Highly Rearranged Karyotypes and Multiple Sex Chromosome Systems in Armored Catfishes from the Genus Harttia (Teleostei, Siluriformes)
Abstract
:1. Introduction
2. Materials and Methods
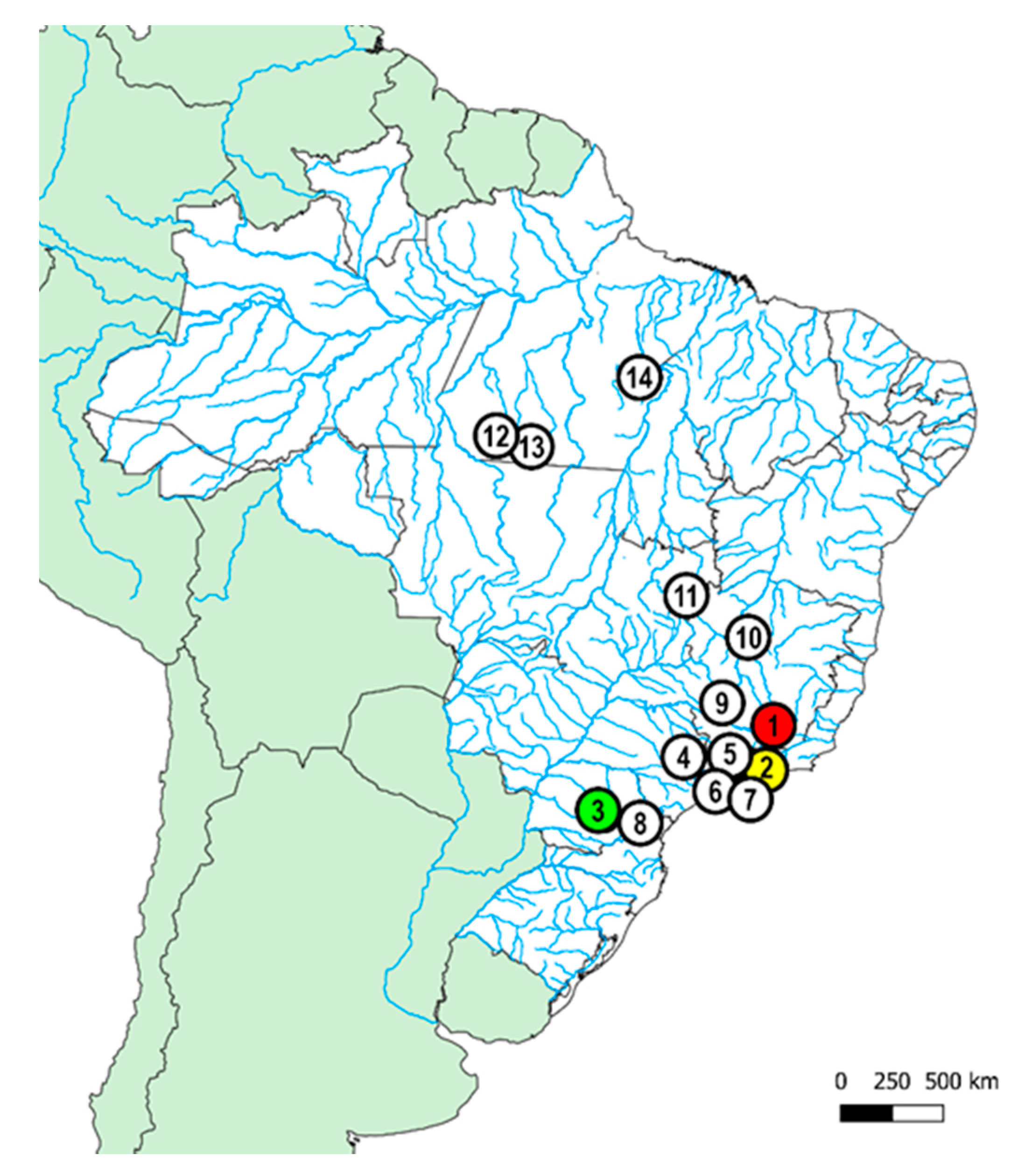
2.1. Specimens
2.2. Chromosome Preparations and C-Banding
2.3. Fluorescence In Situ Hybridization (FISH)
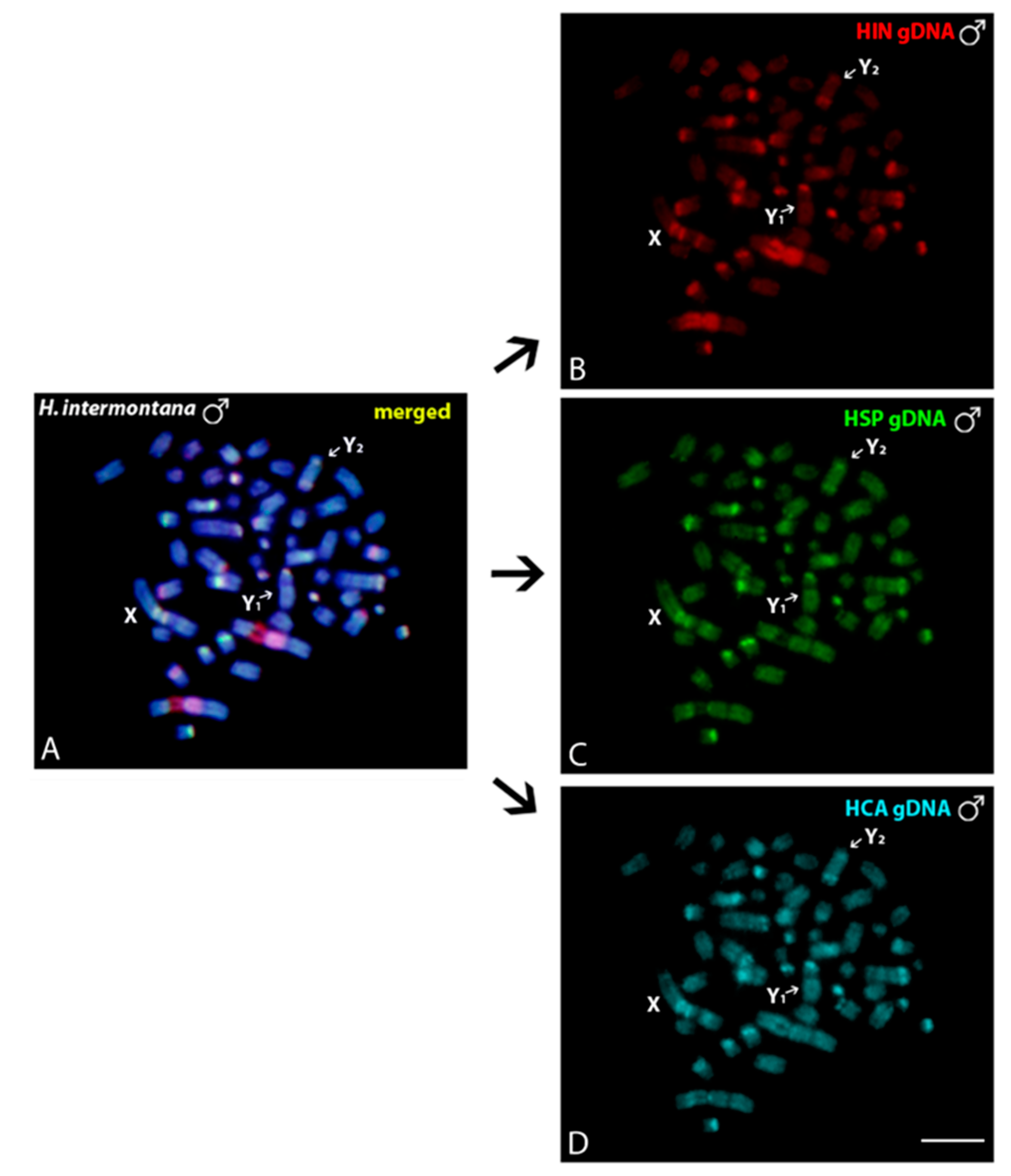
2.4. Comparative Genomic Hybridization (CGH)
2.5. Microscopic Analyses and Image Processing
3. Results
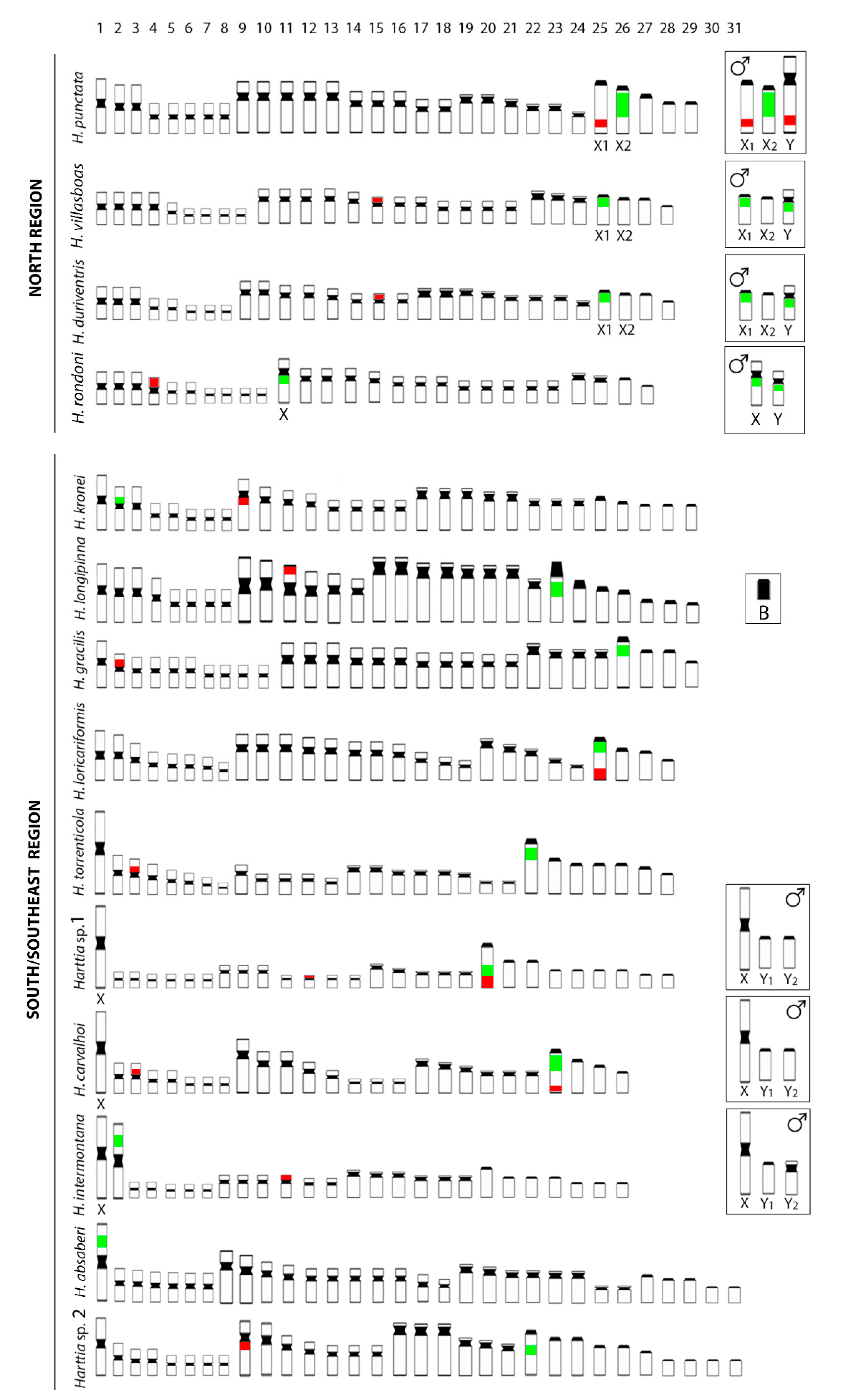
3.1. Karyotypes, C-Banding, and Sex Chromosomes
3.2. Chromosomal Distribution of rDNAs and Telomeric Repeats
3.3. Intraspecific and Interspecific Comparative Genomic Hybridizations
4. Discussion
4.1. Numerical Chromosome Changes in Harttia Species
4.2. Heterochromatin and rDNA Sites Rearrangements in Harttia Species
4.3. The Rare XX/XY1Y2 System in Fish Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, J.S.; Grand, T.C.; Wilson, M.V.H. Fishes of The World, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Albert, J.S.; Tagliacollo, V.A.; Dagosta, F. Diversification of Neotropical Freshwater Fishes. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 27–53. [Google Scholar] [CrossRef]
- Reis, R.E.; Kullander, S.O.; Ferraris, C.J., Jr. Check List of The Freshwater Fishes of South and Central America; Edipucrs: Porto Alegre, Brazil, 2003. [Google Scholar]
- Do Nascimento, V.D.; Coelho, K.A.; Nogaroto, V.; de Almeida, R.B.; Ziemniczak, K.; Centofante, L.; Pavanelli, C.S.; Torres, R.A.; Moreira-Filho, O.; Vicari, M.R. Do multiple karyomorphs and population genetics of freshwater darter characines (Apareiodon affinis) indicate chromosomal speciation? Zool. Anz. 2018, 272, 93–103. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Oyakawa, O.T. New loricariid fishes from headwaters on serra da mantiqueira and complexo do espinhaço, Minas Gerais state, Brazil (Teleostei: Siluriformes: Loricariidae). Zootaxa 2019, 4586, 401–424. [Google Scholar] [CrossRef] [PubMed]
- Buckup, P.A. Sistemática e biogeografia de peixes de riachos. Oecologia Aust. 1999, 06, 91–138. [Google Scholar] [CrossRef]
- Covain, R.; Fisch-Muller, S.; Oliveira, C.; Mol, J.H.; Montoya-Burgos, J.I.; Dray, S. Molecular phylogeny of the highly diversified catfish subfamily Loricariinae (Siluriformes, Loricariidae) reveals incongruences with morphological classification. Mol. Phylogenet. Evol. 2016, 94, 492–517. [Google Scholar] [CrossRef]
- Blanco, D.R.; Vicari, M.R.; Lui, R.L.; Traldi, J.B.; Bueno, V.; Martinez, J.d.F.; Brandão, H.; Oyakawa, O.T.; Moreira Filho, O. Karyotype Diversity and Evolutionary Trends in Armored Catfish Species of the Genus Harttia (Siluriformes: Loricariidae). Zebrafish 2017, 14, 169–176. [Google Scholar] [CrossRef]
- Blanco, D.R.; Vicari, M.R.; Lui, R.L.; Bertollo, L.A.C.; Traldi, J.B.; Moreira-Filho, O. The role of the Robertsonian rearrangements in the origin of the XX/XY1Y2 sex chromosome system and in the chromosomal differentiation in Harttia species (Siluriformes, Loricariidae). Rev. Fish Biol. Fish. 2013, 23, 127–134. [Google Scholar] [CrossRef]
- Blanco, D.R.; Vicari, M.R.; Lui, R.L.; Artoni, R.F.; de Almeida, M.C.; Traldi, J.B.; Margarido, V.P.; Moreira-Filho, O. Origin of the X1X1X2X2/X1X2Y sex chromosome system of Harttia punctata (Siluriformes, Loricariidae) inferred from chromosome painting and FISH with ribosomal DNA markers. Genetica 2014, 142, 119–126. [Google Scholar] [CrossRef]
- Sassi, F.d.M.C.; Deon, G.A.; Moreira-filho, O.; Vicari, M.R.; Bertollo, L.A.C.; Liehr, T.; de Oliveira, E.A.; Cioffi, M.B. Multiple Sex Chromosomes and Evolutionary Relationships in Amazonian Catfishes: The Outstanding Model of the Genus Harttia (Siluriformes: Loricariidae). Genes (Basel) 2020, 11, 1179. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B.; Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity (Edinb) 2005, 95, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Steinemann, S.; Steinemann, M. Retroelements: Tools for sex chromosome evolution. Cytogenet. Genome Res. 2005, 110, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Schemberger, M.O.; Nascimento, V.D.; Coan, R.; Ramos, É.; Nogaroto, V.; Ziemniczak, K.; Valente, G.T.; Moreira-Filho, O.; Martins, C.; Vicari, M.R. DNA transposon invasion and microsatellite accumulation guide W chromosome differentiation in a Neotropical fish genome. Chromosoma 2019, 128, 547–560. [Google Scholar] [CrossRef]
- Moreira-Filho, O.; Bertollo, L.A.C.; Junior, P.M.G. Evidences for a multiple sex chromosome system with female heterogamety in Apareiodon affinis (Pisces, Parodontidae). Caryologia 1980, 33, 83–91. [Google Scholar] [CrossRef]
- Bertollo, L.A.C.; Fontes, M.S.; Fenocchio, A.S.; Cano, J. The X1X2Y sex chromosome system in the fish Hoplias malabaricus. I. G-, C- and chromosome replication banding. Chromosom. Res. 1997, 5, 493–499. [Google Scholar] [CrossRef]
- Schemberger, M.O.; Bellafronte, E.; Nogaroto, V.; Almeida, M.C.; Schühli, G.S.; Artoni, R.F.; Moreira-Filho, O.; Vicari, M.R. Differentiation of repetitive DNA sites and sex chromosome systems reveal closely related group in Parodontidae (Actinopterygii: Characiformes). Genetica 2011, 139, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Kitano, J.; Peichel, C.L. Turnover of sex chromosomes and speciation in fishes. Environ. Biol. Fishes 2012, 94, 549–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, E.A.; Sember, A.; Bertollo, L.A.C.; Yano, C.F.; Ezaz, T.; Moreira-Filho, O.; Hatanaka, T.; Trifonov, V.; Liehr, T.; Al-Rikabi, A.B.H.; et al. Tracking the evolutionary pathway of sex chromosomes among fishes: Characterizing the unique XX/XY1Y2 system in Hoplias malabaricus (Teleostei, Characiformes). Chromosoma 2017, 127, 115–128. [Google Scholar] [CrossRef]
- Cioffi, M.d.B.; Yano, C.F.; Sember, A.; Bertollo, L.A.C. Chromosomal evolution in lower vertebrates: Sex chromosomes in neotropical fishes. Genes (Basel). 2017, 8, 258. [Google Scholar] [CrossRef]
- Yano, C.F.; Bertollo, L.A.C.; Ezaz, T.; Trifonov, V.; Sember, A.; Liehr, T.; Cioffi, M.d.B. Highly conserved Z and molecularly diverged W chromosomes in the fish genus Triportheus (Characiformes, Triportheidae). Heredity (Edinb). 2017, 118, 276–283. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, R.L.; Sember, A.; Bertollo, L.A.; de Oliveira, E.A.; Rab, P.; Hatanaka, T.; Marinho, M.M.; Liehr, T.; Al-Rikabi, A.B.; Feldberg, E.; et al. Comparative cytogenetics and neo-Y formation in small-sized fish species of the genus Pyrrhulina (Characiformes, Lebiasinidae). Front. Genet. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.M. Estudos Cromossômicos e Moleculares em Loricariinae com ênfase em espécies de Rineloricaria (Silurifomes, Loricariidae): Uma perspectiva evolutiva. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2010. [Google Scholar]
- Blanco, D.R.; Vicari, M.R.; Artoni, R.F.; Traldi, J.B.; Moreira-Filho, O. Chromosomal characterization of armored catfish Harttia longipinna (Siluriformes, Loricariidae): First report of B chromosomes in the genus. Zoolog. Sci. 2012, 29, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Kavalco, K.F.; Pazza, R.; Bertollo, L.A.C.; Moreira-Filho, O. Heterochromatin characterization of four fish species of the family Loricariidae (Siluriformes). Hereditas 2004, 141, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.A.C.; de Bello Cioffi, M.; Galetti, P.M.; Moreira-Filho, O. Contributions to the cytogenetics of the Neotropical fish fauna. Comp. Cytogenet. 2017, 11, 665–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Lui, R.L.; Blanco, D.R.; Moreira-Filho, O.; Margarido, V.P. Propidium iodide for making heterochromatin more evident in the C-banding technique. Biotech. Histochem. 2012, 87, 433–438. [Google Scholar] [CrossRef]
- Pendas, A.M.; Moran, P.; Freije, J.P.; Garcia-Vazquez, E. Chromosomal mapping and nucleotide sequence of two tandem repeats of Atlantic salmon 5S rDNA. Cytogenet. Genome Res. 1994, 67, 31–36. [Google Scholar] [CrossRef]
- Cioffi, M.d.B.; Martins, C.; Centofante, L.; Jacobina, U.; Bertollo, L.A.C. Chromosomal variability among allopatric populations of Erythrinidae fish Hoplias malabaricus: Mapping of three classes of repetitive DNAs. Cytogenet. Genome Res. 2009, 125, 132–141. [Google Scholar] [CrossRef]
- Yano, C.F.; Bertollo, L.A.C.; Cioffi, M.d.B. Fish-FISH: Molecular Cytogenetics in Fish Species. In Fluorescence In Situ Hybridization (FISH) Application Guide, 2nd ed.; Liehr, T., Ed.; Springer: Berlin, Germany, 2017; ISBN 9783662529577. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A laboratory Manual; Sambrook, J., Russell, D.W., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Zwick, M.S.; Hanson, R.E.; McKnight, T.D.; Islam-Faridi, M.N.; Stelly, D.M.; Wing, R.A.; Price, H.J. A rapid procedure for the isolation of C0t-1 DNA from plants. Genome 1997, 40, 138–142. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, R.L.R.; Bertollo, L.A.C.; Marinho, M.M.F.; Yano, C.F.; Hatanaka, T.; Barby, F.F.; Troy, W.P.; Cioffi, M.d.B. Evolutionary relationships and cytotaxonomy considerations in the genus Pyrrhulina (Characiformes, Lebiasinidae). Zebrafish 2017, 14, 536–546. [Google Scholar] [CrossRef]
- Sassi, F.d.M.C.; de Oliveira, E.A.; Bertollo, L.A.C.; Nirchio, M.; Hatanaka, T.; Marinho, M.M.F.; Moreira-Filho, O.; Aroutiounian, R.; Liehr, T.; Al-Rikabi, A.B.H.; et al. Chromosomal evolution and evolutionary relationships of Lebiasina species (Characiformes, Lebiasinidae). Int. J. Mol. Sci. 2019, 20, 2944. [Google Scholar] [CrossRef] [Green Version]
- Toma, G.A.; de Moraes, R.L.R.; Sassi, F.d.M.C.; Bertollo, L.A.C.; de Oliveira, E.A.; Rab, P.; Sember, A.; Liehr, T.; Hatanaka, T.; Viana, P.F.; et al. Cytogenetics of the small-sized fish, Copeina guttata (Characiformes, Lebiasinidae): Novel insights into the karyotype differentiation of the family. PLoS ONE 2019, 14, e0226746. [Google Scholar] [CrossRef]
- Symonová, R.; Sember, A.; Maitánová, Z.; Ráb, P. Characterization of Fish Genome by GISH and CGH. In Fish Cytogenetic Techniques. Ray-Fin Fishes and Chondrichthyans; Ozouf-Costaz, C., Pisano, E., Foresti, F., Almeida Toledo, L.F., Eds.; CRC Press: Boca Ranton, FL, USA, 2015; pp. 118–131. [Google Scholar]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for Centromeric Position on Chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Mariotto, S.; Centofante, L.; Vicari, M.R.; Artoni, R.F.; Moreira-Filho, O. Chromosomal diversification in ribosomal DNA sites in Ancistrus Kner, 1854 (Loricariidae, Ancistrini) from three hydrographic basins of Mato Grosso, Brazil. Comp. Cytogenet. 2011, 5, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.V.; Wolski, M.A.V.; Nogaroto, V.; Almeida, M.C.; Moreira-Filho, O.; Vicari, M.R. Fragile sites, dysfunctional telomere and chromosome fusions: What is 5S rDNA role? Gene 2017, 608, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Glugoski, L.; Giuliano-Caetano, L.; Moreira-Filho, O.; Vicari, M.R.; Nogaroto, V. Co-located hAT transposable element and 5S rDNA in an interstitial telomeric sequence suggest the formation of Robertsonian fusion in armored catfish. Gene 2018, 650, 49–54. [Google Scholar] [CrossRef]
- Glugoski, L.; Deon, G.; Schott, S.; Vicari, M.R.; Nogaroto, V.; Moreira-Filho, O. Comparative cytogenetic analyses in Ancistrus species (Siluriformes: Loricariidae). Neotrop. Ichthyol. 2020, 18, 1–16. [Google Scholar] [CrossRef]
- Pinna, M.C.C. Phylogenetic Relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): Historical Overview and Synthesis of Hypotheses. In Phylogeny and Classification of Neotropical Fishes; Malabarba, L.R., Reis, R.E., Vari, R.P., Lucena, Z.M., Lucena, C.A.S., Eds.; Edipucrs: Porto Alegre, Brazil, 1998; pp. 279–330. [Google Scholar]
- Wright, S. Breeding Structure of Populations in Relation to Speciation. Amer Natur 1940, 74, 232–248. [Google Scholar] [CrossRef]
- King, M. Species Evolution; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Rieseberg, L.H. Chromosomal rearrangements and speciation. TRENDS Ecol. Evol. 2001, 16, 351–358. [Google Scholar] [CrossRef]
- Faria, R.; Navarro, A. Chromosomal speciation revisited: Rearranging theory with pieces of evidence. Trends Ecol. Evol. 2010, 25, 660–669. [Google Scholar] [CrossRef]
- Rosa, K.O.; Ziemniczak, K.; de Barros, A.V.; Nogaroto, V.; Almeida, M.C.; Cestari, M.M.; Artoni, R.F.; Vicari, M.R. Numeric and structural chromosome polymorphism in Rineloricaria lima (Siluriformes: Loricariidae): Fusion points carrying 5S rDNA or telomere sequence vestiges. Rev. Fish Biol. Fish. 2012, 22, 739–749. [Google Scholar] [CrossRef]
- Porto, F.E.; de Rossi Vieira, M.M.; Barbosa, L.M.; Borin-Carvalho, L.A.; Vicari, M.R.; De Brito Portela-Castro, A.L.; Martins-Santos, I.C. Chromosomal polymorphism in Rineloricaria lanceolata Günther, 1868 (Loricariidae: Loricariinae) of the paraguay basin (Mato Grosso do Sul, Brazil): Evidence of fusions and their consequences in the population. Zebrafish 2014, 11, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Primo, C.C.; Glugoski, L.; Almeida, M.C.; Zawadzki, C.H.; Moreira-Filho, O.; Vicari, M.R.; Nogaroto, V. Mechanisms of Chromosomal Diversification in Species of Rineloricaria (Actinopterygii: Siluriformes: Loricariidae). Zebrafish 2017, 14, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, A.; Nergadze, S.G.; Santagostino, M.; Giulotto, E. Telomeric repeats far from the ends: Mechanisms of origin and role in evolution. Cytogenet. Genome Res. 2008, 122, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Marajó, L.; Viana, P.F.; Ferreira, M.; Py-Daniel, L.H.R.; Feldberg, E. Cytogenetics of two Farlowella species (Loricariidae: Loricariinae): Implications on the taxonomic status of the species. Neotrop. Ichthyol. 2018, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ocalewicz, K. Telomeres in fishes. Cytogenet. Genome Res. 2013, 141, 114–125. [Google Scholar] [CrossRef]
- Garagna, S.; Broccoli, D.; Redi, C.A.; Searle, J.B.; Cooke, H.J.; Capanna, E. Robertsonian metacentrics of the house mouse lose telomeric sequences but retain some minor satellite DNA in the pericentromeric area. Chromosoma 1995, 103, 685–692. [Google Scholar] [CrossRef]
- Slijepcevic, P. Telomere length and telomere-centromere relationships? Mutat. Res.-Fundam. Mol. Mech. Mutagen. 1998, 404, 215–220. [Google Scholar] [CrossRef]
- Bolzán, A.D. Interstitial telomeric sequences in vertebrate chromosomes: Origin, function, instability and evolution. Mutat. Res.-Rev. Mutat. Res. 2017, 773, 51–65. [Google Scholar] [CrossRef]
- Slijepcevic, P.; Hande, M.P.; Bouffler, S.D.; Lansdorp, P.; Bryant, P.E. Telomere length, chromatin structure and chromosome fusigenic potential. Chromosoma 1997, 106, 413–421. [Google Scholar] [CrossRef]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Sfeir, A.; Kosiyatrakul, S.T.; Hockemeyer, D.; MacRae, S.L.; Karlseder, J.; Schildkraut, C.L.; de Lange, T. Mammalian Telomeres Resemble Fragile Sites and Require TRF1 for Efficient Replication. Cell 2009, 138, 90–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.; Slater, H.R.; Choo, K.H.A. Centric fission—Simple and complex mechanisms. Chromosom. Res. 2004, 12, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Slijepcevic, P. Mechanisms of the evolutionary chromosome plasticity: Integrating the “centromere-from-telomere” hypothesis with telomere length regulation. Cytogenet. Genome Res. 2016, 148, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Barton, N.H. Accumulating postzygotic isolation genes in parapatry: A new twist on chromosomal speciation. Evolution (N. Y.) 2003, 57, 447–459. [Google Scholar] [CrossRef]
- Lin, K.W.; Yan, J. Endings in the middle: Current knowledge of interstitial telomeric sequences. Mutat. Res.-Rev. Mutat. Res. 2008, 658, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, A.; Castresana, J.; Robinson, T.J. Is mammalian chromosomal evolution driven by regions of genome fragility? Genome Biol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Carbone, L.; Harris, R.A.; Vessere, G.M.; Mootnick, A.R.; Humphray, S.; Rogers, J.; Kim, S.K.; Wall, J.D.; Martin, D.; Jurka, J.; et al. Evolutionary breakpoints in the gibbon suggest association between cytosine methylation and karyotype evolution. PLoS Genet. 2009, 5, 1–10. [Google Scholar] [CrossRef]
- Longo, M.S.; Carone, D.M.; Green, E.D.; O’Neill, M.J.; O’Neill, R.J. Distinct retroelement classes define evolutionary breakpoints demarcating sites of evolutionary novelty. BMC Genom. 2009, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Farré, M.; Bosch, M.; López-Giráldez, F.; Ponsà, M.; Ruiz-Herrera, A. Assessing the role of tandem repeats in shaping the genomic architecture of great apes. PLoS ONE 2011, 6, e27239. [Google Scholar] [CrossRef] [Green Version]
- Arai, R. Fish Karyotypes: A Check List; Springer Science & Business Media: Tokyo, Japan, 2011. [Google Scholar]
- Pennell, M.W.; Kirkpatrick, M.; Otto, S.P.; Vamosi, J.C.; Peichel, C.L.; Valenzuela, N.; Kitano, J. Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles. PLoS Genet. 2015, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Mank, J.E.; Avise, J.C. Evolutionary diversity and turn-over of sex determination in teleost fishes. Sex. Dev. 2009, 3, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Frolov, S.V. Differentiation of sex chromosomes in the Salmonidae. III. Multiple sex chromosomes in Coregonus sardinella. Tsitologiya 1990, 32, 659–663. [Google Scholar]
- Ozouf-Costaz, C.; Hureau, J.; Beaunier, M. Chromosome studies on fish of the suborder Notothenioidei collected in the weddell sea during epos 3 cruise. Cybium (Paris) 1991, 15, 271–289. [Google Scholar]
- Sember, A.; Bohlen, J.; Šlechtová, V.; Altmanová, M.; Symonová, R.; Ráb, P. Karyotype differentiation in 19 species of river loach fishes (Nemacheilidae, Teleostei): Extensive variability associated with rDNA and heterochromatin distribution and its phylogenetic and ecological interpretation. BMC Evol. Biol. 2015, 15, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertollo, L.A.C.; Filho, O.M.; Takahashi, C.S. Multiple Sex Chromosomes in the Genus Hoplias (Pisces: Erythrinidae). Cytologia (Tokyo). 1983, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, R.R.; Feldberg, E.; Dos Anjos, M.B.; Zuanon, J. Occurrence of multiple sexual chromosomes (XX/XY1Y2 and Z1Z1Z2Z2/Z1Z2W1W2) in catfishes of the genus Ancistrus (Siluriformes: Loricariidae) from the Amazon basin. Genetica 2008, 134, 243–249. [Google Scholar] [CrossRef]
- Almeida, J.S.; Migues, V.H.; Diniz, D.; Affonso, P.R.A.M. A unique sex chromosome system in the knifefish Gymnotus bahianus with inferences about chromosomal evolution of gymnotidae. J. Hered. 2015, 106, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Favarato, R.M.; Da Silva, M.; De Oliveira, R.R.; Artoni, R.F.; Feldberg, E.; Matoso, D.A. Cytogenetic Diversity and the Evolutionary Dynamics of rDNA Genes and Telomeric Sequences in the Ancistrus Genus (Loricariidae: Ancistrini). Zebrafish 2016, 13, 103–111. [Google Scholar] [CrossRef]
- Bertollo, L.A.C.; Born, G.G.; Dergam, J.A.; Fenocchio, A.S.; Moreira-Filho, O. A biodiversity approach in the neotropical Erythrinidae fish, Hoplias malabaricus. Karyotypic survey, geographic distribution of cytotypes and cytotaxonomic considerations. Chromosom. Res. 2000, 8, 603–613. [Google Scholar] [CrossRef]
- Centofante, L.; Bertollo, L.A.C.; Moreira-Filho, O. Cytogenetic characterization and description of an XX/XY1Y2 sex chromosome system in catfish Harttia carvalhoi (Siluriformes, Loricariidae). Cytogenet. Genome Res. 2006, 112, 320–324. [Google Scholar] [CrossRef]
- Traldi, J.B.; Lui, R.L.; Martinez, J.d.F.; Vicari, M.R.; Nogaroto, V.; Moreira-Filho, O.; Blanco, D.R. Chromosomal distribution of the retroelements Rex1, Rex3 and Rex6 in species of the genus Harttia and Hypostomus (Siluriformes: Loricariidae). Neotrop. Ichthyol. 2019, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]



| Species | 2n | Karyotype | FN 1 | References |
|---|---|---|---|---|
| Harttia absaberi | ♀♂62 | 13m + 23sm + 16st + 10a | 114 | [23] |
| Harttia carvalhoi | 52♀, 53♂ | 16m + 16sm + 12st + 8a ♀ 15m + 16sm + 12st + 10a ♂ | 96 96 | [9] |
| Harttia duriventris | 56♀, 55♂ | 16m + 16sm + 16st + 8a ♀ 17m + 16sm + 16st + 6a ♂ | 104 104 | [11] |
| Harttia gracilis | ♀♂58 | 20m + 22sm + 8st + 8a | 108 | [8] |
| Harttia intermontana | 52♀, 53♂ | 14m + 12sm + 12st + 14a ♀ 13m + 12sm + 13st + 15a ♂ | 90 91 | Present study |
| Harttia kronei | ♀♂58 | 16m + 16sm + 16st + 10a | 106 | [8] |
| Harttia longipinna | ♀♂58 + 0 − 2 Bs | 16m + 12sm + 16st + 14a | 102 | [24] |
| Harttia loricariformis | ♀♂56 | 16m + 22sm + 10st + 8a | 104 | [25] |
| Harttia punctata | 58♀, 57♂ | 16m + 20sm + 12st + 10a ♀ 16m + 21sm + 12st + 8a ♂ | 106 106 | [10] |
| Harttia rondoni | ♀♂54 | 20m + 26sm + 4st + 4a | 104 | [11] |
| Harttia torrenticola | ♀♂56 | 16m + 10sm + 16st + 14a | 98 | [9] |
| Harttia villasboas | 56♀, 55♂ | 18m + 24sm + 6st + 8a ♀ 19m + 24sm + 6st + 6a ♂ | 104 104 | [11] |
| Harttia sp. 1 (Macacos stream) | 56♀, 57♂ | 14m + 14sm + 10st + 18a ♀ 13m + 14sm + 10st + 20a ♂ | 94 94 | Present study |
| Harttia sp. 2 (Barra Grande river) | ♀♂62 | 16m + 14sm + 12st + 20a | 104 | Present study |
| Species | Locality | n |
|---|---|---|
| Piranga river, Carandaí, MG (Brazil) (20°59’34.0″ S, 43°43′30.0″ W) | 20♀, 13♂ |
| Macacos stream, Silveira, SP (Brazil) (22°40’43.0″ S, 44°51′25.0″ W) | 10♀, 7♂ |
| Barra Grande river, Prudentópolis, PR (Brazil) (24°58′40.72″ S, 51°7′34.25″ W) | 17♀, 11♂ |
| Species | 2n | Mechanism of Origin | Reference |
|---|---|---|---|
| Bathydraco marri | 38♀, 39♂ | Y-chromosome fission | [72] |
| Coregonus sardinella | 80♀, 81♂ | Y-chromosome fission | [71] |
| Schistura cf. fasciolata | 50♀, 51♂ | Y-chromosome fission | [73] |
| Hoplias malabaricus (karyomorph G) | 40♀, 41♂ | Tandem fusion X-A | [19,74,78] |
| Gymnotus bahianus | 36♀, 37♂ | Tandem fusion X-A | [76] |
| Ancistrus dubius | 38♀, 39♂ | X-A tandem fusion and further neo-Y chromosome fission | [75,77] |
| Harttia carvalhoi | 52♀, 53♂ | Y-chromosome fission | [8,9,79,80] |
| Harttia intermontana | 52♀, 53♂ | X-A tandem fusion and further neo-Y chromosome fission | Present study |
| Harttia sp. 1 | 56♀, 57♂ | X-A tandem fusion and further neo-Y chromosome fission | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deon, G.A.; Glugoski, L.; Vicari, M.R.; Nogaroto, V.; Sassi, F.d.M.C.; Cioffi, M.d.B.; Liehr, T.; Bertollo, L.A.C.; Moreira-Filho, O. Highly Rearranged Karyotypes and Multiple Sex Chromosome Systems in Armored Catfishes from the Genus Harttia (Teleostei, Siluriformes). Genes 2020, 11, 1366. https://doi.org/10.3390/genes11111366
Deon GA, Glugoski L, Vicari MR, Nogaroto V, Sassi FdMC, Cioffi MdB, Liehr T, Bertollo LAC, Moreira-Filho O. Highly Rearranged Karyotypes and Multiple Sex Chromosome Systems in Armored Catfishes from the Genus Harttia (Teleostei, Siluriformes). Genes. 2020; 11(11):1366. https://doi.org/10.3390/genes11111366
Chicago/Turabian StyleDeon, Geize Aparecida, Larissa Glugoski, Marcelo Ricardo Vicari, Viviane Nogaroto, Francisco de Menezes Cavalcante Sassi, Marcelo de Bello Cioffi, Thomas Liehr, Luiz Antonio Carlos Bertollo, and Orlando Moreira-Filho. 2020. "Highly Rearranged Karyotypes and Multiple Sex Chromosome Systems in Armored Catfishes from the Genus Harttia (Teleostei, Siluriformes)" Genes 11, no. 11: 1366. https://doi.org/10.3390/genes11111366
APA StyleDeon, G. A., Glugoski, L., Vicari, M. R., Nogaroto, V., Sassi, F. d. M. C., Cioffi, M. d. B., Liehr, T., Bertollo, L. A. C., & Moreira-Filho, O. (2020). Highly Rearranged Karyotypes and Multiple Sex Chromosome Systems in Armored Catfishes from the Genus Harttia (Teleostei, Siluriformes). Genes, 11(11), 1366. https://doi.org/10.3390/genes11111366







