Exploring the Role of Contactins across Psychological, Psychiatric and Cardiometabolic Traits within UK Biobank
Abstract
1. Introduction
2. Materials and Methods
2.1. Genes
2.2. Cohort
2.3. Genotyping
2.4. Phenotypes
2.5. Genetic Analyses
2.6. Data Mining
3. Results
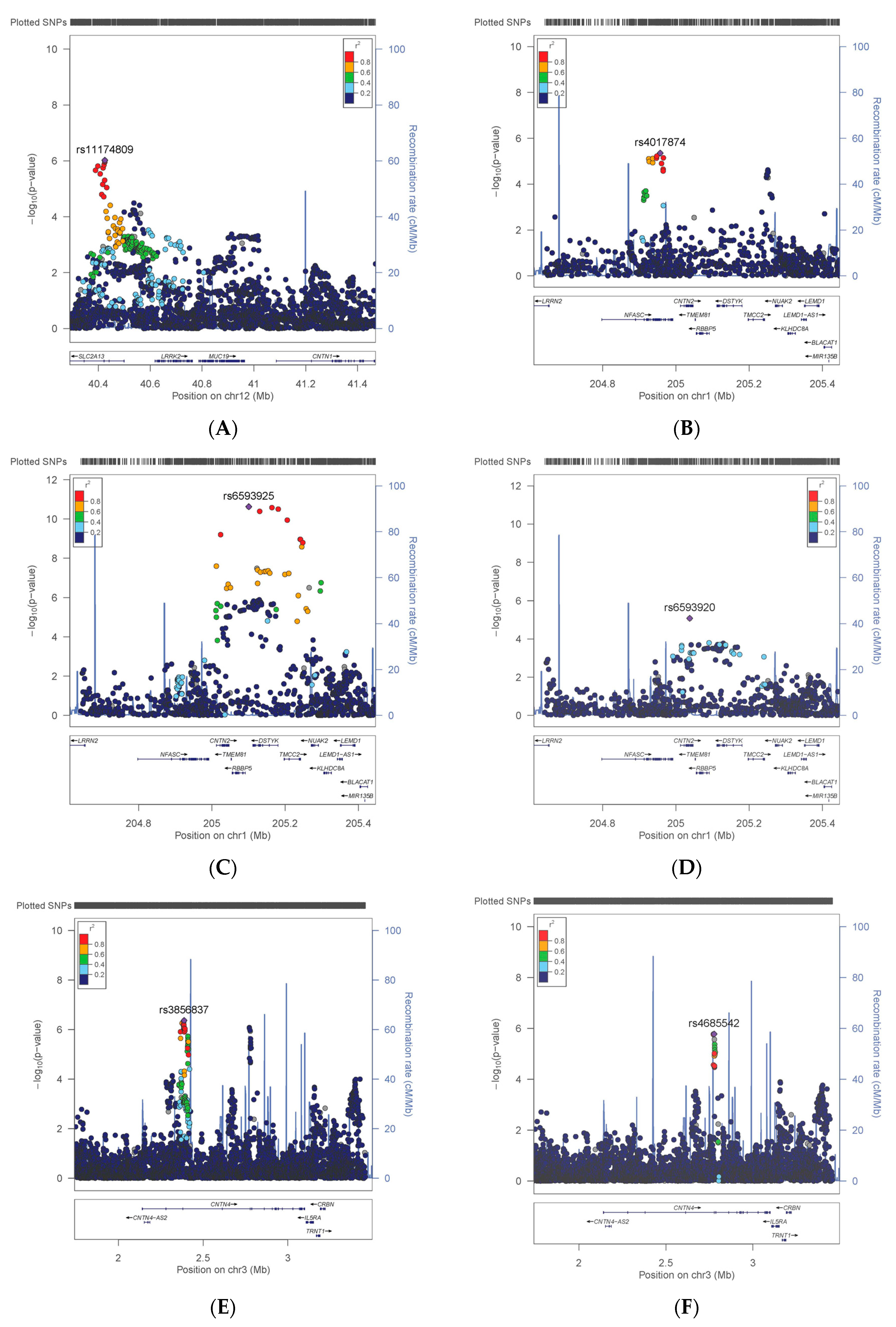
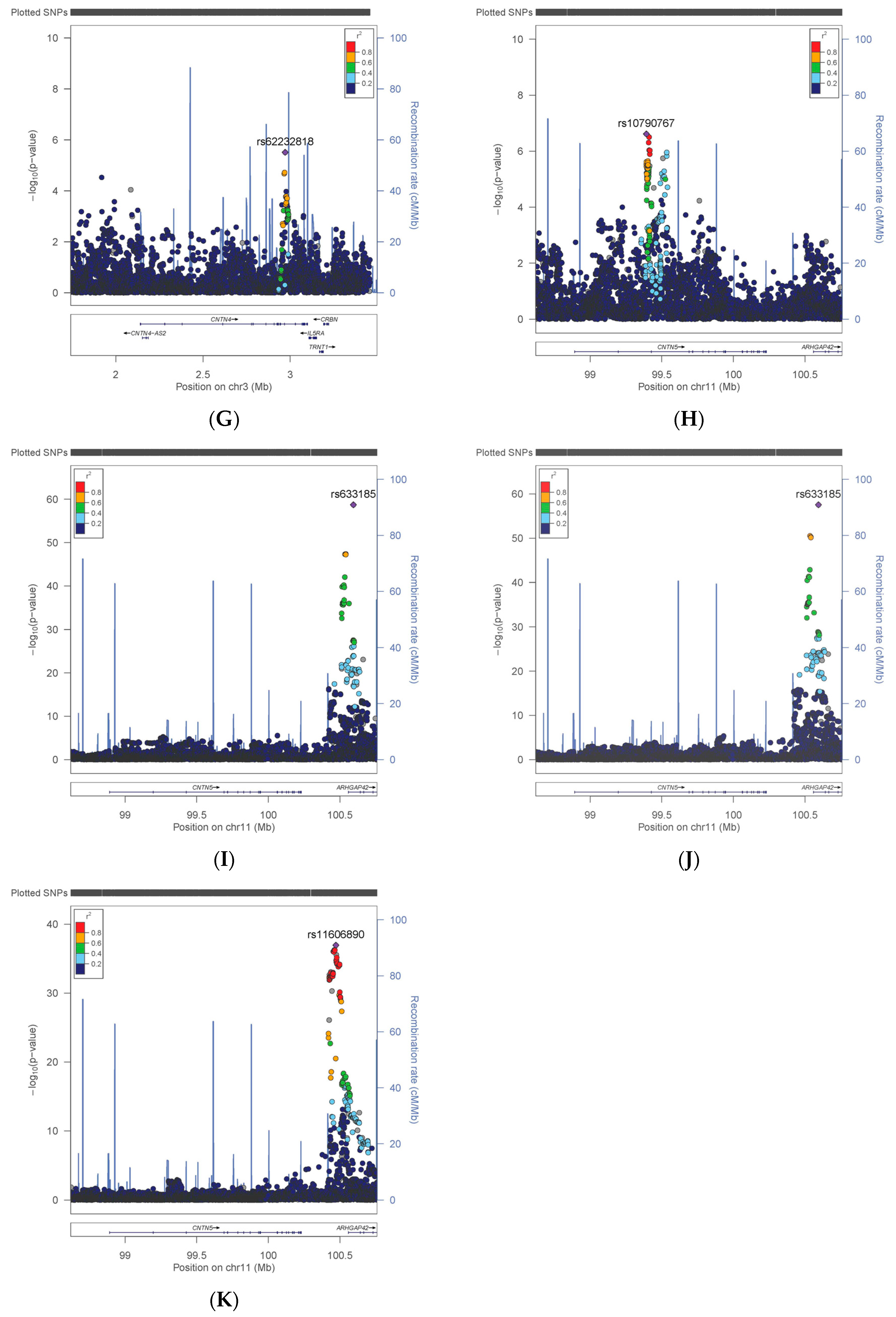
3.1. Variation in CNTN Genes
3.2. Comparison with Previous Findings
3.3. Effect Prediction in VEP
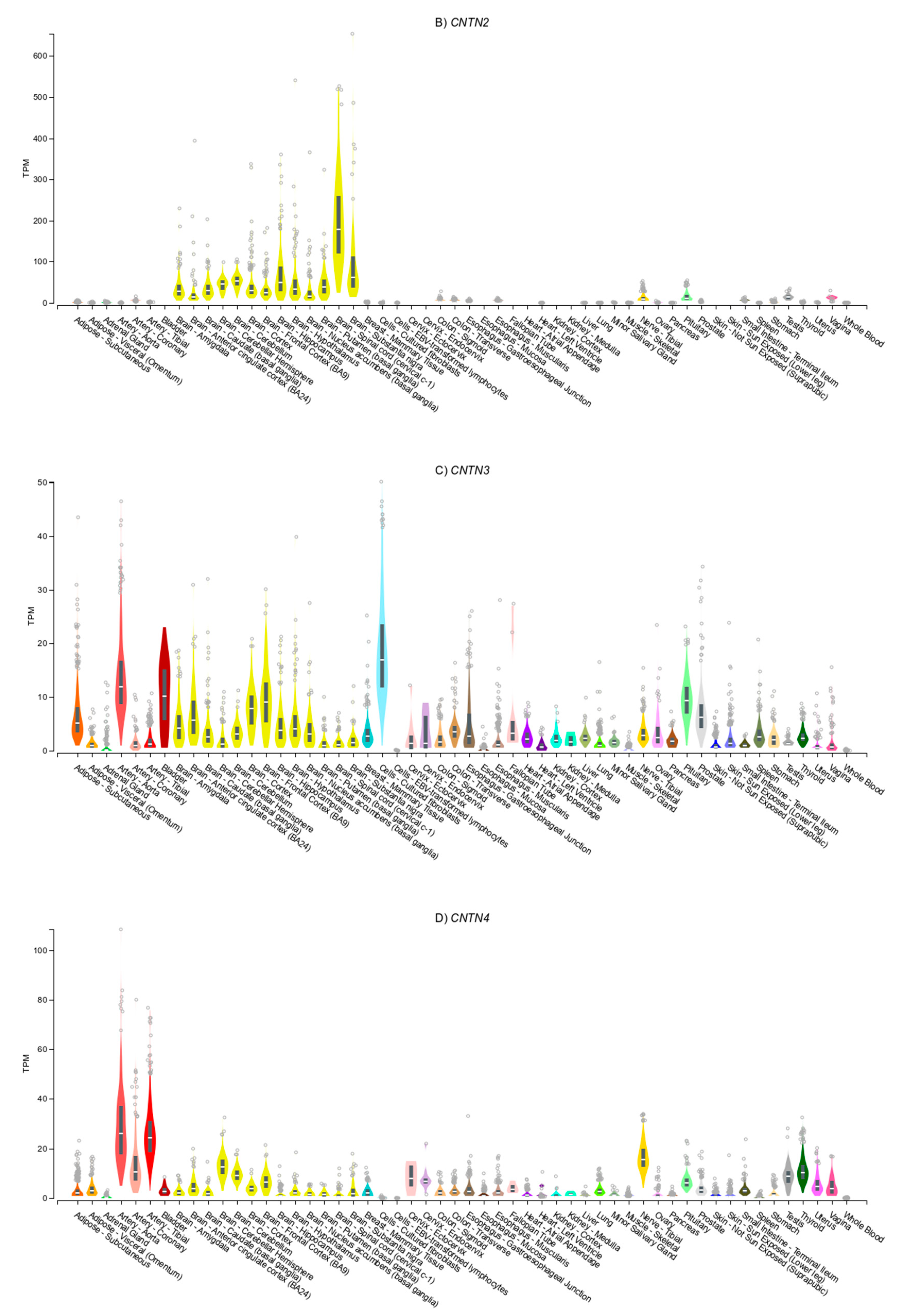
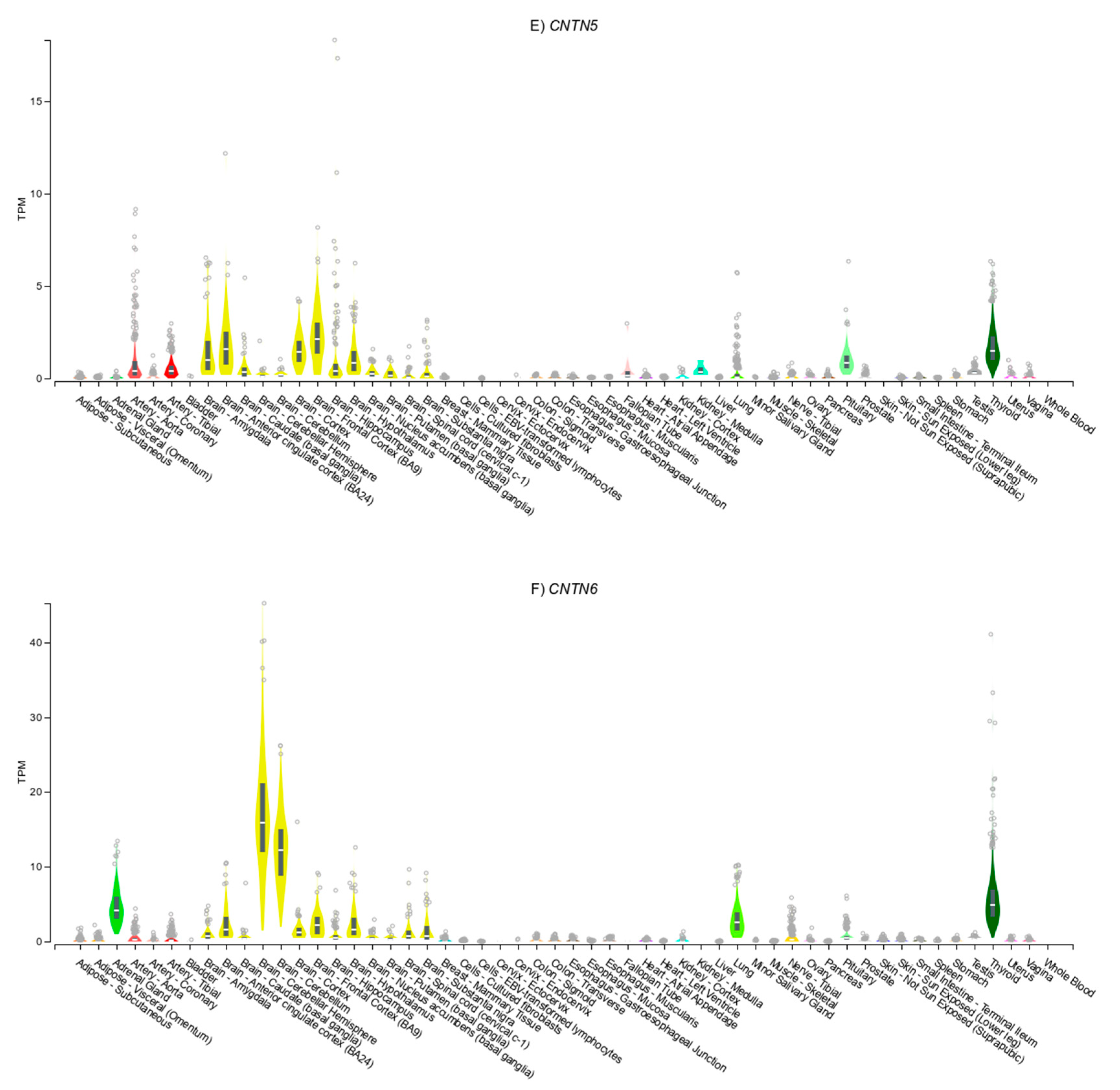
3.4. Gene Expression Patterns
3.5. Genotype-Specific Expression Patterns for Associated SNPs (eQTLs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amare, A.T.; Schubert, K.O.; Klingler-Hoffmann, M.; Cohen-Woods, S.; Baune, B.T. The genetic overlap between mood disorders and cardiometabolic diseases: A systematic review of genome wide and candidate gene studies. Transl. Psychiatry 2017, 7, e1007. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.; Mairs, H. Obesity and Serious Mental Ill Health: A Critical Review of the Literature. Healthcare 2014, 2, 166–182. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.; Asai, I.; Detraux, J.; Gautam, S.; Möller, H.-J.; Ndetei, D.M.; et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011, 10, 52–77. [Google Scholar] [CrossRef] [PubMed]
- So, H.-C.; Chau, K.-L.; Ao, F.-K.; Mo, C.-H.; Sham, P.C. Exploring shared genetic bases and causal relationships of schizophrenia and bipolar disorder with 28 cardiovascular and metabolic traits. Psychol. Med. 2019, 49, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Mulugeta, A.; Zhou, A.; King, C.; Hyppönen, E. Association between major depressive disorder and multiple disease outcomes: A phenome-wide Mendelian randomisation study in the UK Biobank. Mol. Psychiatry 2020, 25, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yuan, S.; Xiong, Y.; He, Q.; Larsson, S.C. Major depressive disorder and cardiometabolic diseases: A bidirectional Mendelian randomisation study. Diabetologia 2020, 63, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Bailey, M.E.S.; Baldassarre, D.; Cullen, B.; De Faire, U.; Ferguson, A.; Gigante, B.; Giral, P.; Goel, A.; Graham, N.; et al. Genetic variation in CADM2 as a link between psychological traits and obesity. Sci. Rep. 2019, 9, 7339. [Google Scholar] [CrossRef]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; De Jonge, P.; Beekman, A.T.F.; Penninx, B.W.J.H. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 2013, 18, 692–699. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Chatterjee, M.; Schild, D. Contactins in the central nervous system: Role in health and disease. Neural Regen. Res. 2019, 14, 206–216. [Google Scholar] [CrossRef]
- Matthews, P.M.; Sudlow, C.L. The UK Biobank. Brain 2015, 138 Pt 12, 3463–3465. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, R.J.; Ward, J.; Cullen, B.; Tunbridge, E.M.; Hartz, S.; Bierut, L.; Horton, A.; Bailey, M.E.S.; Graham, N.; Ferguson, A.; et al. Genome-wide analysis of self-reported risk-taking behaviour and cross-disorder genetic correlations in the UK Biobank cohort. Transl. Psychiatry 2018, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Strawbridge, R.J.; Bailey, M.E.S.; Graham, N.; Ferguson, A.; Lyall, D.M.; Cullen, B.; Pidgeon, L.M.; Cavanagh, J.; Mackay, D.F.; et al. Genome-wide analysis in UK Biobank identifies four loci associated with mood instability and genetic correlation with major depressive disorder, anxiety disorder and schizophrenia. Transl. Psychiatry 2017, 7, 1264. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.; Pedroni, A.; Mata, R.; Rieskamp, J.; Hertwig, R. Risk preference shares the psychometric structure of major psychological traits. Sci. Adv. 2017, 3, e1701381. [Google Scholar] [CrossRef]
- Davis, K.A.S.; Coleman, J.R.I.; Adams, M.; Allen, N.; Breen, G.; Cullen, B.; Dickens, C.; Fox, E.; Graham, N.; Holliday, J.; et al. Erratum: Mental health in UK Biobank: Development, implementation and results from an online questionnaire completed by 157,366 participants-CORRIGENDUM. BJPsych Open 2018, 4, 136. [Google Scholar] [CrossRef]
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Mägi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nat. Cell Biol. 2015, 518, 187–196. [Google Scholar] [CrossRef]
- Ehret, G.B.; CHARGE-EchoGen Consortium; Ferreira, T.; Chasman, D.I.; Jackson, A.U.; Schmidt, E.M.; Johnson, T.; Thorleifsson, G.; Luan, J.; Donnelly, L.A.; et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 2016, 48, 1171–1184. [Google Scholar] [CrossRef]
- Eastwood, S.V.; Mathur, R.; Atkinson, M.; Brophy, S.; Sudlow, C.; Flaig, R.; De Lusignan, S.; Allen, N.; Chaturvedi, N. Algorithms for the Capture and Adjudication of Prevalent and Incident Diabetes in UK Biobank. PLoS ONE 2016, 11, e0162388. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, 7. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.D.M.J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–2655. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.; Jansen, P.R.; Stringer, S.; Watanabe, K.; De Leeuw, C.A.; Bryois, J.; Savage, J.E.; Hammerschlag, A.R.; Skene, N.G.; Muñoz-Manchado, A.B.; et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 2018, 50, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, Y.; Wedow, R.; Li, Y.; Brazel, D.M.; Chen, F.; Datta, G.; Davila-Velderrain, J.; McGuire, D.; Tian, C.; et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019, 51, 237–244. [Google Scholar] [CrossRef]
- Wheeler, E.; Leong, A.; Liu, C.-T.; Hivert, M.-F.; Strawbridge, R.J.; Podmore, C.; Li, M.; Yao, J.; Sim, X.; Hong, J.; et al. Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis. PLoS Med. 2017, 14, e1002383. [Google Scholar] [CrossRef]
- Vujkovic, M.; The HPAP Consortium; Keaton, J.M.; Lynch, J.A.; Miller, D.R.; Zhou, J.; Tcheandjieu, C.; Huffman, J.E.; Assimes, T.L.; Lorenz, K.; et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 2020, 52, 680–691. [Google Scholar] [CrossRef]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Smith, G.D.; Holmes, M.V. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020, 17, e1003062. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nat. Cell Biol. 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Vacic, V.; Ozelius, L.J.; Clark, L.N.; Bar-Shira, A.; Gana-Weisz, M.; Gurevich, T.; Gusev, A.; Kedmi, M.; Kenny, E.E.; Liu, X.; et al. Genome-wide mapping of IBD segments in an Ashkenazi PD cohort identifies associated haplotypes. Hum. Mol. Genet. 2014, 23, 4693–4702. [Google Scholar] [CrossRef] [PubMed]
- Beecham, G.W.; Hamilton, K.; Naj, A.C.; Martin, E.R.; Huentelman, M.; Myers, A.J.; Corneveaux, J.J.; Hardy, J.; Vonsattel, J.P.; Younkin, S.G.; et al. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genet. 2014, 10, e1004606. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Woo, H.G.; Greenwood, T.A.; Kripke, D.F.; Kelsoe, J. A genome-wide association study of seasonal pattern mania identifies NF1A as a possible susceptibility gene for bipolar disorder. J. Affect. Disord. 2013, 145, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, E.M.; Hafferty, J.D.; Hall, L.S.; Howard, D.M.; Clarke, T.-K.; Fabbri, C.; Lewis, C.M.; Uher, R.; Navrady, L.B.; Adams, M.J.; et al. Genome-wide association study of antidepressant treatment resistance in a population-based cohort using health service prescription data and meta-analysis with GENDEP. Pharm. J. 2020, 20, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Linnér, R.K.; Biroli, P.; Kong, E.; Meddens, S.F.W.; Wedow, R.; Fontana, M.A.; Lebreton, M.; Tino, S.P.; Abdellaoui, A.; Hammerschlag, A.R.; et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat. Genet. 2019, 51, 245–257. [Google Scholar] [CrossRef]
- Goes, F.S.; McGrath, J.; Avramopoulos, D.; Wolyniec, P.S.; Pirooznia, M.; Ruczinski, I.; Nestadt, G.; Kenny, E.E.; Vacic, V.; Peters, I.; et al. Genome-wide association study of schizophrenia in Ashkenazi Jews. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 649–659. [Google Scholar] [CrossRef]
- Ikeda, M.; Takahashi, A.; Kamatani, Y.; Momozawa, Y.; Saito, T.; Kondo, K.; Shimasaki, A.; Kawase, K.; Sakusabe, T.; Iwayama, Y.; et al. Genome-Wide Association Study Detected Novel Susceptibility Genes for Schizophrenia and Shared Trans-Populations/Diseases Genetic Effect. Schizophr. Bull. 2019, 45, 824–834. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Yu, H.; He, L.; Xu, Y.; Zhang, D.; Yi, Q.; Li, C.; Li, X.; Shen, J.; et al. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat. Genet. 2017, 49, 1576–1583. [Google Scholar] [CrossRef]
- McDonald, M.-L.N.; Won, S.; Mattheisen, M.; Castaldi, P.J.; Cho, M.H.; Rutten, E.; Hardin, M.; Yip, W.; Rennard, S.I.; Lomas, D.A.; et al. Body mass index change in gastrointestinal cancer and chronic obstructive pulmonary disease is associated with Dedicator of Cytokinesis 1. J. Cachex Sarcopenia Muscle 2017, 8, 428–436. [Google Scholar] [CrossRef]
- Strawbridge, R.J.; Ward, J.; Lyall, L.M.; Tunbridge, E.M.; Cullen, B.; Graham, N.; Ferguson, A.; Johnston, K.J.A.; Lyall, D.M.; Mackay, D.; et al. Genetics of self-reported risk-taking behaviour, trans-ethnic consistency and relevance to brain gene expression. Transl. Psychiatry 2018, 8, 178. [Google Scholar] [CrossRef]
- Smirnov, A.; Kontsevaya, G.V.; Feofanova, N.A.; Anisimova, M.V.; Serova, I.A.; Gerlinskaya, L.A.; Battulin, N.R.; Moshkin, M.P.; Serov, O.L. Unexpected phenotypic effects of a transgene integration causing a knockout of the endogenous Contactin-5 gene in mice. Transgenic Res. 2017, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fried, E.I. Moving forward: How depression heterogeneity hinders progress in treatment and research. Expert Rev. Neurother. 2017, 17, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.; Ellison, W.; Young, D.; Chelminski, I.; Dalrymple, K. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Compr. Psychiatry 2015, 56, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Olbert, C.M.; Gala, G.J.; Tupler, L.A. Quantifying heterogeneity attributable to polythetic diagnostic criteria: Theoretical framework and empirical application. J. Abnorm. Psychol. 2014, 123, 452–462. [Google Scholar] [CrossRef]
- Van Loo, H.M.; De Jonge, P.; Romeijn, J.-W.; Kessler, R.C.; Schoevers, R.A. Data-driven subtypes of major depressive disorder: A systematic review. BMC Med. 2012, 10, 156. [Google Scholar] [CrossRef]
- Power, R.A.; Tansey, K.E.; Buttenschøn, H.N.; Cohen-Woods, S.; Bigdeli, T.; Hall, L.S.; Kutalik, Z.; Lee, S.H.; Ripke, S.; Steinberg, S.; et al. Genome-wide Association for Major Depression Through Age at Onset Stratification: Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Biol. Psychiatry 2017, 81, 325–335. [Google Scholar] [CrossRef]
- Cai, N.; Choi, K.W.; Fried, E.I. Reviewing the genetics of heterogeneity in depression: Operationalizations, manifestations and etiologies. Hum. Mol. Genet. 2020, 29, R10–R18. [Google Scholar] [CrossRef]
- Bosaipo, N.B.; Foss, M.P.; Young, A.H.; Juruena, M.F. Neuropsychological changes in melancholic and atypical depression: A systematic review. Neurosci. Biobehav. Rev. 2017, 73, 309–325. [Google Scholar] [CrossRef]

| Locus | DISEASE/TRAIT | PMID |
|---|---|---|
| CNTN1 | Antidepressant treatment resistance (>2 drugs prescribed) | 30700811 |
| Bipolar disorder | 22925353 | |
| Blood protein levels | 30072576 | |
| Cerebral amyloid deposition in APOEe4 non-carriers (PET imaging) | 26252872 | |
| Cognitive ability in schizophrenia | 31206164 | |
| Dementia and core Alzheimer’s disease neuropathologic changes | 25188341 | |
| IgG glycosylation | 23382691 | |
| Metabolite levels (X-11787) | 23934736 | |
| Parkinson’s disease | 24842889 | |
| Reaction time | 29844566 | |
| CNTN2 | Adventurousness | 30643258 |
| Blood protein levels | 29875488, 28240269 | |
| Cerebrospinal fluid biomarker levels | 28031287 | |
| Highest math class taken | 30038396, 30038396 | |
| Red blood cell count | 27863252 | |
| Schizophrenia | 30285260, 26198764, 28991256 | |
| High density lipoprotein cholesterol levels | 32203549 | |
| Type 2 Diabetes | 32541925 | |
| Waist–hip ratio | 30239722 | |
| CNTN3 | Age at first sexual intercourse | 27089180 |
| Economic and political preferences | 22566634 | |
| Educational attainment (years of education) | 30038396 | |
| Frontal fibrosing alopecia | 30850646 | |
| Heart rate response to recovery post exercise | 29769521, 29497042 | |
| Paediatric bone mineral content (hip) | 28181694 | |
| Peripheral arterial disease (traffic-related air pollution interaction) | 27082954 | |
| Smoking initiation and quantity | 30643251, 24665060 | |
| Systolic blood pressure | 30224653 | |
| CNTN4 | Adolescent idiopathic scoliosis | 30019117 |
| Amyotrophic lateral sclerosis | 19451621 | |
| Atypical femoral fracture in phosphonate treatment | 31006051 | |
| White blood cell phenotypes | 27863252, 29403010 | |
| Bisphosphonate-associated atypical femoral fracture | 31006051 | |
| Blood pressure | 17903302, 25189868 | |
| Blood protein levels | 30072576, 29875488 | |
| Body Mass Index (change over time) in chronic obstructive pulmonary disease | 28044437 | |
| Brain connectivity | 23471985 | |
| Chronotype and sleep variables | 30696823, 27126917, 30804565, 30595370 | |
| Cognitive ability, years of educational attainment or schizophrenia (pleiotropy) | 31374203 | |
| Multiple psychiatric disorders | 31835028, 28540026, 26198764, 30285260, 28991256, 30285260, 31268507, 31740837, 25056061 | |
| DNA methylation variation (age effect) | 30348214 | |
| Gallbladder cancer | 22318345 | |
| Gut microbiota | 32572223 | |
| Immune biomaker levels | 32066700, 27989323 | |
| Intelligence | 22449649 | |
| Metabolite levels | 23823483, 31005972, 24379826 | |
| Middle childhood and early adolescence aggressive behaviour | 26087016 | |
| Monoclonal gammopathy of undetermined significance | 30134812 | |
| Pit-and-fissure caries | 24556642 | |
| Post bronchodilator FEV1/FVC ratio | 26634245 | |
| Response to aripiprazole in schizophrenia | 29503163 | |
| Self-reported math ability | 30038396, 30038396 | |
| Sensorimotor dexterity | 31596458 | |
| Smoking initiation (ever regular vs. never regular) | 30643251 | |
| Stimulated adipocyte lipolysis | 2180562 | |
| Tuberculosis | 28928442 | |
| CNTN5 | Blood cell phenotypes | 32888493, 27863252, 30595370, 28957414 |
| Protein levels in obesity | 29234017 | |
| Bipolar and/or Depressive disorders | 31926635, 31969693, 27089181, 29942085, 30643256, 29292387 | |
| Carotid intima media thickness interaction with smoking | 32117412 | |
| Adolescent idiopathic scoliosis | 30019117 | |
| Alcohol dependence | 26365420 | |
| Atrial fibrillation | 17903304 | |
| Blood protein levels | 29875488, 30072576, 28240269 | |
| Bone mineral density (femoral neck) | 26911590 | |
| Chronotype and sleep phenotypes | 30696823, 30804565, 30595370 | |
| Core binding factor acute myeloid leukaemia | 27903959 | |
| Educational attainment (MTAG) | 30038396 | |
| Factor VII activity | 30642921 | |
| Feeling miserable | 29500382 | |
| Glycated haemoglobin levels | 28898252 | |
| Gout vs. Hyperuricemia | 31289104 | |
| Immune response to smallpox (secreted IL-2) | 22610502 | |
| Interleukin-2 receptor antagonist levels | 27989323 | |
| Life satisfaction | 30643256 | |
| Loneliness (multivariate analysis) | 27629369 | |
| Lung function (FEV1/FVC) | 30595370 | |
| Menarche (age at onset) | 23667675 | |
| Metastasis in stage I-III microsatellite instability low/stable colorectal cancer (time to event) | 30738427 | |
| Myopia (pathological) | 23049088 | |
| Neurological blood protein biomarker levels | 31320639 | |
| Neuroticism | 30595370, 29942085, 29292387, 30643256, 27089181, 29292387 | |
| Objective response to lithium treatment in bipolar disorder | 26503763 | |
| Plasma kynurenine levels in major depressive disorder | 29317604 | |
| Positive affect | 30643256 | |
| Post bronchodilator FEV1/FVC ratio in COPD | 26634245 | |
| Reaction time | 29844566 | |
| Smoking initiation (ever regular vs. never regular) (MTAG) | 30643251 | |
| Spatial memory | 31596458 | |
| Suicidality | 30745170 | |
| Systolic blood pressure | 30595370 | |
| Trans fatty acid levels | 25646338 | |
| Waist–hip ratio | 25673412 | |
| Well-being spectrum (multivariate analysis) | 30643256 | |
| Volumetric brain MRI | 17903297 | |
| CNTN6 | Caudate activity during reward | 28927378 |
| Depressive symptoms (stressful life events interaction) | 27529621 | |
| Diastolic blood pressure | 28270201 | |
| DNA methylation variation (age effect) | 30348214 | |
| Gut microbiota | 31519223, 27723756, 32572223 | |
| Iris colour (L* coordinate) | 30895295 | |
| Loneliness | 27629369 | |
| Metabolite levels | 23823483, 22675492 | |
| Neurocognitive impairment in HIV-1 infection (continuous) | 28447399 | |
| PR interval in Tripanosoma cruzi seropositivity | 24324551 | |
| Prostate cancer (SNP x SNP interaction) | 22219177 | |
| Subjective response to placebo treatment in childhood asthma (change in cough/wheeze) | 31557306 | |
| Systemic lupus erythematosus | 24871463 | |
| Visceral adipose tissue/subcutaneous adipose tissue ratio | 22589738 |
| Gene | Chr | Start | End | −400 kb | +400 kb |
|---|---|---|---|---|---|
| CNTN1 | 12 | 40,692,442 | 41,072,412 | 40,292,442 | 41,472,412 |
| CNTN2 | 1 | 205,042,937 | 205,078,272 | 204,642,937 | 205,478,272 |
| CNTN3 | 3 | 74,262,568 | 74,521,140 | 73,862,568 | 74,921,140 |
| CNTN4 | 3 | 2,098,803 | 3,057,961 | 1,698,803 | 3,457,961 |
| CNTN5 | 11 | 99,021,190 | 100,358,885 | 98,621,190 | 100,758,885 |
| CNTN6 | 3 | 1,092,661 | 1,403,594 | 692,661 | 1,803,594 |
| Men | Women | All | |
|---|---|---|---|
| N (% men) | 185,228 | 217,635 | 402,863 (46.0) |
| Age (years) | 57.1 (8.1) | 56.7 (7.9) | 56.9 (8.0) |
| WHR | 0.94 (0.06) | 0.82 (0.07) | 0.87 (0.09) |
| BMI (kg/m2) | 27.8 (4.2) | 27.0 (5.1) | 27.4 (4.8) |
| SBP (mmHg) | 141 (17) | 136 (19) | 138 (19) |
| DBP (mmHg) | 84 (10) | 81 (10) | 82 (10) |
| SBP * (mmHg) | 145 (19) | 138 (21) | 141 (21) |
| DBP * (mmHg) | 87 (11) | 82 (11) | 84 (11) |
| HbA1C (mmol/mol) | 36.3 (7.3) | 35.7 (5.7) | 36.0 (6.5) |
| Neuroticism score | 3.6 (3.2) | 4.6 (3.2) | 4.1 (3.3) |
| CVD | 5886 (3.2) | 2294 (1.1) | 8180 (2.03) |
| T2D | 11,189 (6.0) | 6232 (2.9) | 17421 (4.3) |
| BD | 875 (1.5) | 999 (1.4) | 1874 (1.5) |
| GAD | 3069 (7.2) | 6029 (12.8) | 9098 (10.1) |
| MDD | 9684 (19.8) | 21,199 (35.0) | 30,883 (28.2) |
| Addiction | 3895 (6.9) | 3580 (4.9) | 7475 (5.8) |
| Mood instability | 77,173 (41.7) | 100,841 (46.3) | 178,014 (44.2) |
| Risk-taking | 18,519 (33.2) | 13,702 (19.4) | 32,221 (25.5) |
| Current Smoking | 21,821 (11.8) | 18,803 (8.7) | 40,624 (10.1) |
| Gene | Trait | Lead SNP | A1 | A1F | N | β/OR | SE | P |
|---|---|---|---|---|---|---|---|---|
| CNTN1 | Smoking | rs11174809 | T | 0.35 | 398,675 | 1.025 | 0.005 | 9.52 × 10−7 |
| CNTN2 | Risks | rs35068223 | T | 0.19 | 377,622 | 1.036 | 0.007 | 1.77 × 10−7 |
| CNTN2 | WHRadjBMI | rs6593925 | G | 0.09 | 401,149 | −0.001 | 0.000 | 2.38 × 10−11 |
| CNTN2 | WHRadjBMI * | rs11240349 | A | 0.46 | 400,770 | −0.001 | 0.000 | 3.98 × 10−8 |
| CNTN4 | BMI | rs3856837 | C | 0.48 | 394,050 | −0.054 | 0.011 | 4.28 × 10−7 |
| CNTN4 | BMI * | rs4685542 | C | 0.14 | 391,199 | −0.075 | 0.016 | 1.66 × 10−6 |
| CNTN4 | Risks | rs62232818 | T | 0.10 | 383,065 | 1.042 | 0.009 | 3.09 × 10−6 |
| CNTN5 | Neuroticism | rs10790767 | T | 0.40 | 324,775 | 0.042 | 0.008 | 2.43 × 10−7 |
| CNTN5 | HbA1C | rs11606890 | C | 0.10 | 384,094 | −0.007 | 0.001 | 1.14 × 10−37 |
| CNTN5 | DBP | rs633185 | G | 0.29 | 370,841 | −0.459 | 0.028 | 1.94 × 10−59 |
| CNTN5 | SBP | rs633185 | G | 0.29 | 370,770 | −0.787 | 0.049 | 2.85 × 10−58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, J.; Leung, S.S.Y.; Bailey, M.E.S.; Cullen, B.; Ferguson, A.; Graham, N.; Johnston, K.J.A.; Lyall, D.M.; Lyall, L.M.; Ward, J.; et al. Exploring the Role of Contactins across Psychological, Psychiatric and Cardiometabolic Traits within UK Biobank. Genes 2020, 11, 1326. https://doi.org/10.3390/genes11111326
Morris J, Leung SSY, Bailey MES, Cullen B, Ferguson A, Graham N, Johnston KJA, Lyall DM, Lyall LM, Ward J, et al. Exploring the Role of Contactins across Psychological, Psychiatric and Cardiometabolic Traits within UK Biobank. Genes. 2020; 11(11):1326. https://doi.org/10.3390/genes11111326
Chicago/Turabian StyleMorris, Julia, Soddy Sau Yu Leung, Mark E.S. Bailey, Breda Cullen, Amy Ferguson, Nicholas Graham, Keira J. A. Johnston, Donald M. Lyall, Laura M. Lyall, Joey Ward, and et al. 2020. "Exploring the Role of Contactins across Psychological, Psychiatric and Cardiometabolic Traits within UK Biobank" Genes 11, no. 11: 1326. https://doi.org/10.3390/genes11111326
APA StyleMorris, J., Leung, S. S. Y., Bailey, M. E. S., Cullen, B., Ferguson, A., Graham, N., Johnston, K. J. A., Lyall, D. M., Lyall, L. M., Ward, J., Smith, D. J., & Strawbridge, R. J. (2020). Exploring the Role of Contactins across Psychological, Psychiatric and Cardiometabolic Traits within UK Biobank. Genes, 11(11), 1326. https://doi.org/10.3390/genes11111326





