Effect of 20(S)-Hydroxycholesterol on Multilineage Differentiation of Mesenchymal Stem Cells Isolated from Compact Bones in Chicken
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Compact Bone-Derived Mesenchymal Stem Cells
2.2. Cell Culture
2.3. Osteogenic Differentiation of cBMSCs
2.4. Adipogenic Differentiation of cBMSCs
2.5. Myogenic Differentiation of cBMSCS
2.6. Adipocyte Differentiation—Oil Red O Stain
2.7. Mineralization of Osteoblasts—Alizarin Red
2.8. Osteogenic Differentiation and Calcium Deposition—Von Kossa Stain
2.9. Quantification of mRNA Expression Using qRT-PCR
2.10. Statistical Analysis
3. Results
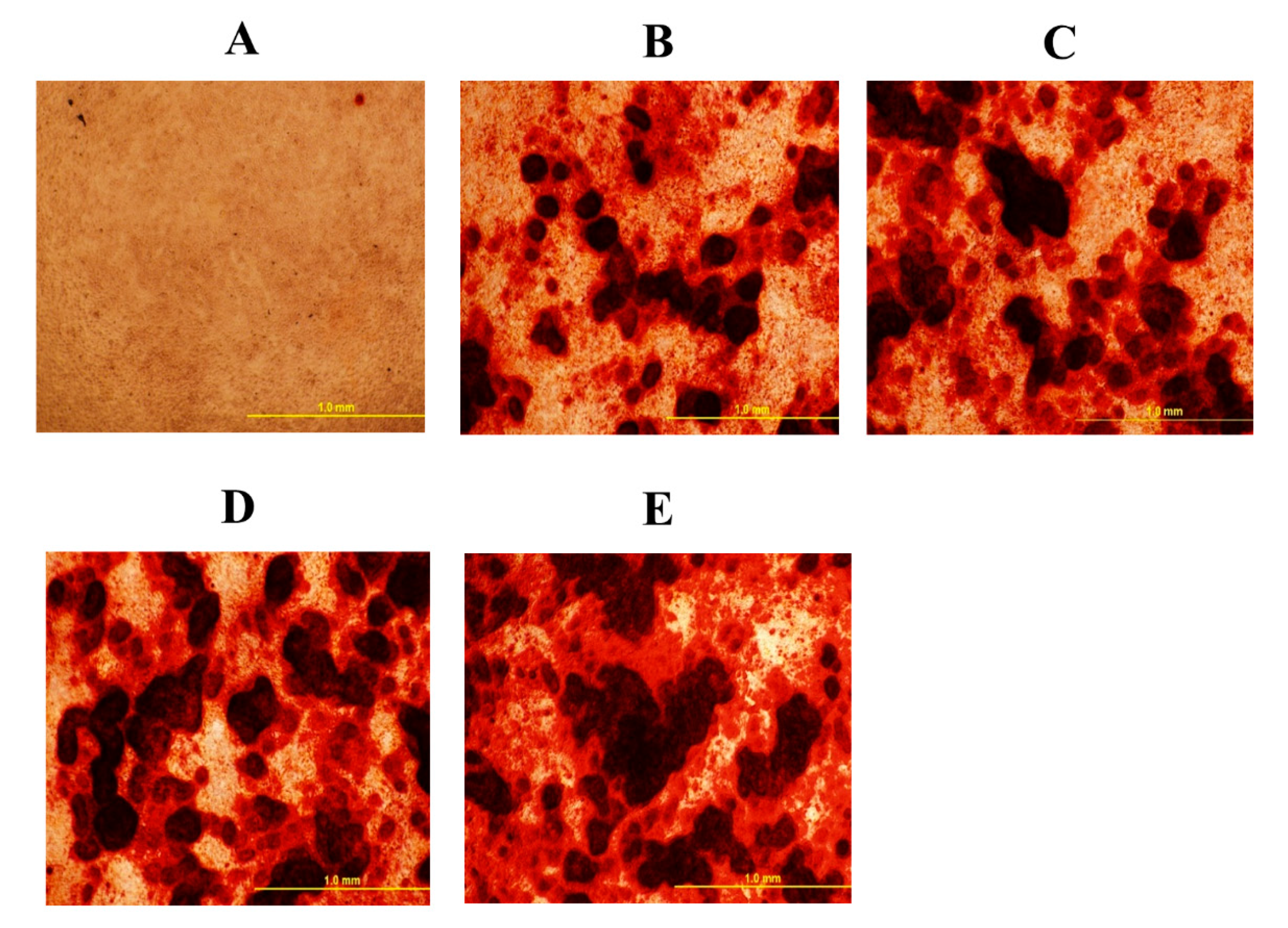
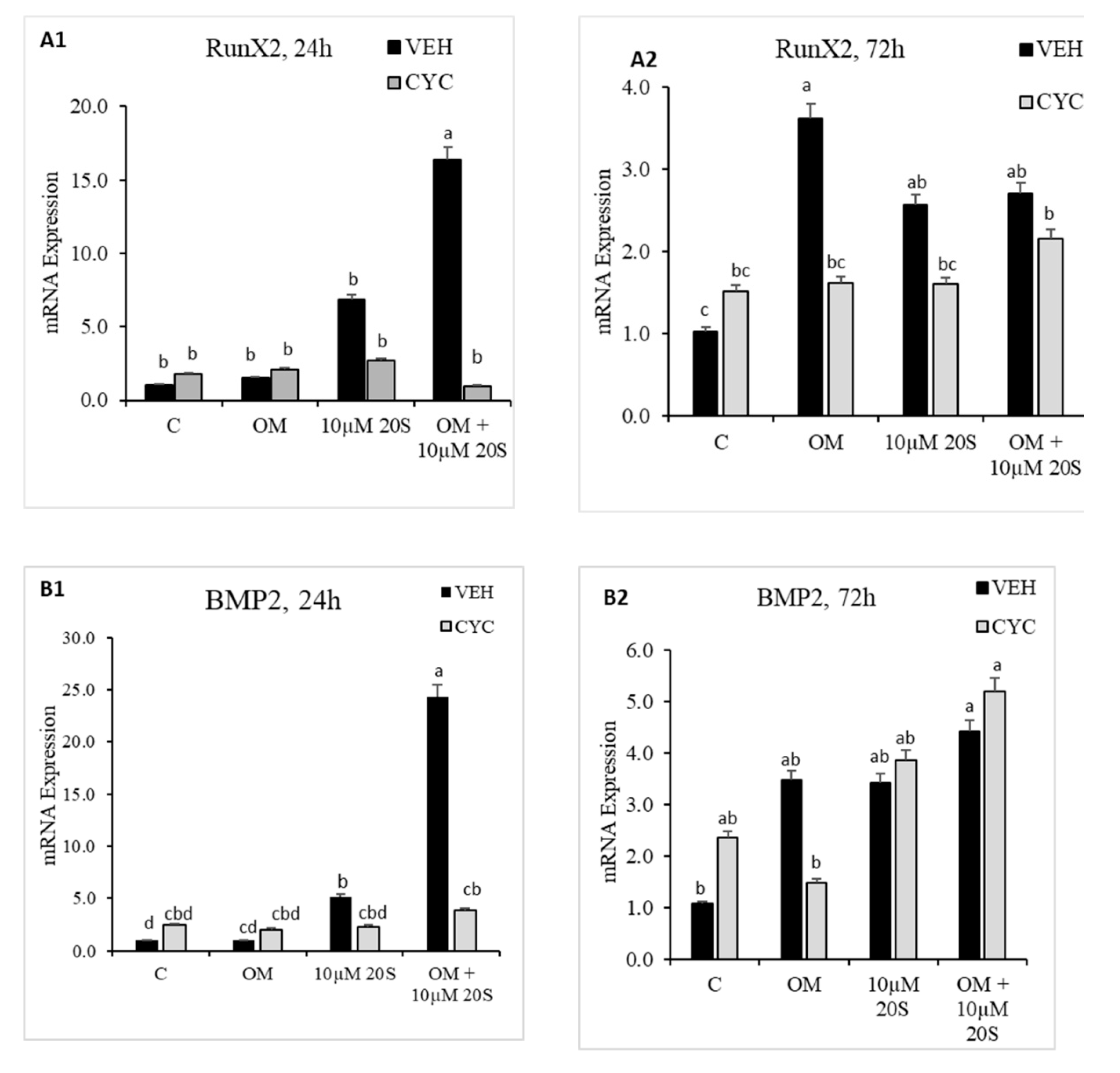
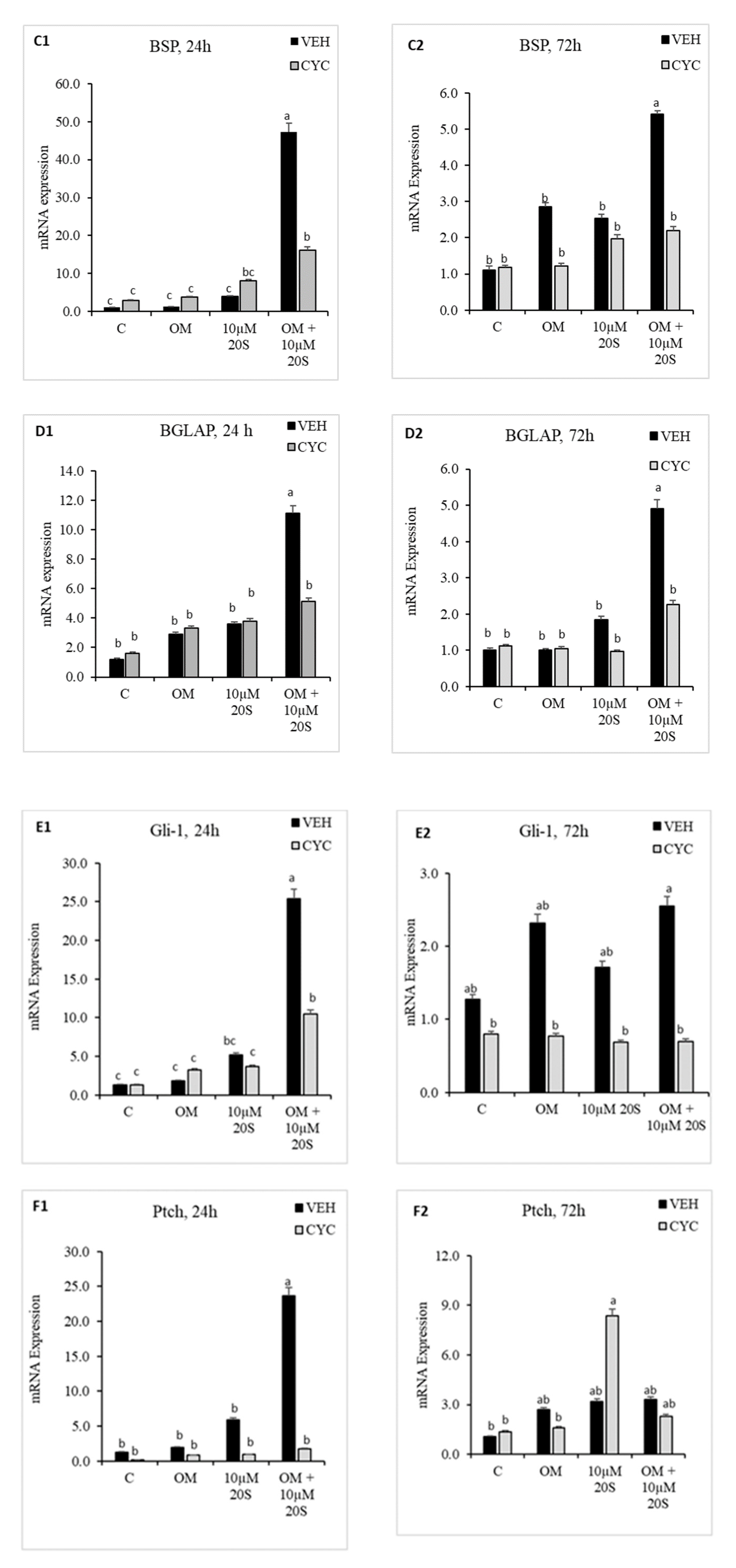
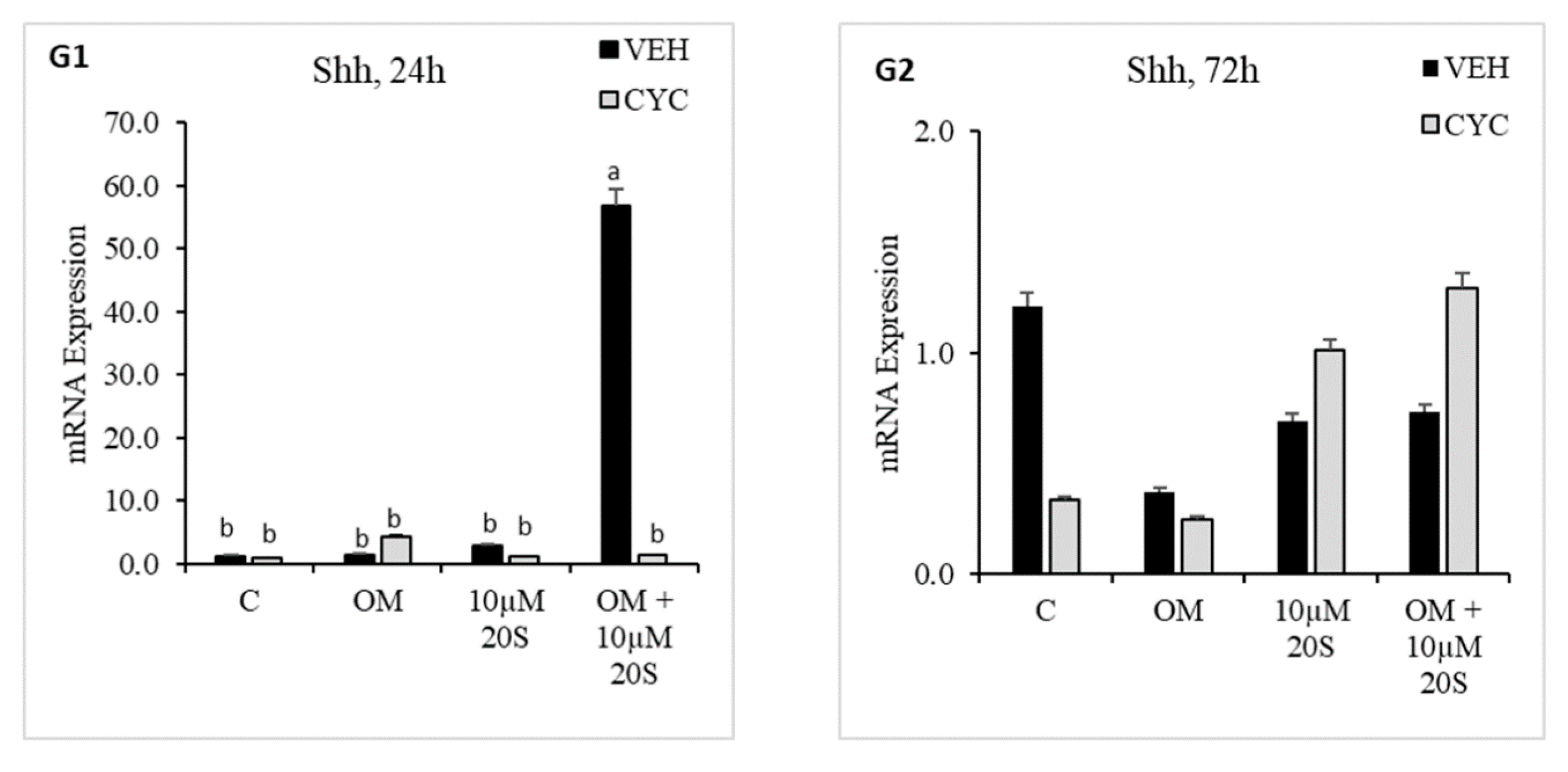
3.1. Oxysterol Induced Osteogenic Differentiation of cBMSCs
3.2. Role of Hh Signaling in Osteogenic Differentiation of cBMSCs
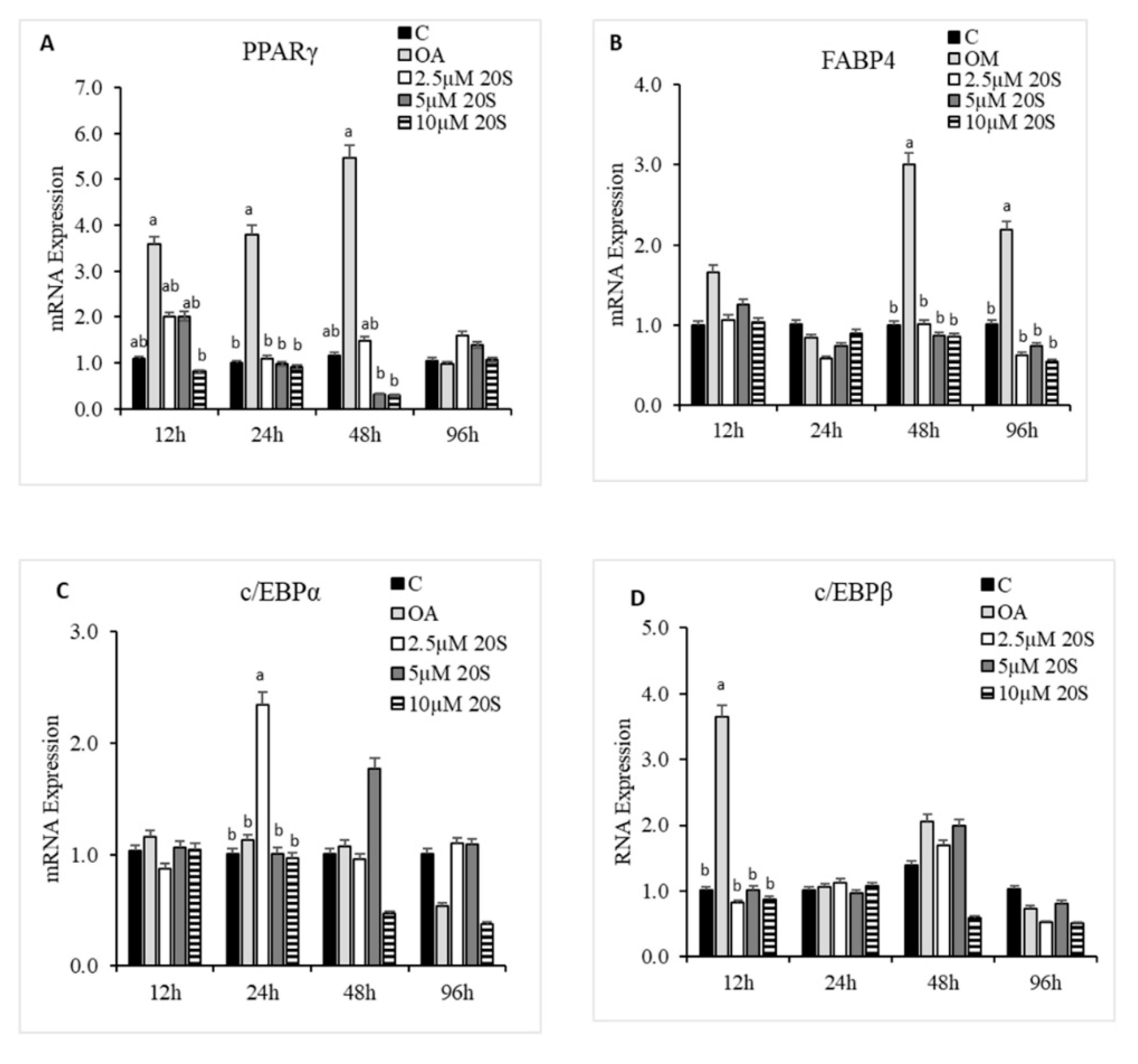
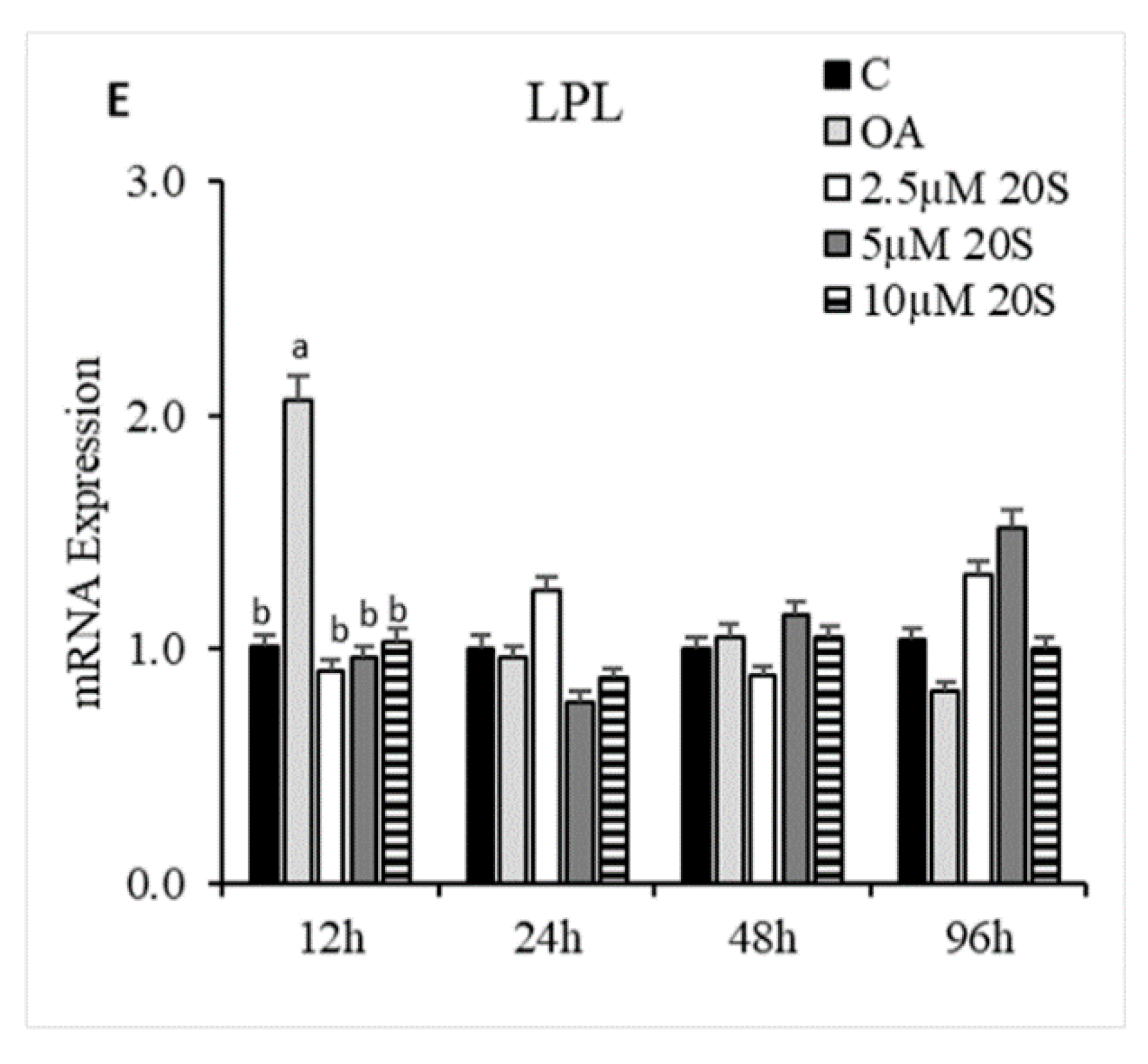
3.3. Effects of 20S on Adipogenic Differentiation of cBMSCs
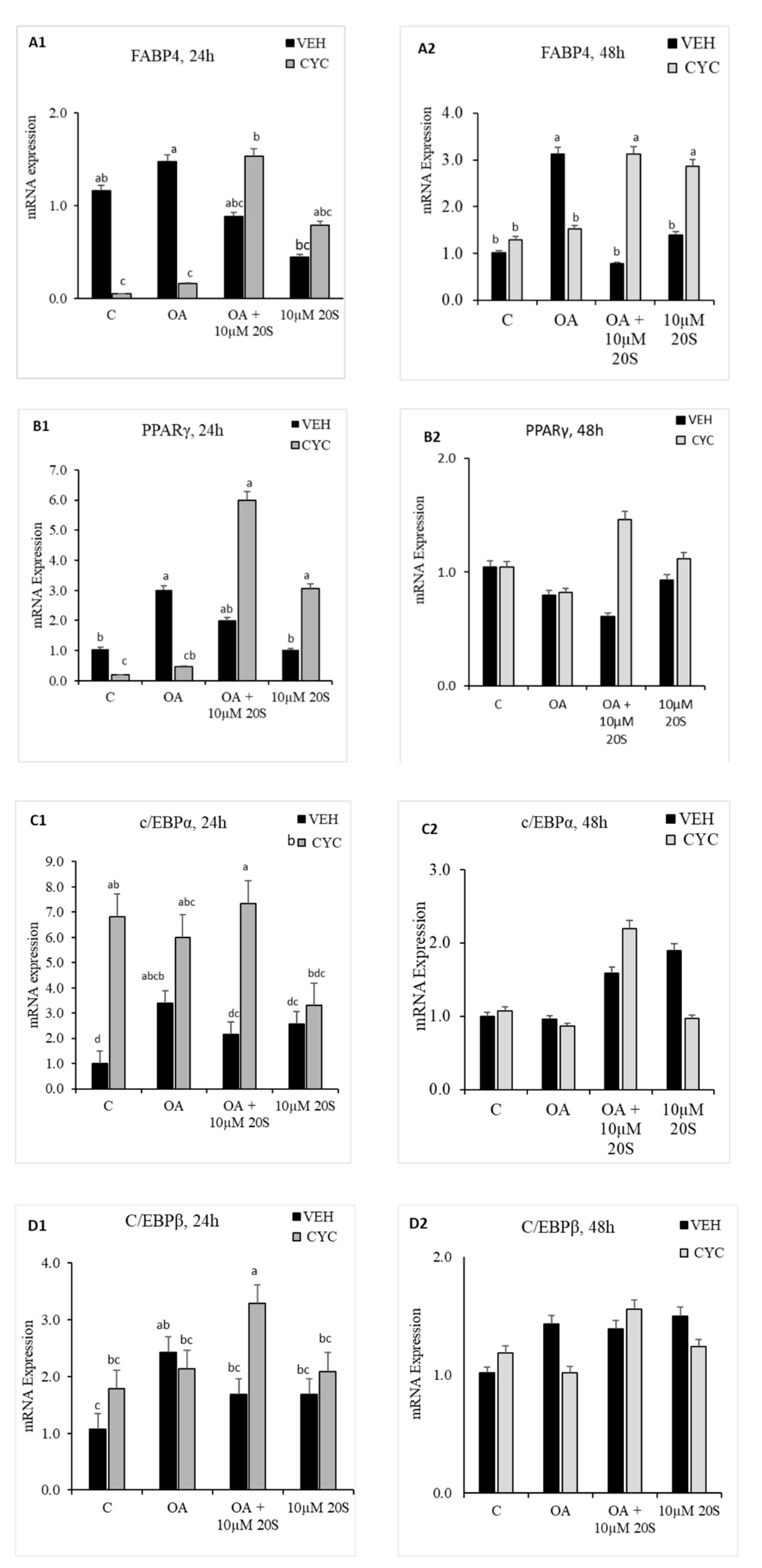
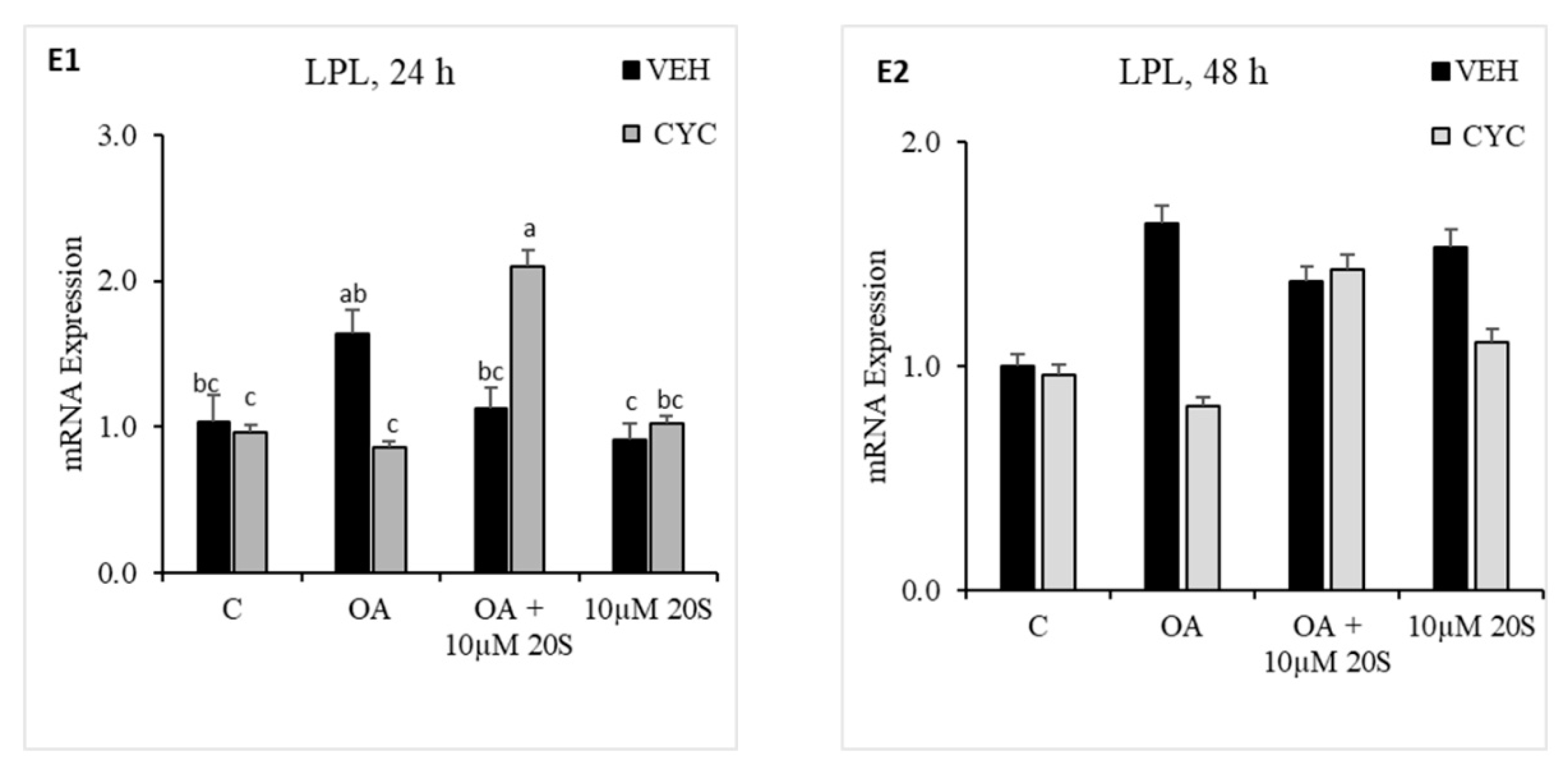
3.4. Role of Hh Signaling Mechanism in Adipogenic Signaling Pathway
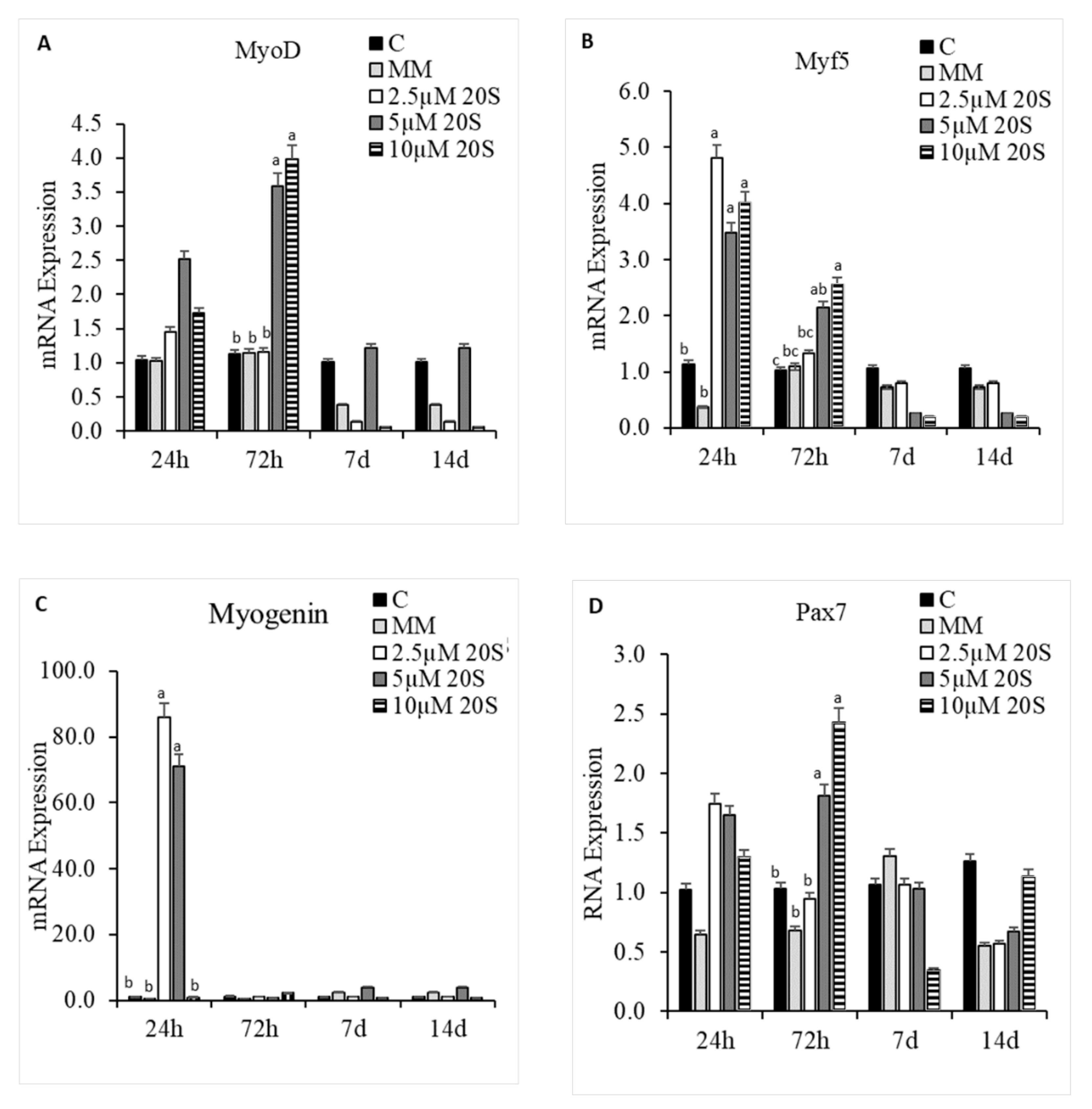
3.5. Effect of 20S in Myogenic Differentiation of cBMSCs
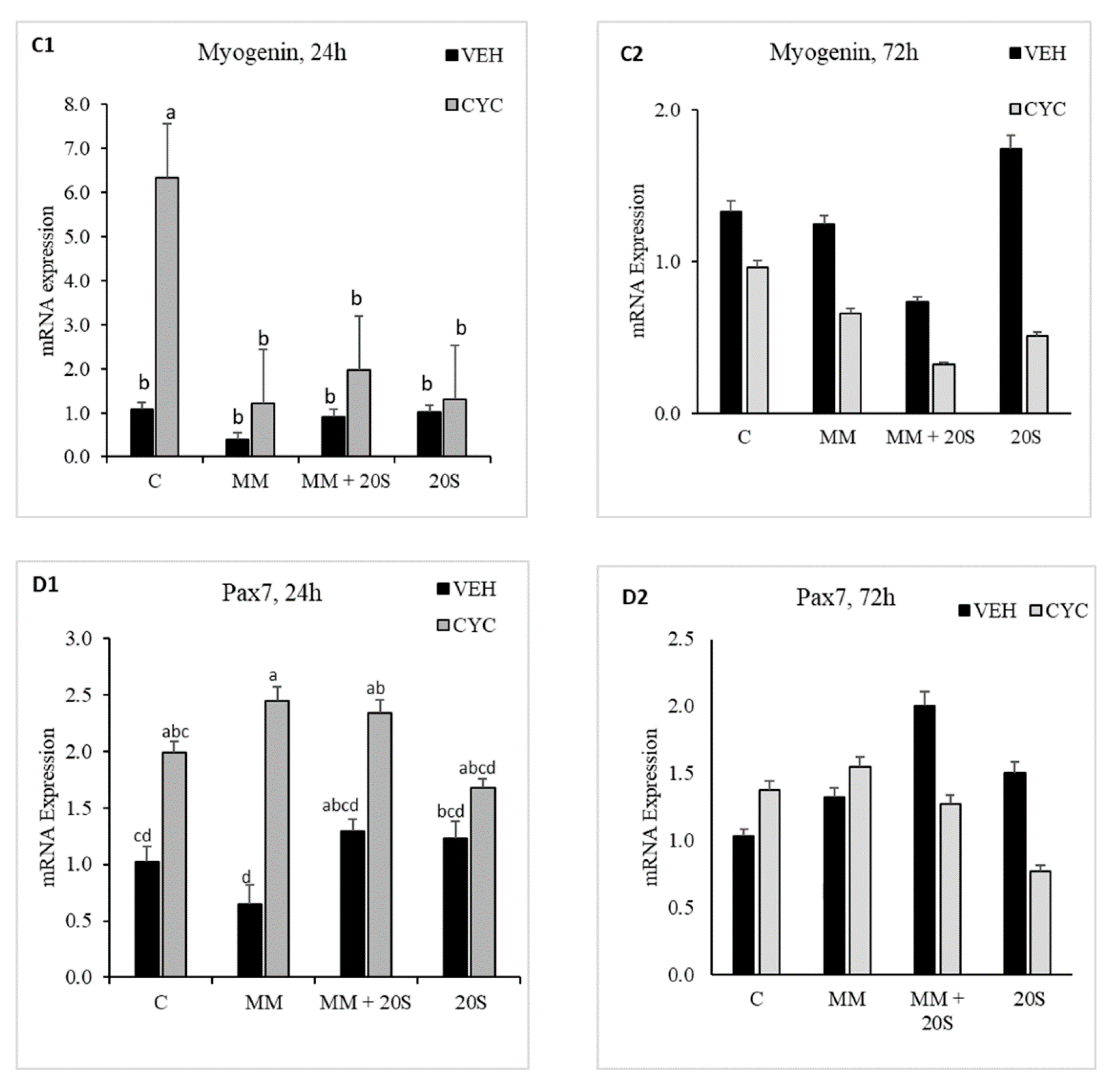
3.6. Role of Hh Signaling Mechanism in Myogenic Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bailey, R.A.; Watson, K.A.; Bilgili, S.F.; Avendano, S. The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poult. Sci. 2015, 94, 2870–2879. [Google Scholar] [CrossRef] [PubMed]
- Julian, R.J. Rapid growth problems: Ascites and skeletal deformities in broilers. Poult. Sci. 1998, 77, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.G.; Kestin, S.C.; Haslam, S.M.; Brown, S.N.; Green, L.E.; Butterworth, A.; Pope, S.J.; Pfeiffer, D.; Nicol, C.J. Leg Disorders in Broiler Chickens: Prevalence, Risk Factors and Prevention. PLoS ONE 2008, 3, e1545. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.J.; Richards, J.D.; Kitchell, M.L.; Quiroz, M.A. Metabolic Challenges and Early Bone Development. J. Appl. Poult. Res. 2007, 16, 126–137. [Google Scholar] [CrossRef]
- Nasr, M.A.; Murrell, J.; Nicol, C.J. The effect of keel fractures on egg production, feed and water consumption in individual laying hens. Br. Poult. Sci 2013, 54, 165–170. [Google Scholar] [CrossRef]
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef]
- Neijat, M.; House, J.D.; Guenter, W.; Kebreab, E. Calcium and phosphorus dynamics in commercial laying hens housed in conventional or enriched cage systems. Poult. Sci. 2011, 90, 2383–2396. [Google Scholar] [CrossRef]
- Kim, W.K.; Bloomfield, S.A.; Sugiyama, T.; Ricke, S.C. Concepts and methods for understanding bone metabolism in laying hens. World’s Poult. Sci. J. 2012, 68, 71–82. [Google Scholar] [CrossRef]
- Whitehead, C.C.; Fleming, R.H. Osteoporosis in cage layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef]
- Regmi, P.; Nelson, N.; Steibel, J.P.; Anderson, K.E.; Karcher, D.M. Comparisons of bone properties and keel deformities between strains and housing systems in end-of-lay hens. Poult. Sci. 2016, 95, 2225–2234. [Google Scholar] [CrossRef]
- Nicol, C.J.; Brown, S.N.; Glen, E.; Pope, S.J.; Short, F.J.; Warriss, P.D.; Zimmerman, P.H.; Wilkins, L.J. Effects of stocking density, flock size and management on the welfare of laying hens in single-tier aviaries. Br. Poult. Sci 2006, 47, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.T.; Guerin, M.T.; Widowski, T.M. On-farm comparison of keel fracture prevalence and other welfare indicators in conventional cage and floor-housed laying hens in Ontario, Canada. Poult. Sci. 2015, 94, 579–585. [Google Scholar] [CrossRef]
- Heerkens, J.L.; Delezie, E.; Rodenburg, T.B.; Kempen, I.; Zoons, J.; Ampe, B.; Tuyttens, F.A. Risk factors associated with keel bone and foot pad disorders in laying hens housed in aviary systems. Poult. Sci. 2016, 95, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.K.; Duque, G. Age-related bone loss: Old bone, new facts. Gerontology 2002, 48, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, M.E.; Gimble, J.M. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 2000, 27, 177–184. [Google Scholar] [CrossRef]
- Stenderup, K.; Justesen, J.; Clausen, C.; Kassem, M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003, 33, 919–926. [Google Scholar] [CrossRef]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef]
- Takada, I.; Kouzmenko, A.P.; Kato, S. Molecular switching of osteoblastogenesis versus adipogenesis: Implications for targeted therapies. Expert Opin. Ther. Targets 2009, 13, 593–603. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef]
- Bellotti, C.; Duchi, S.; Bevilacqua, A.; Lucarelli, E.; Piccinini, F. Long term morphological characterization of mesenchymal stromal cells 3D spheroids built with a rapid method based on entry-level equipment. Cytotechnology 2016, 68, 2479–2490. [Google Scholar] [CrossRef]
- Sanjurjo-Rodriguez, C.; Castro-Vinuelas, R.; Hermida-Gomez, T.; Fernandez-Vazquez, T.; Fuentes-Boquete, I.M.; de Toro-Santos, F.J.; Diaz-Prado, S.M.; Blanco-Garcia, F.J. Ovine Mesenchymal Stromal Cells: Morphologic, Phenotypic and Functional Characterization for Osteochondral Tissue Engineering. PLoS ONE 2017, 12, e0171231. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef]
- Fellows, C.R.; Matta, C.; Zakany, R.; Khan, I.M.; Mobasheri, A. Adipose, Bone Marrow and Synovial Joint-Derived Mesenchymal Stem Cells for Cartilage Repair. Front. Genet. 2016, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Floerkemeier, T.; Melzer, C.; Hass, R. Comparison of in vitro-cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. J. Tissue Eng. Regen. Med. 2017, 11, 2565–2581. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Oreffo, R.O.; Triffitt, J.T. Interconversion potential of cloned human marrow adipocytes in vitro. Bone 1999, 24, 549–554. [Google Scholar] [CrossRef]
- Kassem, M.; Abdallah, B.M.; Saeed, H. Osteoblastic cells: Differentiation and trans-differentiation. Arch. Biochem. Biophys. 2008, 473, 183–187. [Google Scholar] [CrossRef]
- Jilka, R.L.; Weinstein, R.S.; Takahashi, K.; Parfitt, A.M.; Manolagas, S.C. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J. Clin. Investig. 1996, 97, 1732–1740. [Google Scholar] [CrossRef]
- Nuttall, M.E.; Patton, A.J.; Olivera, D.L.; Nadeau, D.P.; Gowen, M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: Implications for osteopenic disorders. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1998, 13, 371–382. [Google Scholar] [CrossRef]
- Singh, L.; Brennan, T.A.; Russell, E.; Kim, J.H.; Chen, Q.; Brad Johnson, F.; Pignolo, R.J. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone 2016, 85, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; O’Brien, T.D.; Goyal, S.M.; Sharma, J.M. Isolation and characterization of chicken lung mesenchymal stromal cells and their susceptibility to avian influenza virus. Dev. Comp. Immunol. 2010, 34, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; O’Brien, T.D.; Sharma, J.M. Isolation and differentiation of chicken mesenchymal stem cells from bone marrow. Stem. Cells Dev. 2009, 18, 1485–1492. [Google Scholar] [CrossRef]
- Li, L.; Ma, Y.; Li, X.; Li, X.; Bai, C.; Ji, M.; Zhang, S.; Guan, W.; Li, J. Isolation, Culture, and Characterization of Chicken Cartilage Stem/Progenitor Cells. Biomed. Res. Int. 2015, 2015, 586290. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Hou, L.; Ma, Y.; Chen, L.; Zhang, M.; Guan, W. Isolation and characterization of mesenchymal stem cells from chicken bone marrow. Cell Tissue Bank. 2013, 14, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Hou, L.; Bai, C.; Jin, D.; He, X.; Guan, W.; Ma, Y. Isolation and biological characteristics of chicken adipose-derived progenitor cells. DNA Cell Biol. 2011, 30, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Bjorkhem, I.; Meaney, S.; Diczfalusy, U. Oxysterols in human circulation: Which role do they have? Curr. Opin. Lipidol. 2002, 13, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Meliton, V.; Amantea, C.M.; Hahn, T.J.; Parhami, F. 20(S)-hydroxycholesterol inhibits PPARgamma expression and adipogenic differentiation of bone marrow stromal cells through a hedgehog-dependent mechanism. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Kha, H.T.; Basseri, B.; Shouhed, D.; Richardson, J.; Tetradis, S.; Hahn, T.J.; Parhami, F. Oxysterols regulate differentiation of mesenchymal stem cells: Pro-bone and anti-fat. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Meliton, V.; Tetradis, S.; Weinmaster, G.; Hahn, T.J.; Carlson, M.; Nelson, S.F.; Parhami, F. Osteogenic Oxysterol, 20(S)-Hydroxycholesterol, Induces Notch Target Gene Expression in Bone Marrow Stromal Cells. J. Bone Miner. Res. 2010, 25, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Amantea, C.M.; Cowan, C.M.; Richardson, J.A.; Wu, B.M.; Parhami, F.; Tetradis, S. Oxysterols enhance osteoblast differentiation in vitro and bone healing in vivo. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2007, 25, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F.; Mody, N.; Gharavi, N.; Ballard, A.J.; Tintut, Y.; Demer, L.L. Role of the cholesterol biosynthetic pathway in osteoblastic differentiation of marrow stromal cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002, 17, 1997–2003. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.K.; Jiang, X.X.; Li, H.; Wang, X.Y.; Yao, H.Y.; Zhang, Y.; Mao, N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 2010, 5, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F.; Jackson, S.M.; Tintut, Y.; Le, V.; Balucan, J.P.; Territo, M.; Demer, L.L. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1999, 14, 2067–2078. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef]
- Regassa, A.; Kim, W.K. Effects of oleic acid and chicken serum on the expression of adipogenic transcription factors and adipogenic differentiation in hen preadipocytes. Cell Biol. Int. 2013, 37, 961–971. [Google Scholar] [CrossRef]
- Sławińska, A.; Brzezińska, J.; Siwek, M.; Elminowska-Wenda, G. Expression of myogenic genes in chickens stimulated in ovo with light and temperature. Reprod. Biol. 2013, 13, 161–165. [Google Scholar] [CrossRef]
- Freeman, B.T.; Jung, J.P.; Ogle, B.M. Single-Cell RNA-Seq of Bone Marrow-Derived Mesenchymal Stem Cells Reveals Unique Profiles of Lineage Priming. PLoS ONE 2015, 10, e0136199. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Hokugo, A.; Segovia, L.A.; Yalom, A.; Rezzadeh, K.; Zhou, S.; Zhang, Z.; Parhami, F.; Stappenbeck, F.; Jarrahy, R. Oxy133, a novel osteogenic agent, promotes bone regeneration in an intramembranous bone-healing model. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.J.; Lee, K.Y.; Choi, N.S.; Lee, M.H.; Kim, H.N.; Jin, Y.H.; Ryoo, H.M.; Choi, J.Y.; Yoshida, M.; Nishino, N.; et al. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J. Biol. Chem. 2006, 281, 16502–16511. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Dwyer, J.R.; Sever, N.; Carlson, M.; Nelson, S.F.; Beachy, P.A.; Parhami, F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem. 2007, 282, 8959–8968. [Google Scholar] [CrossRef]
- Richardson, J.A.; Amantea, C.M.; Kianmahd, B.; Tetradis, S.; Lieberman, J.R.; Hahn, T.J.; Parhami, F. Oxysterol-induced osteoblastic differentiation of pluripotent mesenchymal cells is mediated through a PKC- and PKA-dependent pathway. J. Cell. Biochem. 2007, 100, 1131–1145. [Google Scholar] [CrossRef]
- Montgomery, S.R.; Nargizyan, T.; Meliton, V.; Nachtergaele, S.; Rohatgi, R.; Stappenbeck, F.; Jung, M.E.; Johnson, J.S.; Aghdasi, B.; Tian, H.; et al. A Novel Osteogenic Oxysterol Compound for Therapeutic Development to Promote Bone Growth: Activation of Hedgehog Signaling and Osteogenesis through Smoothened Binding. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 1872–1885. [Google Scholar] [CrossRef]
- Hokugo, A.; Sorice, S.; Yalom, A.; Lee, J.C.; Li, A.; Zuk, P.; Jarrahy, R. In vitro study of a novel oxysterol for osteogenic differentiation on rabbit bone marrow stromal cells. Plast. Reconstr. Surg. 2013, 132, 70e–80e. [Google Scholar] [CrossRef]
- Matsubara, Y.; Endo, T.; Kano, K. Fatty acids but not dexamethasone are essential inducers for chick adipocyte differentiation in vitro. Comp. Biochem. Physiol. Part. AMol. Integr. Physiol. 2008, 151, 511–518. [Google Scholar] [CrossRef]
- Matsubara, Y.; Sato, K.; Ishii, H.; Akiba, Y. Changes in mRNA expression of regulatory factors involved in adipocyte differentiation during fatty acid induced adipogenesis in chicken. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 108–115. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Graves, R.A.; Budavari, A.I.; Spiegelman, B.M. mPPAR γ 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.I.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakamura, K.; et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 2004, 113, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kim, J.K.; Mortensen, R.; Mutyaba, L.P.; Hankenson, K.D.; Krebsbach, P.H. Osteoblast-targeted suppression of PPARgamma increases osteogenesis through activation of mTOR signaling. Stem Cells (Dayt. Ohio) 2013, 31, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Lecka-Czernik, B.; Gubrij, I.; Moerman, E.J.; Kajkenova, O.; Lipschitz, D.A.; Manolagas, S.C.; Jilka, R.L. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J. Cell. Biochem. 1999, 74, 357–371. [Google Scholar] [CrossRef]
- Spinella-Jaegle, S.; Rawadi, G.; Kawai, S.; Gallea, S.; Faucheu, C.; Mollat, P.; Courtois, B.; Bergaud, B.; Ramez, V.; Blanchet, A.M.; et al. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J. Cell Sci. 2001, 114, 2085–2094. [Google Scholar] [PubMed]
- Gupta, S.; Takebe, N.; LoRusso, P. Targeting the Hedgehog pathway in cancer. Ther. Adv. Med. Oncol. 2010, 2, 237–250. [Google Scholar] [CrossRef]
- Chiang, C.; Litingtung, Y.; Lee, E.; Young, K.E.; Corden, J.L.; Westphal, H.; Beachy, P.A. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 1996, 383, 407–413. [Google Scholar] [CrossRef]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef]
- Nakamura, T.; Aikawa, T.; Iwamoto-Enomoto, M.; Iwamoto, M.; Higuchi, Y.; Pacifici, M.; Kinto, N.; Yamaguchi, A.; Noji, S.; Kurisu, K.; et al. Induction of osteogenic differentiation by hedgehog proteins. Biochem. Biophys. Res. Commun. 1997, 237, 465–469. [Google Scholar] [CrossRef]
- Wang, C.; Shan, S.; Wang, C.; Wang, J.; Li, J.; Hu, G.; Dai, K.; Li, Q.; Zhang, X. Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp. Cell Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, T.; Kataoka, H.; Kinto, N.; Iwamoto, M.; Enomoto-Iwamoto, M.; Iemura, S.; Ueno, N.; Shibata, Y.; Kurosawa, H.; Yamaguchi, A. Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J. Cell. Physiol. 2002, 193, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.X.; Du, S.X.; Liu, D.Z.; Hu, Q.X.; Yu, G.Y.; Wu, C.C.; Zheng, G.Z.; Xie, D.; Li, X.D.; Chang, B. Naringin promotes osteogenic differentiation of bone marrow stromal cells by up-regulating Foxc2 expression via the IHH signaling pathway. Am. J. Transl Res. 2016, 8, 5098–5107. [Google Scholar]
- Johnson, J.S.; Meliton, V.; Kim, W.K.; Lee, K.B.; Wang, J.C.; Nguyen, K.; Yoo, D.; Jung, M.E.; Atti, E.; Tetradis, S.; et al. Novel oxysterols have pro-osteogenic and anti-adipogenic effects in vitro and induce spinal fusion in vivo. J. Cell. Biochem. 2011, 112, 1673–1684. [Google Scholar] [CrossRef]
- Hokugo, A.; Sorice, S.; Parhami, F.; Yalom, A.; Li, A.; Zuk, P.; Jarrahy, R. A novel oxysterol promotes bone regeneration in rabbit cranial bone defects. J. Tissue Eng. Regen. Med. 2016, 10, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Amantea, C.M.; Kim, W.-K.; Meliton, V.; Tetradis, S.; Parhami, F. Oxysterol-Induced Osteogenic Differentiation of Marrow Stromal Cells is Regulated by Dkk-1 Inhibitable and PI3-Kinase Mediated Signaling. J. Cell. Biochem. 2008, 105, 424–436. [Google Scholar] [CrossRef]
- Suh, J.M.; Gao, X.; McKay, J.; McKay, R.; Salo, Z.; Graff, J.M. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006, 3, 25–34. [Google Scholar] [CrossRef]
- Song, L.; Tuan, R.S. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 980–982. [Google Scholar] [CrossRef]
- Hong, J.-H.; Hwang, E.S.; McManus, M.T.; Amsterdam, A.; Tian, Y.; Kalmukova, R.; Mueller, E.; Benjamin, T.; Spiegelman, B.M.; Sharp, P.A.; et al. TAZ, a Transcriptional Modulator of Mesenchymal Stem Cell Differentiation. Science 2005, 309, 1074. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Jensen, C.H.; Gutierrez, G.; Leslie, R.G.; Jensen, T.G.; Kassem, M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 841–852. [Google Scholar] [CrossRef]
- Gang, E.J.; Jeong, J.A.; Hong, S.H.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells (Dayt. Ohio) 2004, 22, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Arnold, H.H. Myf-5 and myoD genes are activated in distinct mesenchymal stem cells and determine different skeletal muscle cell lineages. EMBO J. 1996, 15, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Gang, E.J.; Bosnakovski, D.; Simsek, T.; To, K.; Perlingeiro, R.C. Pax3 activation promotes the differentiation of mesenchymal stem cells toward the myogenic lineage. Exp. Cell Res. 2008, 314, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Sabourin, L.A.; Rudnicki, M.A. The molecular regulation of myogenesis. Clin. Genet. 2000, 57, 16–25. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Vandenabeele, F.; Vermeesch, J.R.; Raymackers, J.-M.; Luyten, F.P. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J. Cell Biol. 2003, 160, 909–918. [Google Scholar] [CrossRef]
- Jalali Tehrani, H.; Parivar, K.; Ai, J.; Kajbafzadeh, A.; Rahbarghazi, R.; Hashemi, M.; Sadeghizadeh, M. Effect of Dexamethasone, Insulin and EGF on the Myogenic Potential on Human Endometrial Stem Cell. Iran. J. Pharm. Res. IJPR 2014, 13, 659–664. [Google Scholar]
- Yablonka-Reuveni, Z.; Day, K.; Vine, A.; Shefer, G. Defining the transcriptional signature of skeletal muscle stem cells. J. Anim. Sci. 2008, 86, E207–E216. [Google Scholar] [CrossRef]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef]
- Lindon, C.; Montarras, D.; Pinset, C. Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J. Cell Biol. 1998, 140, 111–118. [Google Scholar] [CrossRef]
- Zammit, P.S.; Partridge, T.A.; Yablonka-Reuveni, Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2006, 54, 1177–1191. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer Sequence (5′-3′) | Product Length (bp) | Annealing Temperature (°C) | Accession # |
|---|---|---|---|---|
| GAPDH | Fwd: GCTAAGGCTGTGGGGAAAGT | 161 | 55 | [49] |
| Rev: TCAGCAGCAGCCTTCACTAC | ||||
| BSP | Fwd: CAGTGGGAGTACGAGGTGAC | 141 | 55 | NM_205162.1 |
| Rev: CAGTGGGAGTACGAGGTGAC | ||||
| Gli1 | Fwd: GCACAGCTCCAACGACCGCT | 205 | 57 | NM_001305245.1 |
| Rev: GTTGCCGTCGGAAGCACCCA | ||||
| BMP2 | Fwd: TCAGCTCAGGCCGTTGTTAG | 163 | 57 | NM_204358.1 |
| Rev: GTCATTCCACCCCACGTCAT | ||||
| BGLAP | Fwd: GACGGCTCGGATGCTCGCAG | 226 | 56 | [49] |
| Rev: CAGACGGGGCCGTAGAAGCG | ||||
| Ptch | Fwd: GGCGTTCGCGGTGGGACTAC | 205 | 56 | NM_204960.2 |
| Rev: GGTGCTGCCGGAGTGCTTCT | ||||
| Shh | Fwd: TGC TAG GGA TCG GTG GAT AG | 197 | 56 | NM_204821.1 |
| Rev: ACA AGT CAG CCC AGA GGA GA | ||||
| RunX2 | Fwd: ACTTTGACAATAACTGTCCT | 192 | 52 | NM_204821.1 |
| Rev: GACCCCTACTCTCATACTGG | ||||
| FABP4 | Fwd: TGCTGGGCATCTCAATCACA | 106 | 57 | [49] |
| Rev: GCATTAGTCAGAACGGGCCT | ||||
| PPARγ | Fwd: TGAATGTCGTGTGTGTGGGG | 229 | 55 | [49] |
| Rev: GCATTCGCCCAAACCTGATG | ||||
| C/EBPα | Fwd: CCTACGGCTACAGAGAGGCT | 205 | 55 | [49] |
| Rev: GAAATCGAAATCCCCGGCCA | ||||
| C/EBPβ | Fwd: CCGCTCCATGACCGAACTTA | 204 | 55 | [49] |
| Rev: GCCGCTGCCTTTATAGTCCT | ||||
| LPL | Fwd: TGCCCCTATCCGCCTCTCCC | 297 | 57 | [49] |
| Rev: GTTGCAGCGGTAGGCCATGCT | ||||
| Col1A2 | Fwd: AGAAAGGAATCCAGCCCAAT | 237 | 58 | NM_204426.1 |
| Rev: ACACCTGCCAGATTGATTCC | ||||
| MyoD | Fwd: CAGCAGCTACTACACGGAATCA | 102 | 57 | [50] |
| Rev: GGAAATCCTCTCCACAATGCTT | ||||
| Myogenin | Fwd: AGCAGCCTCAACCAGCAGGA | 179 | 58 | NM_204184.1 |
| Rev: TCTGCCTGGTCATCGCTCAG | ||||
| Pax7 | Fwd: AGGCTGACTTCTCCATCTCTCCT | 156 | 57 | XM_015296832.1 |
| Rev: TGTAACTGGTGGTGCTGTAGGTG | ||||
| Myf5 | Fwd: GAGGAACGCCATCAGGTACATC | 126 | 57 | NM_001030363.1 |
| Rev: ACATCGGAGCAGCTGGAGCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, R.; Chen, C.; Kim, W.K. Effect of 20(S)-Hydroxycholesterol on Multilineage Differentiation of Mesenchymal Stem Cells Isolated from Compact Bones in Chicken. Genes 2020, 11, 1360. https://doi.org/10.3390/genes11111360
Adhikari R, Chen C, Kim WK. Effect of 20(S)-Hydroxycholesterol on Multilineage Differentiation of Mesenchymal Stem Cells Isolated from Compact Bones in Chicken. Genes. 2020; 11(11):1360. https://doi.org/10.3390/genes11111360
Chicago/Turabian StyleAdhikari, Roshan, Chongxiao Chen, and Woo Kyun Kim. 2020. "Effect of 20(S)-Hydroxycholesterol on Multilineage Differentiation of Mesenchymal Stem Cells Isolated from Compact Bones in Chicken" Genes 11, no. 11: 1360. https://doi.org/10.3390/genes11111360
APA StyleAdhikari, R., Chen, C., & Kim, W. K. (2020). Effect of 20(S)-Hydroxycholesterol on Multilineage Differentiation of Mesenchymal Stem Cells Isolated from Compact Bones in Chicken. Genes, 11(11), 1360. https://doi.org/10.3390/genes11111360






