Identification of Bradyrhizobium elkanii USDA61 Type III Effectors Determining Symbiosis with Vigna mungo
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbiological and Molecular Techniques
2.2. Plant Assays
2.3. GUS Assay and Microscopic Analysis
2.4. Bioinformatics and Statistical Analyses
3. Results
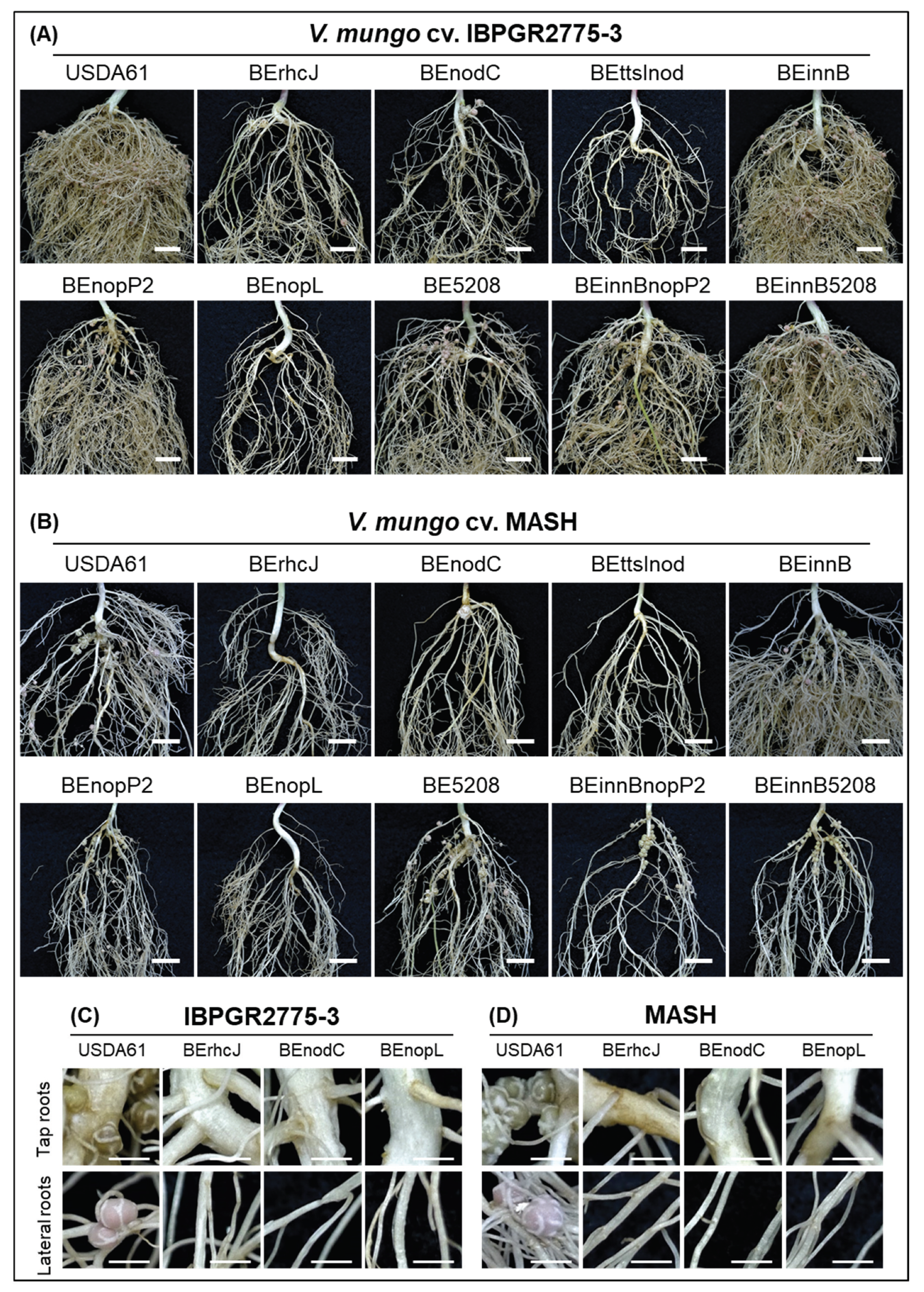
3.1. T3SS of B. elkanii USDA61 Played Pivotal Roles in Host-Specific Symbioses with Different Vigna Species and V. mungo Cultivars
3.2. Identification of B. elkanii USDA61 T3Es Involved in Determining Symbiotic Efficiency on V. mungo
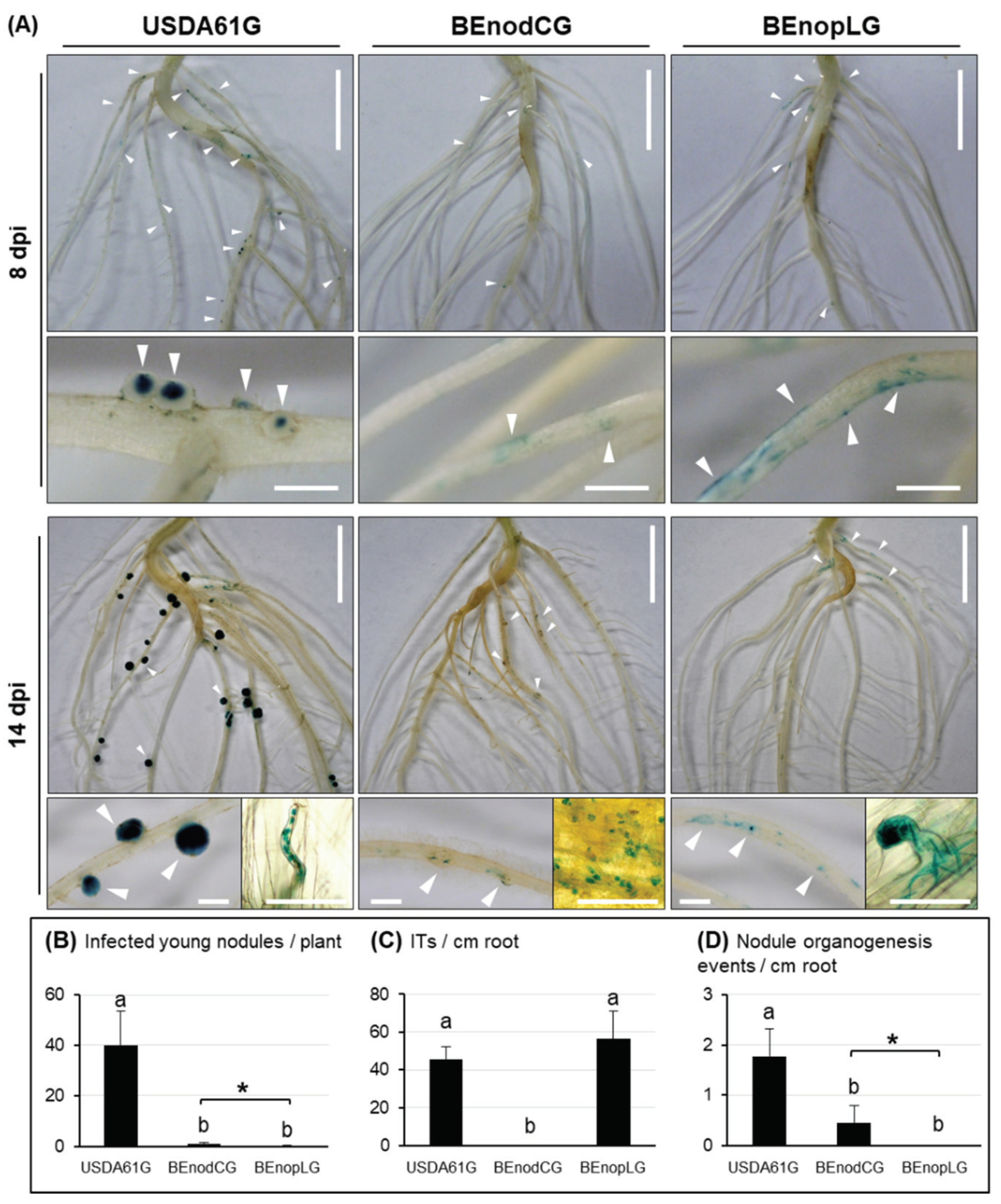
3.3. NopL of B. elkanii USDA61 is a Key Determinant for T3SS-Dependent Nodulation in South Asiatic V. mungo Cultivars
3.4. B. elkanii USDA61 NopL is Required for Early Nodule Development
3.5. Structural Features of B. elkanii USDA61 NopL
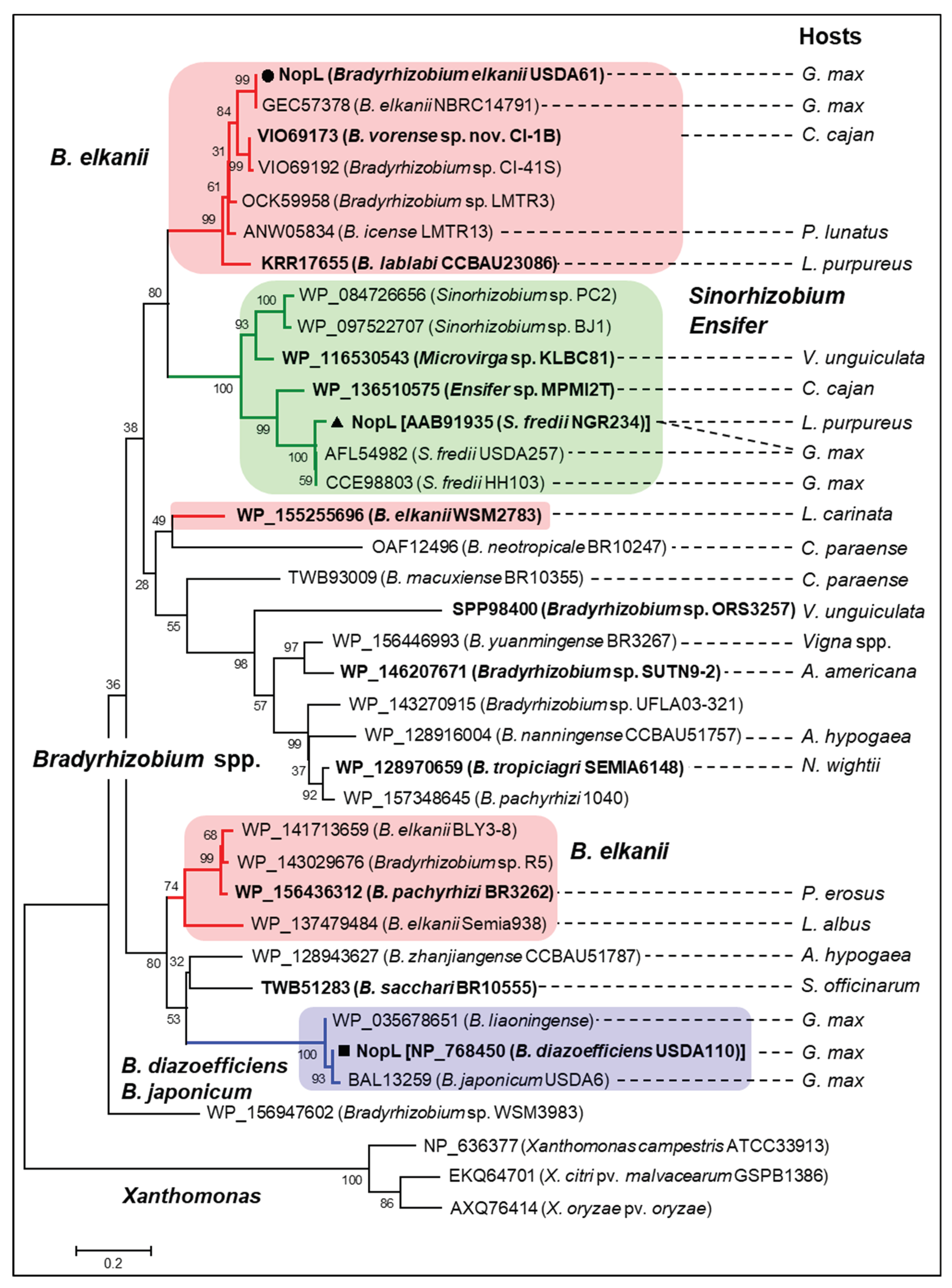
3.6. Phylogenetic Analyses of Rhizobial NopLs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deakin, W.J.; Broughton, W.J. Symbiotic use of pathogenic strategies: Rhizobial protein secretion systems. Nat. Rev. Microbiol. 2009, 7, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Kimbrel, J.A.; Thomas, W.J.; Jiang, Y.; Creason, A.L.; Thireault, C.A.; Sachs, J.L.; Chang, J.H. Mutualistic co-evolution of type III effector genes in Sinorhizobium fredii and Bradyrhizobium japonicum. PLoS Pathog. 2013, 9, e1003204. [Google Scholar] [CrossRef] [PubMed]
- Angus, A.A.; Agapakis, C.M.; Fong, S.; Yerrapragada, S.; Estrada-de los Santos, P.; Yang, P.; Song, N.; Kano, S.; Caballero-Mellado, J.; de Faria, S.M.; et al. Plant-associated symbiotic Burkholderia species lack hallmark strategies required in mammalian pathogenesis. PLoS ONE 2014, 9, e83779. [Google Scholar] [CrossRef] [PubMed]
- Viprey, V.; Del Greco, A.; Golinowski, W.; Broughton, W.J.; Perret, X. Symbiotic implications of type III protein secretion machinery in Rhizobium. Mol. Microbiol. 1998, 28, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Tampakaki, A.P. Commonalities and differences of T3SSs in rhizobia and plant pathogenic bacteria. Front. Plant Sci. 2014, 5, 1–19. [Google Scholar] [CrossRef]
- Nelson, M.S.; Sadowsky, M.J. Secretion systems and signal exchange between nitrogen-fixing rhizobia and legumes. Front. Plant Sci. 2015, 6, 491. [Google Scholar] [CrossRef]
- Staehelin, C.; Krishnan, H.B. Nodulation outer proteins: Double-edged swords of symbiotic rhizobia. Biochem. J. 2015, 470, 263–274. [Google Scholar] [CrossRef]
- Keyser, H.H.; van Berkum, P.; Weber, D.F. A comparative study of the physiology of symbioses formed by Rhizobium japonicum with Glycine max, Vigna unguiculata, and Macroptilium atropurpurem. Plant Physiol. 1982, 70, 1626–1630. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Ratu, S.T.N.; Yasuda, M.; Göttfert, M.; Okazaki, S. InnB, a novel Type III effector of Bradyrhizobium elkanii USDA61, controls symbiosis with Vigna species. Front. Microbiol. 2018, 9, 3155. [Google Scholar] [CrossRef]
- Miwa, H.; Okazaki, S. How effectors promote beneficial interactions. Curr. Opin. Plant Biol. 2017, 38, 148–154. [Google Scholar] [CrossRef]
- Okazaki, S.; Zehner, S.; Hempel, J.; Lang, K.; Göttfert, M. Genetic organization and functional analysis of the type III secretion system of Bradyrhizobium elkanii. FEMS Microbiol. Lett. 2009, 295, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Deakin, W.J.; Marie, C.; Saad, M.M.; Krishnan, H.B.; Broughton, W.J. NopA is associated with cell surface appendages produced by the type III secretion system of Rhizobium sp. strain NGR234. Mol. Plant-Microbe Interact. 2005, 18, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Medina, C.; Ollero, F.J.; López-Baena, F.J. NopC is a Rhizobium-specific Type 3 secretion system effector secreted by Sinorhizobium (Ensifer) fredii HH103. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, M.J.; Tully, R.E.; Cregan, P.B.; Keyser, H.H. Genetic diversity in Bradyrhizobium japonicum serogroup 123 and its relation to genotype-specific nodulation of soybean. Appl. Environ. Microbiol. 1987, 53, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Miwa, H.; Kaneko, T.; Sato, S.; Okazaki, S. Identification of Bradyrhizobium elkanii genes involved in incompatibility with Vigna radiata. Genes (Basel) 2017, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Sakanaka, M.; Fukuma, H.; Murayama, H.; Kano, Y.; Fukiya, S.; Yokota, A. Development of a double-crossover markerless gene deletion system in Bifidobacterium longum: Functional analysis of the α-galactosidase gene for raffinose assimilation. Appl. Environ. Microbiol. 2012, 78, 4984–4994. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.; Tauch, A.; Jager, W.; Kalinowski, J.; Thierbach, G.; Puhler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Krause, A.; Doerfel, A.; Göttfert, M. Mutational and transcriptional analysis of the type III secretion system of Bradyrhizobium japonicum. Mol. Plant-Microbe Interact. 2002, 15, 1228–1235. [Google Scholar] [CrossRef]
- Okazaki, S.; Kaneko, T.; Sato, S.; Saeki, K. Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system. Proc. Natl. Acad. Sci. USA 2013, 110, 17131–17136. [Google Scholar] [CrossRef]
- Broughton, W.J.; Dilworth, M.J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef]
- Yasuda, M.; Miwa, H.; Masuda, S.; Takebayashi, Y.; Sakakibara, H.; Okazaki, S. Effector-triggered immunity determines host genotype-specific incompatibility in legume-Rhizobium symbiosis. Plant Cell Physiol. 2016, 57, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.J.; Sessitsch, A.; Corbo, J.C.; Giller, K.E.; Akkermans, A.D.L.; Jefferson, R.A. β-Glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other Gram-negative bacteria. Microbiology 1995, 141, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Hattori, Y.; Saeki, K. The Mesorhizobium loti purB gene is involved in infection thread formation and nodule development in Lotus japonicus. J. Bacteriol. 2007, 189, 8347–8352. [Google Scholar] [CrossRef] [PubMed]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, E.T.; Tian, C.F.; Wang, F.Q.; Han, L.L.; Chen, W.F.; Chen, W.X. Bradyrhizobium elkanii, Bradyrhizobium yuanmingense and Bradyrhizobium japonicum are the main rhizobia associated with Vigna unguiculata and Vigna radiata in the subtropical region of China. FEMS Microbiol. Lett. 2008, 285, 146–154. [Google Scholar] [CrossRef]
- Appunu, C.; N’Zoue, A.; Moulin, L.; Depret, G.; Laguerre, G. Vigna mungo, V. radiata and V. unguiculata plants sampled in different agronomical–ecological–climatic regions of India are nodulated by Bradyrhizobium yuanmingense. Syst. Appl. Microbiol. 2009, 32, 460–470. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.-J.; Lu, H.-B.; Xie, Z.-P.; Staehelin, C. Functional analysis of the type 3 effector nodulation outer protein L (NopL) from Rhizobium sp. NGR234: Symbiotic effects, phosphorylation, and interference with mitogen-activated protein kinase signaling. J. Biol. Chem. 2011, 286, 32178–32187. [Google Scholar] [CrossRef]
- Ge, Y.-Y.; Xiang, Q.-W.; Wagner, C.; Zhang, D.; Xie, Z.-P.; Staehelin, C. The type 3 effector NopL of Sinorhizobium sp. strain NGR234 is a mitogen-activated protein kinase substrate. J. Exp. Bot. 2016, 67, 2483–2494. [Google Scholar] [CrossRef]
- Skorpil, P.; Saad, M.M.; Boukli, N.M.; Kobayashi, H.; Ares-Orpel, F.; Broughton, W.J.; Deakin, W.J. NopP, a phosphorylated effector of Rhizobium sp. strain NGR234, is a major determinant of nodulation of the tropical legumes Flemingia congesta and Tephrosia vogelii. Mol. Microbiol. 2005, 57, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Medina, C.; Ollero, F.J.; López-Baena, F.J. The Sinorhizobium (Ensifer) fredii HH103 nodulation outer protein NopI is a determinant for efficient nodulation of soybean and cowpea plants. Appl. Environ. Microbiol. 2017, 83, e02770-16. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bahena, M.H.; Peix, A.; Rivas, R.; Camacho, M.; Rodríguez-Navarro, D.N.; Mateos, P.F.; Martínez-Molina, E.; Willems, A.; Velázquez, E. Bradyrhizobium pachyrhizi sp. nov. and Bradyrhizobium jicamae sp. nov., isolated from effective nodules of Pachyrhizus erosus. Int. J. Syst. Evolut. Microbiol. 2009, 59, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Okabe, S.; Higashi, M.; Shimoda, Y.; Sato, S.; Tabata, S.; Hashiguchi, M.; Akashi, R.; Göttfert, M.; Saeki, K. Identification and functional analysis of type III effector proteins in Mesorhizobium loti. Mol. Plant-Microbe Interact. 2010, 23, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Moulin, L.; Vallenet, D.; Barbe, V.; Cytryn, E.; Avarre, J.-C.; Jaubert, M.; Simon, D.; Cartieaux, F.; Prin, Y.; et al. Legumes symbioses: Absence of Nod genes in photosynthetic bradyrhizobia. Science 2007, 316, 1307–1312. [Google Scholar] [CrossRef] [PubMed]

| Strains | Characteristics a | References |
|---|---|---|
| USDA61 | Wild-type strain, Polr | USDA b |
| BErhcJ | USDA61 derivative harboring insertion in rhcJ encoding a membrane protein of the type III secretion apparatus, defective in type III protein secretion, Polr, Kmr, Tcr | [11] |
| BEnodC | USDA61 derivative harboring insertion in nodC gene, Polr, Kmr, Tcr | [19] |
| BEttsInod | USDA61 derivative harboring insertion in ttsI and nodC genes, Polr, Kmr, Smr, Tcr | [19] |
| BEnopL | USDA61 derivative with the nopL gene deleted via double-crossover, Polr | This study |
| BEnopP1 | USDA61 derivative harboring insertion of the plasmid pSUPSCAKm::nopP1 in the nopP1 gene via single-crossover, Polr, Kmr | This study |
| BEnopP2 | USDA61 derivative with the nopP2 gene deleted via double-crossover, Polr | This study |
| BE5208 | USDA61 derivative with the bel2-5 gene deleted via double-crossover, Polr | This study |
| BEinnB | USDA61 derivative with the innB gene deleted via double-crossover, Polr | This study |
| BEnopL | USDA61 derivative with the nopL gene deleted via double-crossover, Polr | This study |
| BEnopP1 | USDA61 derivative harboring insertion of the plasmid pSUPSCAKm::nopP1 in the nopP1 gene via single-crossover, Polr, Kmr | This study |
| BEnopP2 | USDA61 derivative with the nopP2 gene deleted via double-crossover, Polr | This study |
| BEinnBnopP2 | USDA61 derivative with both innB and nopP2 deleted via double-crossover, Polr | This study |
| BEinnB5208 | USDA61 derivative with both innB and bel2-5 deleted via double-crossover, Polr | This study |
| Species/Cultivars | Symbiotic Phenotypes Induced by B. elkanii Strains b | Origins | Regions | ||||
|---|---|---|---|---|---|---|---|
| USDA61 | BErhcJ | BEnodC | BE53/BEinnB | BEnopL | |||
| V. unguiculata | ++ | + | N.O. | +++ | N.O. | Myanmar | Southeast Asia |
| V. trinervia | – | ++ | N.O. | – | N.O. | Myanmar | Southeast Asia |
| V. angularis | ++ | + | N.O. | +++ | N.O. | Japan | East Asia |
| V. aconitifolia | – | + | N.O. | + | N.O. | India | South Asia |
| V. mungo | |||||||
| PI173934 | +++ | + | N.O. | ++ | + | India | South Asia |
| MASH | ++ | – | – | ++ | – | Nepal | South Asia |
| IBPGR2775-3 | +++ | – | – | ++++ | – | Pakistan | South Asia |
| MAFF2002M3 | ++ | + | N.O. | + | N.O. | Myanmar | Southeast Asia |
| OSUM745 | ++ | + | N.O. | ++ | N.O. | Philippines | Southeast Asia |
| VM3003 | – | + | N.O. | – | N.O. | Thailand | Southeast Asia |
| U-THONG2 | – | + | N.O. | – | N.O. | Thailand | Southeast Asia |
| CQ5785 | + | ++ | N.O. | +++ | N.O. | Australia | Oceania |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.P.; Ratu, S.T.N.; Yasuda, M.; Teaumroong, N.; Okazaki, S. Identification of Bradyrhizobium elkanii USDA61 Type III Effectors Determining Symbiosis with Vigna mungo. Genes 2020, 11, 474. https://doi.org/10.3390/genes11050474
Nguyen HP, Ratu STN, Yasuda M, Teaumroong N, Okazaki S. Identification of Bradyrhizobium elkanii USDA61 Type III Effectors Determining Symbiosis with Vigna mungo. Genes. 2020; 11(5):474. https://doi.org/10.3390/genes11050474
Chicago/Turabian StyleNguyen, Hien P., Safirah T. N. Ratu, Michiko Yasuda, Neung Teaumroong, and Shin Okazaki. 2020. "Identification of Bradyrhizobium elkanii USDA61 Type III Effectors Determining Symbiosis with Vigna mungo" Genes 11, no. 5: 474. https://doi.org/10.3390/genes11050474
APA StyleNguyen, H. P., Ratu, S. T. N., Yasuda, M., Teaumroong, N., & Okazaki, S. (2020). Identification of Bradyrhizobium elkanii USDA61 Type III Effectors Determining Symbiosis with Vigna mungo. Genes, 11(5), 474. https://doi.org/10.3390/genes11050474






