Y.; Jung, K.W.; Bahn, Y.S. Targeted gene disruption in Cryptococcus neoformans using double-joint PCR with split dominant selectable markers. Methods Mol. Biol. 2012, 845, 67–84. [Google Scholar] [PubMed]
- Liu, K.H.; Yeh, Y.L.; Shen, W.C. Fast preparation of fungal DNA for PCR screening. J. Microbiol. Methods 2011, 85, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Won, M.; Hoe, K.L.; Cho, Y.S.; Song, K.B.; Yoo, H.S. DNA-induced conformational change of Gaf1, a novel GATA factor in Schizosaccharomyces pombe. Biochem. Cell Biol. 1999, 77, 127–132. [Google Scholar] [CrossRef]
- Kim, L.; Hoe, K.L.; Yu, Y.M.; Yeon, J.H.; Maeng, P.J. The fission yeast GATA factor, Gaf1, modulates sexual development via direct down-regulation of ste11+ expression in response to nitrogen starvation. PLoS ONE 2012, 7, e42409. [Google Scholar] [CrossRef]
- Kmetzsch, L.; Staats, C.C.; Simon, E.; Fonseca, F.L.; Oliveira, D.L.; Joffe, L.S.; Rodrigues, J.; Lourenco, R.F.; Gomes, S.L.; Nimrichter, L.; et al. The GATA-type transcriptional activator Gat1 regulates nitrogen uptake and metabolism in the human pathogen Cryptococcus neoformans. Fungal Genet. Biol. 2011, 48, 192–199. [Google Scholar] [CrossRef]
- Lee, I.R.; Chow, E.W.; Morrow, C.A.; Djordjevic, J.T.; Fraser, J.A. Nitrogen metabolite repression of metabolism and virulence in the human fungal pathogen Cryptococcus neoformans. Genetics 2011, 188, 309–323. [Google Scholar] [CrossRef]
- Jung, K.W.; Yang, D.H.; Maeng, S.; Lee, K.T.; So, Y.S.; Hong, J.; Choi, J.; Byun, H.J.; Kim, H.; Bang, S.; et al. Systematic functional profiling of transcription factor networks in Cryptococcus neoformans. Nat. Commun. 2015, 6, 6757. [Google Scholar] [CrossRef]
- Bayram, O.; Sari, F.; Braus, G.H.; Irniger, S. The protein kinase ImeB is required for light-mediated inhibition of sexual development and for mycotoxin production in Aspergillus nidulans. Mol. Microbiol. 2009, 71, 1278–1295. [Google Scholar] [CrossRef]
- Chen, F.; Chen, X.Z.; Su, X.Y.; Qin, L.N.; Huang, Z.B.; Tao, Y.; Dong, Z.Y. An Ime2-like mitogen-activated protein kinase is involved in cellulase expression in the filamentous fungus Trichoderma reesei. Biotechnol. Lett. 2015, 37, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.E.; Cooper, S. Extreme Diversity in the Regulation of Ndt80-Like Transcription Factors in Fungi. G3 Genes Genomes Genet. 2015, 5, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.W.; Kruzel, E.K.; Shimizu, K.; Hull, C.M. Characterizing the role of the microtubule binding protein Bim1 in Cryptococcus neoformans. Fungal Genet. Biol. 2010, 47, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.B.; Wittenberg, C. Topology and control of the cell-cycle-regulated transcriptional circuitry. Genetics 2014, 196, 65–90. [Google Scholar] [CrossRef]
- Tobe, B.T.; Kitazono, A.A.; Garcia, J.S.; Gerber, R.A.; Bevis, B.J.; Choy, J.S.; Chasman, D.; Kron, S.J. Morphogenesis signaling components influence cell cycle regulation by cyclin dependent kinase. Cell Div. 2009, 4, 12. [Google Scholar] [CrossRef]
- Novak, B.; Csikasz-Nagy, A.; Gyorffy, B.; Chen, K.; Tyson, J.J. Mathematical model of the fission yeast cell cycle with checkpoint controls at the G1/S, G2/M and metaphase/anaphase transitions. Biophys. Chem. 1998, 72, 185–200. [Google Scholar] [CrossRef]
- Kellogg, D.R. Wee1-dependent mechanisms required for coordination of cell growth and cell division. J. Cell Sci. 2003, 116, 4883–4890. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, J.J.; Ying, S.H.; Feng, M.G. Wee1 and Cdc25 control morphogenesis, virulence and multistress tolerance of Beauveria bassiana by balancing cell cycle-required cyclin-dependent kinase 1 activity. Environ. Microbiol. 2015, 17, 1119–1133. [Google Scholar] [CrossRef]
- Sgarlata, C.; Pérez-Martín, J. Inhibitory phosphorylation of a mitotic cyclin-dependent kinase regulates the morphogenesis, cell size and virulence of the smut fungus Ustilago maydis. J. Cell Sci. 2005, 118, 3607–3622. [Google Scholar] [CrossRef]
- Bueno, A.; Richardson, H.; Reed, S.I.; Russell, P. A fission yeast B-type cyclin functioning early in the cell cycle. Cell 1991, 66, 149–159. [Google Scholar] [CrossRef]
- Sveiczer, A.; Csikasz-Nagy, A.; Gyorffy, B.; Tyson, J.J.; Novak, B. Modeling the fission yeast cell cycle: Quantized cycle times in wee1-cdc25∆ mutant cells. Proc. Natl. Acad. Sci. USA 2000, 97, 7865–7870. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Muse, T.; Steinberg, G.; Pérez-Martín, J. Characterization of B-type cyclins in the smut fungus Ustilago maydis: Roles in morphogenesis and pathogenicity. J. Cell Sci. 2004, 117, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Guttmann-Raviv, N.; Martin, S.; Kassir, Y. Ime2, a meiosis-specific kinase in yeast, is required for destabilization of its transcriptional activator, Ime1. Mol. Cell. Biol. 2002, 22, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.P.; Driscoll, S.E.; Smith, H.E. Positive control of sporulation-specific genes by the IME1 and IME2 products in Saccharomyces cerevisiae. Mol. Cell. Biol. 1990, 10, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.E.; Su, S.S.; Neigeborn, L.; Driscoll, S.E.; Mitchell, A.P. Role of IME1 expression in regulation of meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1990, 10, 6103–6113. [Google Scholar] [CrossRef] [PubMed]
- Hoe, K.L.; Won, M.S.; Chung, K.S.; Park, S.K.; Kim, D.U.; Jang, Y.J.; Yoo, O.J.; Yoo, H.S. Molecular cloning of gaf1, a Schizosaccharomyces pombe GATA factor, which can function as a transcriptional activator. Gene 1998, 215, 319–328. [Google Scholar] [CrossRef]
- Hwang, L.H.; Seth, E.; Gilmore, S.A.; Sil, A. SRE1 regulates iron-dependent and –independent pathways in the fungal pathogen Histoplasma capsulatum. Eukaryot. Cell 2012, 11, 16–25. [Google Scholar] [CrossRef]
| Strains | Description | Reference |
|---|---|---|
| JEC20 | MATa | [50] |
| JEC21 | MATα | [50] |
| JEC43 | MATα ura5 | [51] |
| DLC2 | MATα crk1::NATR | [29] |
| DLC4 | MATacrk1::NATR | [29] |
| DLC18 | MATacrk1 + PGPD1::GFP-H2B ura5 | [29] |
| DLC21 | MATa PGPD1::GFP-H2B ura5 | [29] |
| DLC22 | MATαcrk1 + PGPD1::CRK1 | This study |
| DLC23 | MATamat2::HYGR | This study |
| DLC24 | MATα mat2::HYGR | This study |
| DLC25 | MATamat2::HYGRcrk1::NATR | This study |
| DLC26 | MATα mat2::HYGR crk1::NATR | This study |
| DLC27 | MATα PGPD1::MAT2 | This study |
| DLC28 | MATaznf2::HYGR | This study |
| DLC29 | MATα znf2::HYGR | This study |
| DLC30 | MATaznf2::HYGRcrk1::NATR | This study |
| DLC31 | MATα znf2::HYGR crk1::NATR | This study |
| DLC32 | MATagat1::HYGR | This study |
| DLC33 | MATα gat1::HYGR | This study |
| DLC34 | MATagat1 + GAT1::NATR | This study |
| DLC35 | MATα gat1 + GAT1::NATR | This study |
| DLC36 | MATα PGPD1::GAT1 | This study |
| DLC37 | MATα crk1 + PGPD1::CRK1 | This study |
| DLC38 | MATα crk1 + PGPD1::CRK1 + PGPD1::GAT1 | This study |
| DLC39 | MATaGAT1T1164A::HYGR | This study |
| DLC40 | MATα GAT1T1164A::HYGR | This study |
| DLC41 | MATacrk1::NATRgat1::HYGR | This study |
| DLC42 | MATα crk1::NATR gat1::HYGR | This study |
| DLC43 | MATa PGPD1::GAT1 | This study |
| DLC44 | MATaGAT1T1164D::HYGR | This study |
| DLC45 | MATα GAT1T1164D::HYGR | This study |
| DLC46 | MATacrk1 + GAT1T1164D::HYGR | This study |
| DLC47 | MATα crk1 + GAT1T1164D::HYGR | This study |
| DLC48 | MATa PGPD1::CRK1 gat1::HYGR | This study |
| DLC49 | MATα PGPD1::CRK1 gat1::HYGR | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.-H.; Shen, W.-C. Sexual Differentiation Is Coordinately Regulated by Cryptococcus neoformans CRK1 and GAT1. Genes 2020, 11, 669. https://doi.org/10.3390/genes11060669
Liu K-H, Shen W-C. Sexual Differentiation Is Coordinately Regulated by Cryptococcus neoformans CRK1 and GAT1. Genes. 2020; 11(6):669. https://doi.org/10.3390/genes11060669
Chicago/Turabian StyleLiu, Kuang-Hung, and Wei-Chiang Shen. 2020. "Sexual Differentiation Is Coordinately Regulated by Cryptococcus neoformans CRK1 and GAT1" Genes 11, no. 6: 669. https://doi.org/10.3390/genes11060669
APA StyleLiu, K.-H., & Shen, W.-C. (2020). Sexual Differentiation Is Coordinately Regulated by Cryptococcus neoformans CRK1 and GAT1. Genes, 11(6), 669. https://doi.org/10.3390/genes11060669





