Genome-Wide Association Study Confirms Previous Findings of Major Loci Affecting Resistance to Piscine myocarditis virus in Atlantic Salmon (Salmo salar L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Infection Challenge Tests in the SalmoBreed and StofnFiskur Populations
2.3. Genotyping, Estimation of Genetic Parameters and Genome-Wide Association Study
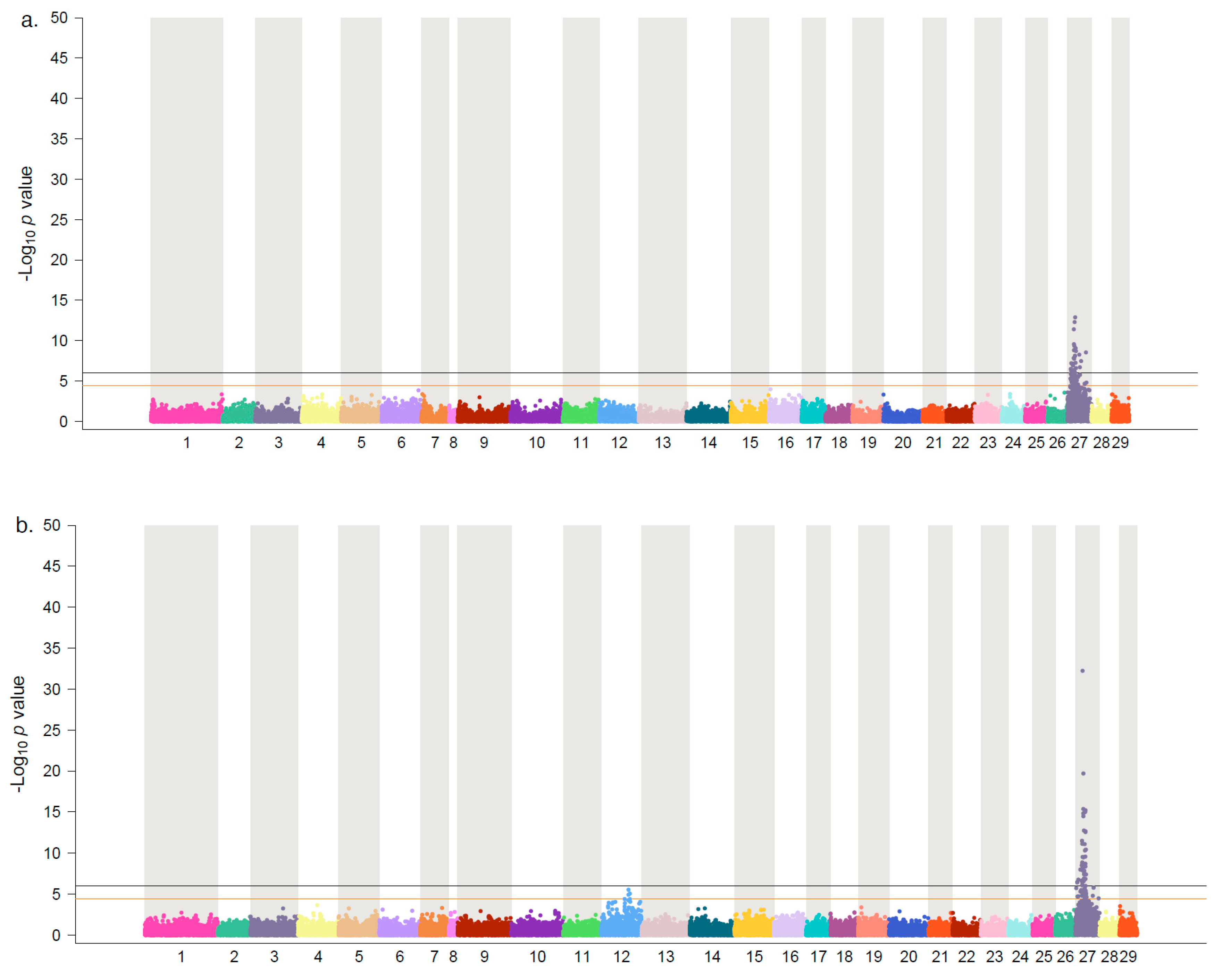
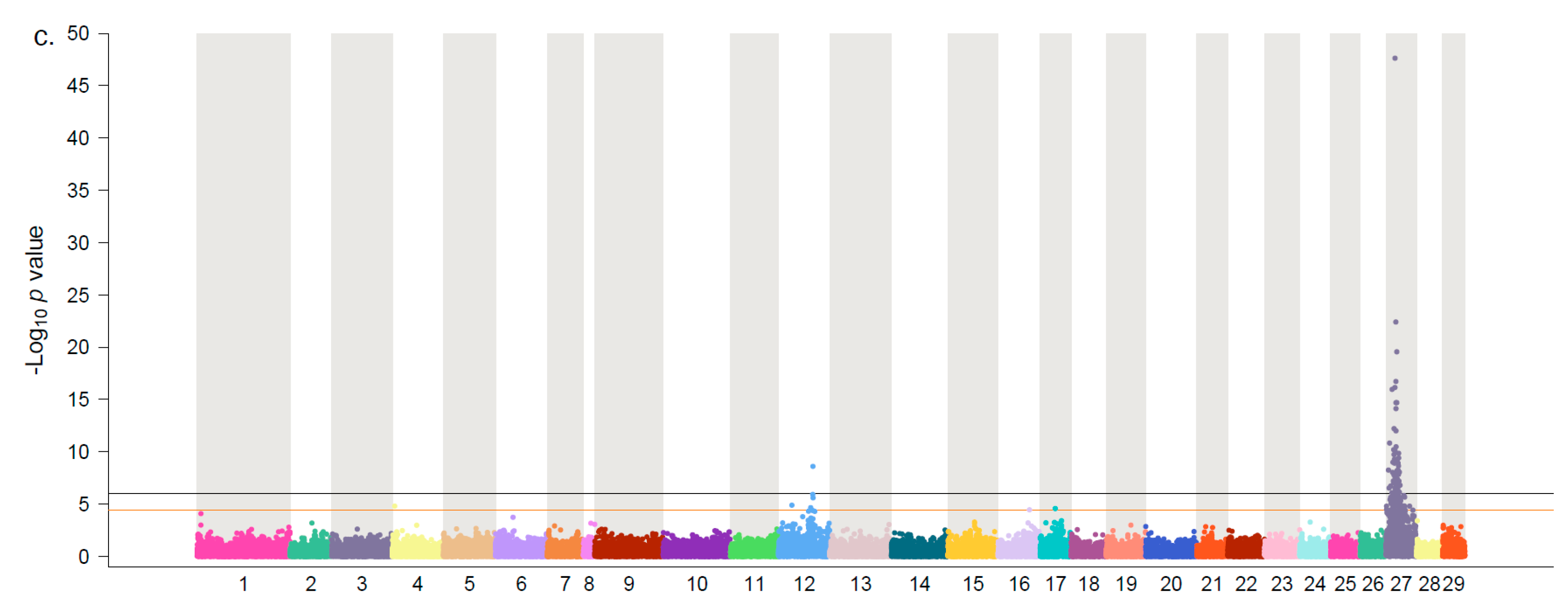
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brun, E.; Poppe, T.; Skrudland, A. Cardiomyopathy syndrome in farmed Atlantic salmon Salmo salar: Occurrence and direct financial losses for Norwegian aquaculture. Dis. Aquat. Organ. 2003, 56, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Garseth, Å.H.; Biering, E.; Tengs, T. Piscine myocarditis virus (PMCV) in wild Atlantic salmon Salmo salar. Dis. Aquat. Organ. 2012, 102, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Garseth, H.; Fritsvold, C.; Svendsen, J.C.; Bang Jensen, B.; Mikalsen, A.B. Cardiomyopathy syndrome in Atlantic salmon Salmo salar L.: A review of the current state of knowledge. J. Fish Dis. 2018, 41, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Løvoll, M.; Wiik-Nielsen, J.; Grove, S.; Wiik-Nielsen, C.R.; Kristoffersen, A.B.; Faller, R.; Poppe, T.; Jung, J.; Pedamallu, C.S.; Nederbragt, A.J.; et al. A novel totivirus and piscine reovirus (PRV) in Atlantic salmon (Salmo salar) with cardiomyopathy syndrome (CMS). Virol. J. 2010, 7, 309. [Google Scholar] [CrossRef] [PubMed]
- Haugland, O.; Mikalsen, A.B.; Nilsen, P.; Lindmo, K.; Thu, B.J.; Eliassen, T.M.; Roos, N.; Rode, M.; Evensen, O. Cardiomyopathy syndrome of Atlantic salmon (Salmo salar L.) is caused by a double-stranded RNA virus of the Totiviridae family. J. Virol. 2011, 85, 5275–5286. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Trasti, J. Endomyocarditis in Atlantic salmon in Norwegian seafarms; a case report. Bull. Eur. Assoc. Fish Pathol. 1988, 8, 70–71. [Google Scholar]
- Fritsvold, C.; Jensen, B.B. The Health Situation in Norwegian Aquaculture; Hjeltnes, B., Bang Jensen, B., Bornø, G., Haukaas, A., Walde, C.S., Eds.; Norwegian Veterinary Institute: Oslo, Norway, 2019. [Google Scholar]
- Svendsen, J.C.; Fritsvold, C. Experimental transmission of cardiomyopathy syndrome (CMS) in Atlantic salmon Salmo salar. Dis. Aquat. Organ. 2009, 87, 225–234. [Google Scholar]
- Norwegian Seafood Research Fund (FHF). Cardiomyopathy Syndrome: A Multi-Task Approach to Reduce Losses and Improve Knowledge. 2011. Available online: https://www.fhf.no/prosjekter/prosjektbasen/900261/ (accessed on 6 May 2020).
- Timmerhaus, G.; Krasnov, A.; Takle, H.; Afanasyev, S.; Nilsen, P.; Rode, M.; Jørgensen, S.M. Comparison of Atlantic salmon individuals with different outcomes of cardiomyopathy syndrome (CMS). BMC Genom. 2012, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Timmerhaus, G.; Krasnov, A.; Nilsen, P.; Alarcon, M.; Afanasyev, S.; Rode, M.; Takle, H.; Jørgensen, S.M. Transcriptome profiling of immune responses to cardiomyopathy syndrome (CMS) in Atlantic salmon. BMC Genom. 2011, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Hillestad, B.; Moghadam, H.K. Genome-wide association study of Piscine myocarditis virus (PMCV) resistance in Atlantic salmon (Salmo salar). J. Hered. 2019, 110, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Boison, S.; Ding, J.; Leder, E.; Gjerde, B.; Bergtun, P.H.; Norris, A.; Baranski, M.; Robinson, N. QTLs associated with resistance to cardiomyopathy syndrome in Atlantic salmon. J. Hered. 2019, 110, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Spets, M.H.; Eriksen, L.B.; Ólafsdóttir, G. The Genetic Background of Stofnfiskur Breeding Lines of Farmed Atlantic Salmon. Norsk Institutt for Naturforskning (NINA) report 4101. 2017. Available online: https://brage.nina.no/nina-xmlui/handle/11250/2459641 (accessed on 6 May 2020).
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Houston, R.D.; Taggart, J.B.; Cézard, T.; Bekaert, M.; Lowe, N.R.; Downing, A.; Talbot, R.; Bishop, S.C.; Archibald, A.L.; Bron, J.E.; et al. Development and validation of a high density SNP genotyping array for Atlantic salmon (Salmo salar). BMC Genom. 2014, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Duggal, P.; Gillanders, E.M.; Holmes, T.N.; Bailey-Wilson, J.E. Establishing an adjusted p-value threshold to control the family-wide type 1 error in genome wide association studies. BMC Genom. 2008, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Sham, P.C.; Purcell, S.M. Statistical power and significance testing in large-scale genetic studies. Nat. Rev. Genet. 2014, 15, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, Y.T.; do Carmo, A.S.; Carvalheiro, R.; Neves, H.H.R.; Matos, M.C.; Zavarez, L.B.; Pérez O’Brien, A.M.; Sölkner, J.; McEwan, J.C.; Cole, J.B.; et al. Genome-wide association study for birth weight in Nellore cattle points to previously described orthologous genes affecting human and bovine height. BMC Genet. 2013, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Grimholt, U.; Larsen, S.; Nordmo, R.; Midtlyng, P.; Kjoeglum, S.; Storset, A.; Saebø, S.; Stet, R.J.M. MHC polymorphism and disease resistance in Atlantic salmon (Salmo salar); facing pathogens with single expressed major histocompatibility class I and class II loci. Immunogenetics 2003, 55, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Dionne, M.; Miller, K.M.; Dodson, J.J.; Bernatchez, L. MHC standing genetic variation and pathogen resistance in wild Atlantic salmon. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Nabar, N.R.; Kehrl, J.H. The transcription factor EB links cellular stress to the immune response. Yale J. Biol. Med. 2017, 90, 301–315. [Google Scholar] [PubMed]


| Parameters | SF-17 | SB-18 |
|---|---|---|
| (se) | 3.11 (0.63) | 3.62 (0.80) |
| (se) | 1.50 (1.13) | 4.28 (1.75) |
| (se) | 3.29 (0.54) | 6.98 (0.98) |
| (se) | 7.91 (0.65) | 6.31 (0.94) |
| (se) | 0.39 (0.07) | 0.57 (0.12) |
| Population | chr | Position | p-Value | maf | VP | Vg |
|---|---|---|---|---|---|---|
| SB-17 | 27 | 10,393,267 | 1.63 × 10−14 | 0.32 | 7.69 | 15.62 |
| SB-17 | 27 | 10,348,407 | 4.51 × 10−15 | 0.48 | 7.71 | 15.68 |
| SB-18 | 27 | 10,052,153 | 2.18 × 10−13 | 0.17 | 7.97 | 13.55 |
| SB-18 | 27 | 10,348,407 | 5.64 × 10−13 | 0.50 | 8.30 | 14.11 |
| SF-17 | 27 | 10,052,153 | 1.51 × 10−35 | 0.39 | 15.70 | 38.80 |
| SF-17 | 27 | 10,413,738 | 3.65 × 10−21 | 0.37 | 9.17 | 22.65 |
| Meta-analysis | 27 | 10,052,153 | 2.06 × 10−48 | 0.27 | 9.57 | 21.91 |
| Meta-analysis | 27 | 10,348,407 | 3.91 × 10−23 | 0.49 | 4.49 | 10.29 |
| SB-17 | 12 | 61,702,164 | 9.65 × 10−07 | 0.12 | 3.03 | 6.16 |
| SB-17 | 12 | 61,701,806 | 5.74 × 10−06 | 0.12 | 2.64 | 5.36 |
| SF-17 | 12 | 61,626,398 | 1.38 × 10−06 | 0.22 | 2.71 | 6.70 |
| SF-17 | 12 | 61,694,062 | 2.97 × 10−06 | 0.39 | 2.87 | 7.09 |
| Meta-analysis | 12 | 61,626,398 | 2.47 × 10−09 | 0.41 | 2.34 | 5.36 |
| Meta-analysis | 12 | 61,443,087 | 1.20 × 10−06 | 0.20 | 1.17 | 2.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillestad, B.; H. Kristjánsson, Ó.; Makvandi-Nejad, S.; Moghadam, H.K. Genome-Wide Association Study Confirms Previous Findings of Major Loci Affecting Resistance to Piscine myocarditis virus in Atlantic Salmon (Salmo salar L.). Genes 2020, 11, 608. https://doi.org/10.3390/genes11060608
Hillestad B, H. Kristjánsson Ó, Makvandi-Nejad S, Moghadam HK. Genome-Wide Association Study Confirms Previous Findings of Major Loci Affecting Resistance to Piscine myocarditis virus in Atlantic Salmon (Salmo salar L.). Genes. 2020; 11(6):608. https://doi.org/10.3390/genes11060608
Chicago/Turabian StyleHillestad, Borghild, Ólafur H. Kristjánsson, Shokouh Makvandi-Nejad, and Hooman K. Moghadam. 2020. "Genome-Wide Association Study Confirms Previous Findings of Major Loci Affecting Resistance to Piscine myocarditis virus in Atlantic Salmon (Salmo salar L.)" Genes 11, no. 6: 608. https://doi.org/10.3390/genes11060608
APA StyleHillestad, B., H. Kristjánsson, Ó., Makvandi-Nejad, S., & Moghadam, H. K. (2020). Genome-Wide Association Study Confirms Previous Findings of Major Loci Affecting Resistance to Piscine myocarditis virus in Atlantic Salmon (Salmo salar L.). Genes, 11(6), 608. https://doi.org/10.3390/genes11060608





