Aquatic Exposure to Abscisic Acid Transstadially Enhances Anopheles stephensi Resistance to Malaria Parasite Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Colony Rearing and Maintenance
2.2. Mouse Infection and Parasite Transmission to A. stephensi
2.3. Treatment of Mosquito Larvae with ABA
2.4. qRT-PCR Assays
2.5. Triacylglycerol (TAG) Quantification
2.6. Statistical Analyses
3. Results
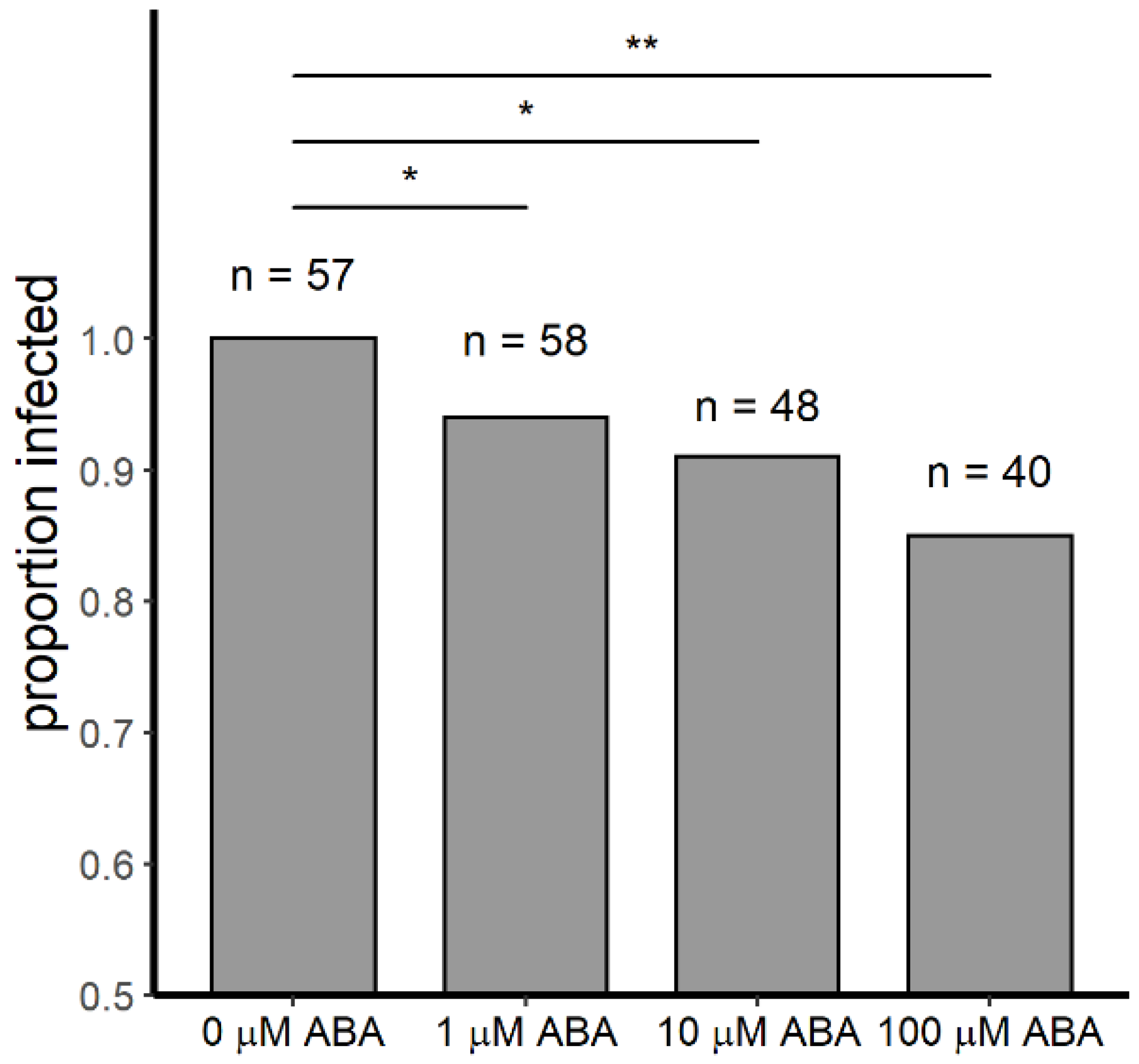
3.1. Relative to Controls, Female A. stephensi Derived from ABA-Treated Larvae Were Significantly More Resistant to P. y. yoelii 17XNL Infection
3.2. TAG Concentration and Expression of apoLp-III Were Unaltered in Adult Female A. stephensi Derived from ABA-Treated Larvae Relative to Mosquitoes Derived from Control Larvae
3.3. Insulin-Like Peptide (ilp) Gene Expression in Female A. stephensi Derived from ABA-Treated Larvae Was Reduced Relative to Females Derived from Control Larvae
3.4. Nitric Oxide Synthase (nos) Gene Expression in Female A. stephensi Derived from ABA-Treated Larvae Was Increased Relative to Mosquitoes Derived from Control Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Malaria Programme. In World Malaria Report 2019; WHO: Geneva, Switzerland, 2019; 232p, ISBN 978-92-4-156572-1. [Google Scholar]
- Carter, T.E.; Yared, S.; Gebresilassie, A.; Bonnell, V.; Damodaran, L.; Lopez, K.; Ibrahim, M.; Mohammed, S.; Janies, D. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018, 188, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Faulde, M.K.; Rueda, L.M.; Khaireh, B.A. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 2014, 139, 39–43. [Google Scholar] [CrossRef]
- Thomas, S.; Ravishankaran, S.; Justin, J.A.; Asokan, A.; Mathai, M.T.; Valecha, N.; Thomas, M.B.; Eapen, A. Overhead tank is the potential breeding habitat of Anopheles stephensi in an urban transmission setting of Chennai, India. Malar. J. 2016, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takken, W.; Lindsay, S. Increased threat of urban malaria from Anopheles stephensi mosquitoes, Africa. Emerg. Infect. Dis. 2019, 25, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Sharma, A.; Rajagopal, R.; Adak, T.; Bhatnagar, R.K. Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol. 2009, 9, 1–22. [Google Scholar] [CrossRef]
- Gimonneau, G.; Tchioffo, M.T.; Abate, L.; Boissière, A.; Awono-ambéné, P.H.; Nsango, S.E.; Christian, R.; Morlais, I. Composition of Anopheles coluzzii and Anopheles gambiae microbiota from larval to adult stages. Infect. Genet. Evol. 2014, 28, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Coon, K.L.; Brown, M.R.; Strand, M.R. Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Mol. Ecol. 2016, 25, 5806–5826. [Google Scholar] [CrossRef]
- Bascuñán, P.; Niño-Garcia, J.P.; Galeano-Castañeda, Y.; Serre, D.; Correa, M.M. Factors shaping the gut bacterial community assembly in two main Colombian malaria vectors. Microbiome 2018, 6, 148. [Google Scholar] [CrossRef]
- Dennison, N.J.; Jupatanakul, N.; Dimopoulos, G. The mosquito microbiota influences vector competence for human pathogens. Curr. Opin. Insect Sci. 2014, 3, 6–13. [Google Scholar] [CrossRef]
- Dickson, L.B.; Jiolle, D.; Minard, G.; Moltini-Conclois, I.; Volant, S.; Ghozlane, A.; Bouchier, C.; Ayala, D.; Paupy, C.; Valiente, C.M.; et al. Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci. Adv. 2017, 3, e1700585. [Google Scholar] [CrossRef]
- Sharma, P.; Rani, J.; Chauhan, C.; Kumari, S.; Tevatiya, S.; Das De, T.; Savargaonkar, D.; Pandey, K.C.; Dixit, R. Altered gut microbiota and immunity defines Plasmodium vivax survival in Anopheles stephensi. Front. Immunol. 2020, 11, 609. [Google Scholar] [CrossRef]
- Yee, D.A.; Kesavaraju, B.; Juliano, S.A. Direct and indirect effects of animal detritus on growth, survival, and mass of invasive container mosquito Aedes albopictus (Diptera: Culicidae). Popul. Community Ecol. 2007, 44, 580–588. [Google Scholar] [CrossRef]
- Jeanrenaud, A.C.S.N.; Brooke, B.D.; Oliver, S.V. The effects of larval organic fertiliser exposure on the larval development, adult longevity and insecticide tolerance of zoophilic members of the Anopheles gambiae complex (Diptera: Culicidae). PLoS ONE 2019, 14, e0215552. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Annu. Rev. Entomol. 2009, 54, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Chandrasegaran, K.; Juliano, S.A. How do trait-mediated non-lethal effects of predation affect population-level performance of mosquitoes? Front. Ecol. Evol. 2019, 7, 25. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef]
- Olds, C.L.; Glennon, E.K.K.; Luckhart, S. Abscisic acid: New perspectives on an ancient universal stress signaling molecule. Microbes Infect. 2018, 20, 484–492. [Google Scholar] [CrossRef]
- Else, M.A.; Hall, K.C.; Arnold, G.M.; Davies, W.J.; Jackson, M.B. Export of abscisic acid, 1-aminocyclopropane-1-carboxylic acid, phosphate, and nitrate from roots to shoots of flooded tomato plants (accounting for effects of xylem sap flow rate on concentration and delivery). Plant Physiol. 1995, 107, 377–384. [Google Scholar] [CrossRef]
- Taylor, D.M.; Olds, C.L.; Haney, R.S.; Torrevillas, B.K.; Luckhart, S. Comprehensive and durable modulation of growth, development, lifespan and fecundity in Anopheles stephensi following larval treatment with the stress signaling molecule and novel antimalarial abscisic acid. Front. Microbiol. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Roy, R.; Schmitt, A.J.; Thomas, J.B.; Carter, C.J. Review: Nectar biology: From molecules to ecosystems. Plant Sci. 2017, 262, 148–164. [Google Scholar] [CrossRef]
- Glennon, E.K.K.; Megawati, D.; Torrevillas, B.K.; Ssewanyana, I.; Huang, L.; Aweeka, F.; Greenhouse, B.; Adams, L.G.; Luckhart, S. Elevated plasma abscisic acid is associated with asymptomatic falciparum malaria and with IgG-/caspase-1-dependent immunity in Plasmodium yoelii-infected mice. Sci. Rep. 2018, 8, 8896. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Emionite, L.; Guida, L.; Vigliarolo, T.; Sturla, L.; Spinelli, S.; Buschiazzo, A.; Marini, C.; Sambuceti, G.; De Flora, A.; et al. Insulin-independent stimulation of skeletal muscle glucose uptake by low-dose abscisic acid via AMPK activation. Sci. Rep. 2020, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Glennon, E.K.; Adams, L.G.; Hicks, D.R.; Dehesh, K.; Luckhart, S. Supplementation with abscisic acid reduces malaria disease severity and parasite transmission. Am. J. Trop. Med. Hyg. 2016, 94, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Glennon, E.K.K.; Torrevillas, B.K.; Morrissey, S.F.; Ejercito, J.M.; Luckhart, S. Abscisic acid induces a transient shift in signaling that enhances NF-κB-mediated parasite killing in the midgut of Anopheles stephensi without reducing lifespan or fecundity. Parasit. Vectors 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Pietri, J.E.; Pietri, E.J.; Potts, R.; Riehle, M.A.; Luckhart, S. Plasmodium falciparum suppresses the host immune response by inducing the synthesis of insulin-like peptides (ILPs) in the mosquito Anopheles stephensi. Dev. Comp. Immunol. 2015, 53, 134–144. [Google Scholar] [CrossRef]
- Rono, M.K.; Whitten, M.M.A.; Oulad-abdelghani, M.; Levashina, E.A.; Marois, E. The major yolk protein vitellogenin interferes with the anti-Plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol. 2010, 8, 1–12. [Google Scholar] [CrossRef]
- Paskewitz, S.M.; Shi, L. The hemolymph proteome of Anopheles gambiae. Insect Biochem. Mol. Biol. 2005, 35, 815–824. [Google Scholar] [CrossRef]
- Gupta, L.; Noh, J.Y.; Jo, Y.H.; Oh, S.H.; Kumar, S.; Noh, M.Y.; Lee, Y.S.; Cha, S.J.; Seo, S.J.; Kim, I.; et al. Apolipophorin-III mediates antiplasmodial epithelial responses in Anopheles gambiae (G3) mosquitoes. PLoS ONE 2010, 5, e15410. [Google Scholar] [CrossRef]
- Dhawan, R.; Gupta, K.; Kajla, M.; Kakani, P.; Choudhury, T.P.; Kumar, S.; Kumar, V.; Gupta, L. Apolipophorin-III acts as a positive regulator of Plasmodium development in Anopheles stephensi. Front. Physiol. 2017, 8, 185. [Google Scholar] [CrossRef]
- Costa, G.; Gildenhard, M.; Lidquist, R.L.; Hauser, A.E.; Sauerwein, R.; Goosemann, C.; Brinkmann, V.; Carillo-Bustamante, P.; Levashina, E.A. Non-competitive resource exploitation within mosquito shapes within-host malaria infectivity and virulence. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Mendes, A.M.; Schlegelmilch, T.; Cohuet, A.; Awono-Ambene, P.; De Iorio, M.; Fontenille, D.; Morlais, I.; Christophides, G.K.; Kafatos, F.C.; Vlachou, D. Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathog. 2008, 4, e1000069. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Drexler, A.; de Jong, L.W.; Antonova, Y.; Pakpour, N.; Ziegler, R.; Ramberg, F.; Lewis, E.E.; Brown, J.M.; Luckhart, S.; et al. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010, 6, e1001003. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Lee, Y.; Coulibaly, C.A.; Rashbrook, V.K.; Cornel, A.J.; Lanzaro, G.C.; Luckhart, S. Identification of three single nucleotide polymorphisms in Anopheles gambiae immune signaling genes that are associated with natural Plasmodium falciparum infection. Malar. J. 2010, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Surachetpong, W.; Pakpour, N.; Cheung, K.W.; Luckhart, S. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid. Redox Signal. 2011, 14, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Pakpour, N.; Corby-Harris, V.; Green, G.P.; Smithers, H.M.; Cheung, K.W.; Riehle, M.A.; Luckhart, S. Ingested human insulin inhibits the mosquito NF-kappaB-dependent immune response to Plasmodium falciparum. Infect. Immun. 2012, 80, 2141–2149. [Google Scholar] [CrossRef]
- Drexler, A.L.; Pietri, J.E.; Pakpour, N.; Hauck, E.; Wang, B.; Glennon, E.K.; Georgis, M.; Riehle, M.A.; Luckhart, S. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathog. 2014, 10, e1004231. [Google Scholar] [CrossRef]
- Pietri, J.E.; Pakpour, N.; Napoli, E.; Song, G.; Pietri, E.; Potts, R.; Cheung, K.W.; Walker, G.; Riehle, M.A.; Starcevich, H.; et al. Two insulin-like peptides differentially regulate malaria parasite infection in the mosquito through effects on intermediary metabolism. Biochem. J. 2016, 473, 3487–3503. [Google Scholar] [CrossRef]
- Luckhart, S.; Giulivi, C.; Drexler, A.L.; Antonova-Koch, Y.; Sakaguchi, D.; Napoli, E.; Wong, S.; Price, M.S.; Eigenheer, R.; Phinney, B.S.; et al. Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum infection in the mosquito host. PLoS Pathog. 2013, 9, e1003180. [Google Scholar] [CrossRef]
- Dong, Y.; Aguilar, R.; Xi, Z.; Warr, E.; Mongin, E.; Dimopoulos, G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006, 2, e52. [Google Scholar] [CrossRef]
- Povelones, M.; Waterhouse, R.M.; Kafatos, F.C.; Christophides, G.K. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 2009, 324, 258–261. [Google Scholar] [CrossRef]
- Jaramillo-Gutierrez, G.; Rodrigues, J.; Ndikuyeze, G.; Povelones, M.; Molina-Cruz, A.; Barillas-Mury, C. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 2009, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.M.; Barry, W.E.; Cox, J.; Thummel, C.S. Methods for studying metabolism in Drosophila. Methods 2014, 68, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, S.E.; Briegel, H. Larval growth and biosynthesis of reserves in mosquitoes. J. Insect Physiol. 1999, 45, 461–470. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Luckhart, S.; Riehle, M.A. Midgut mitochondrial function as a gatekeeper for malaria parasite infection and development in the mosquito host. Front. Cell. Infect. Microbiol. 2020. Accepted for Publication. [Google Scholar]
- Peterson, T.M.; Gow, A.J.; Luckhart, S. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radic. Biol. Med. 2007, 42, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.A.; Hensley, L.; Beier, J.C. Sporogonic development of Plasmodium yoelii in five anopheline species. J. Parasitol. 1994, 80, 674–681. [Google Scholar] [CrossRef]
- Gulia-Nuss, M.; Robertson, A.E.; Brown, M.R.; Strand, M.R. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS ONE 2011, 6, e20401. [Google Scholar] [CrossRef]
- Nuss, A.B.; Brown, M.R. Isolation of an insulin-like peptide from the Asian malaria mosquito, Anopheles stephensi, that acts as a steroidogenic gonadotropin across diverse mosquito taxa. Gen. Comp. Endocrinol. 2018, 258, 140–148. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Ling, L.; Raikhel, A.S. Serotonin signaling regulates insulin-like peptides for growth, reproduction, and metabolism in the disease vector Aedes aegypti. Proc. Natl. Acad. Sci. USA 2018, 115, 9822–9831. [Google Scholar] [CrossRef]
- Gulati, S.; Ekland, E.H.; Ruggles, K.V.; Chan, R.B.; Jayabalasingham, B.; Zhou, B.; Mantel, P.Y.; Lee, M.C.; Spottiswoode, N.; Coburn-Flynn, O.; et al. Profiling the essential nature of lipid metabolism in asexual blood and gametocyte stages of Plasmodium falciparum. Cell Host Microbe 2015, 18, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Canavoso, L.E.; Jouni, Z.E.; Pennington, J.E.; Tsuchida, K.; Wells, M.A. Lipid storage and mobilization in insects: Current status and future directions. Insect Biochem. Mol. Biol. 2001, 31, 7–17. [Google Scholar] [CrossRef]
- Ford, P.S.; Van Heusden, M.C. Triglyceride-rich lipophorin in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1995, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Atella, G.C.; Silva-Neto, M.A.; Golodne, D.M.; Arefin, S.; Shahabuddin, M. Anopheles gambiae lipophorin: Characterization and role in lipid transport to developing oocyte. Insect Biochem. Mol. Biol. 2006, 36, 375–386. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, D.M.; Haney, R.S.; Luckhart, S. Aquatic Exposure to Abscisic Acid Transstadially Enhances Anopheles stephensi Resistance to Malaria Parasite Infection. Genes 2020, 11, 1393. https://doi.org/10.3390/genes11121393
Taylor DM, Haney RS, Luckhart S. Aquatic Exposure to Abscisic Acid Transstadially Enhances Anopheles stephensi Resistance to Malaria Parasite Infection. Genes. 2020; 11(12):1393. https://doi.org/10.3390/genes11121393
Chicago/Turabian StyleTaylor, Dean M., Reagan S. Haney, and Shirley Luckhart. 2020. "Aquatic Exposure to Abscisic Acid Transstadially Enhances Anopheles stephensi Resistance to Malaria Parasite Infection" Genes 11, no. 12: 1393. https://doi.org/10.3390/genes11121393
APA StyleTaylor, D. M., Haney, R. S., & Luckhart, S. (2020). Aquatic Exposure to Abscisic Acid Transstadially Enhances Anopheles stephensi Resistance to Malaria Parasite Infection. Genes, 11(12), 1393. https://doi.org/10.3390/genes11121393




