Overexpression of Two CCCH-type Zinc-Finger Protein Genes Leads to Pollen Abortion in Brassica campestris ssp. chinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. β-glucuronidase (GUS) Histochemical Staining Assay
2.3. Generation of BcMF30a and BcMF30c Overexpression Transgenic Chinese Cabbage
2.4. RNA Extraction and qRT-PCR
2.5. Phenotypic Analyses, Cytological Observation, and Pollen Germination Assay
2.6. Subcellular Localization
3. Results
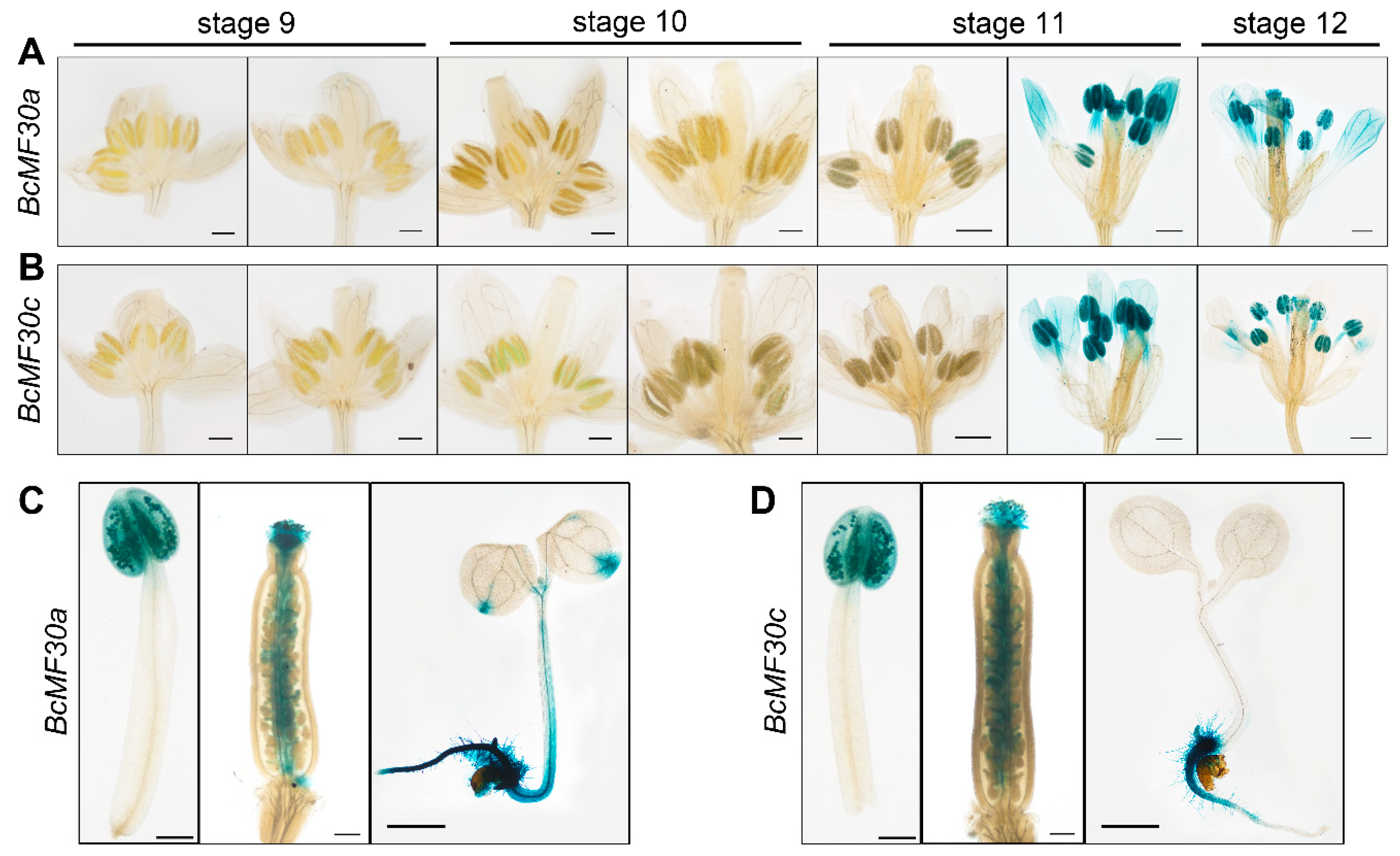
3.1. BcMF30a and BcMF30c Share Similar Gene Expression Patterns and Both Are Highly Expressed in Bicellular Pollen
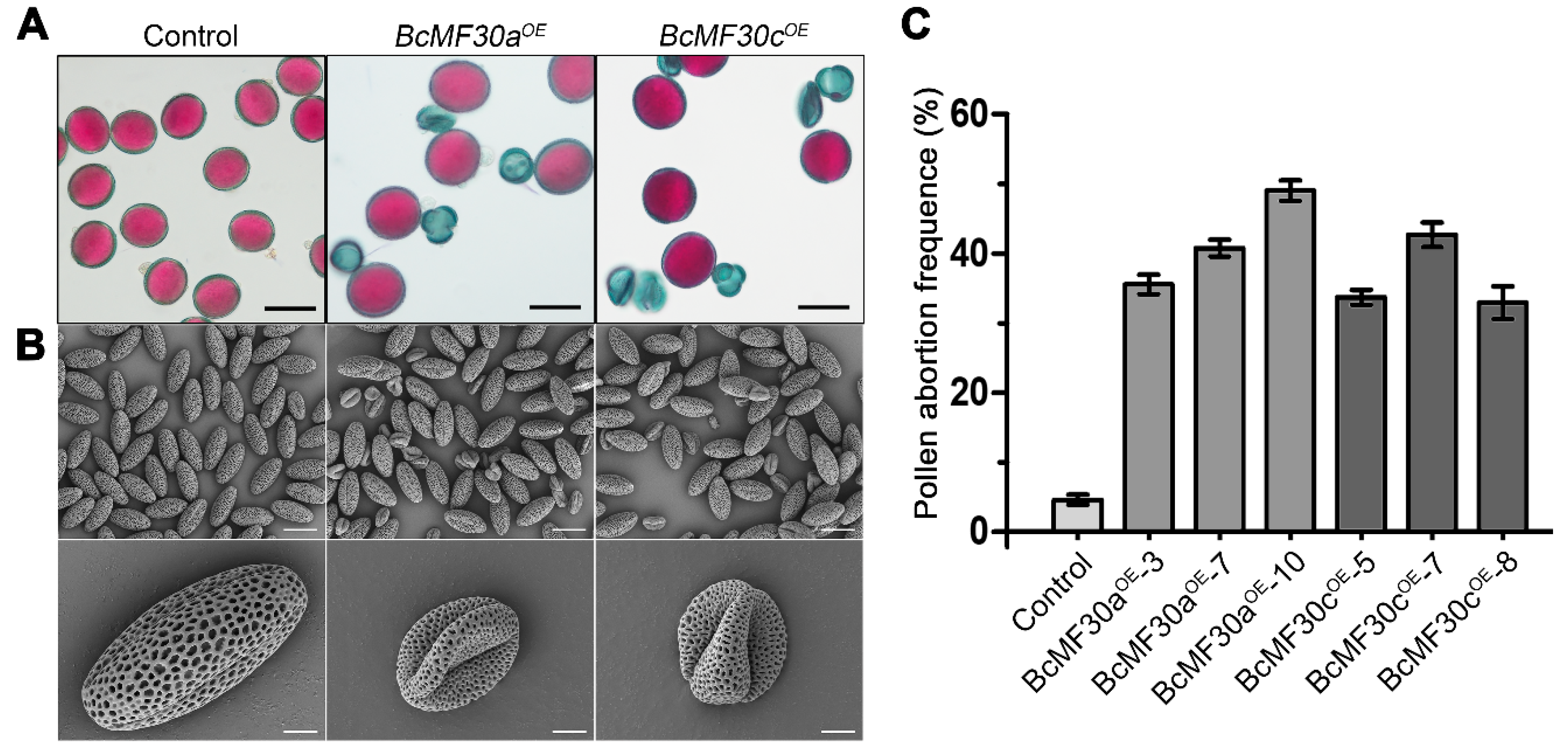
3.2. Overexpression of BcMF30a and BcMF30c in B. campestris Causes Reduced Male Fertility
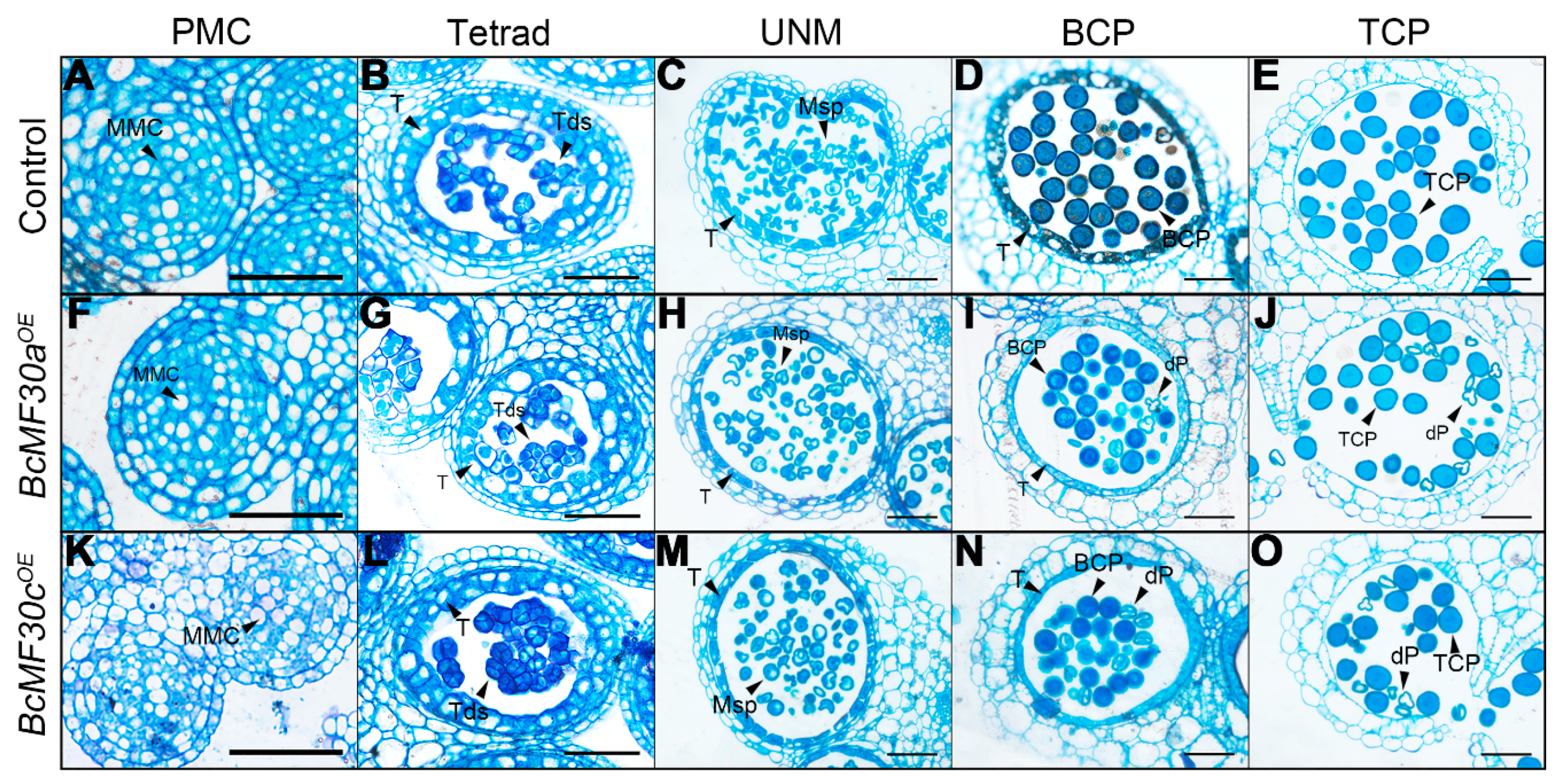
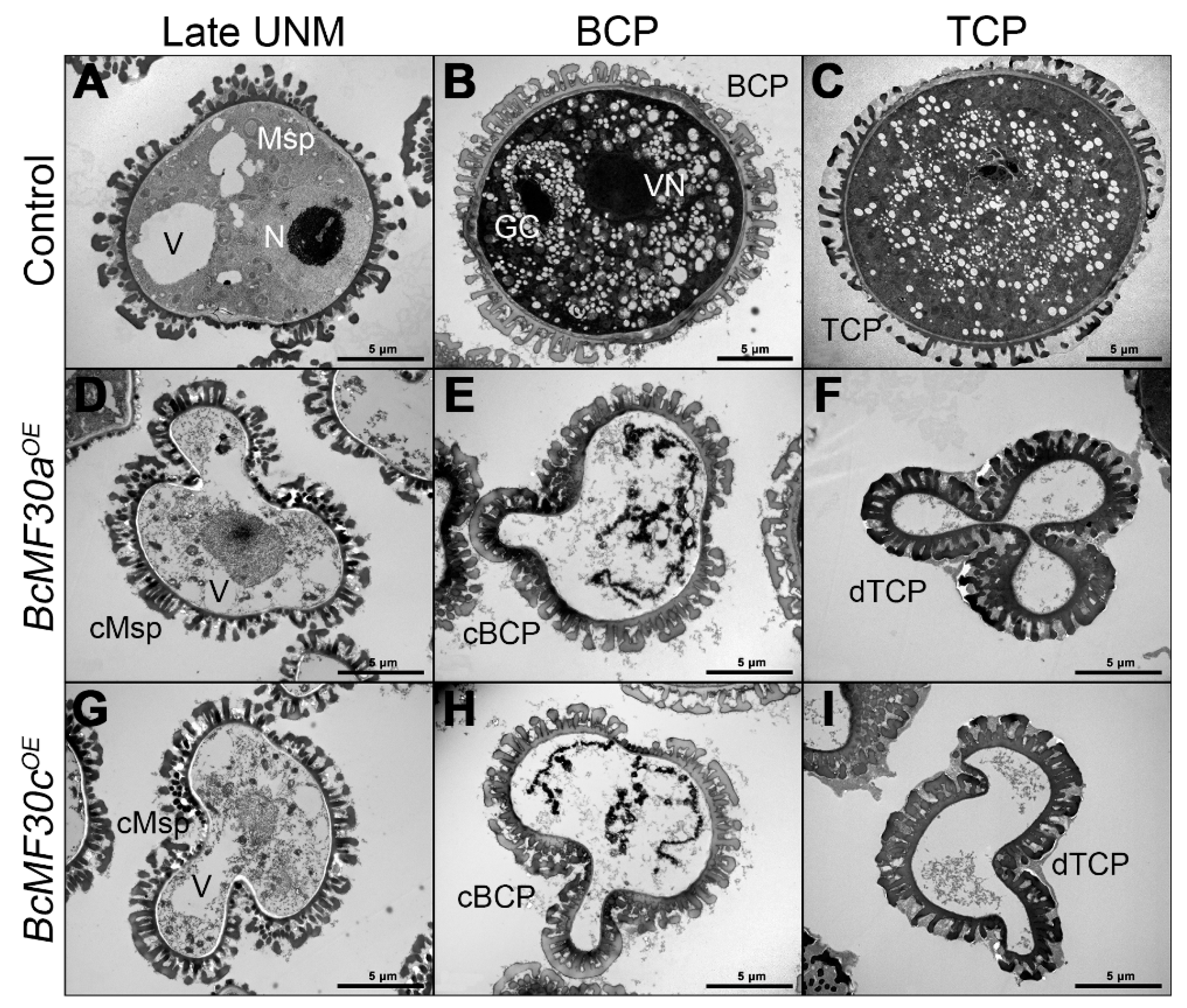
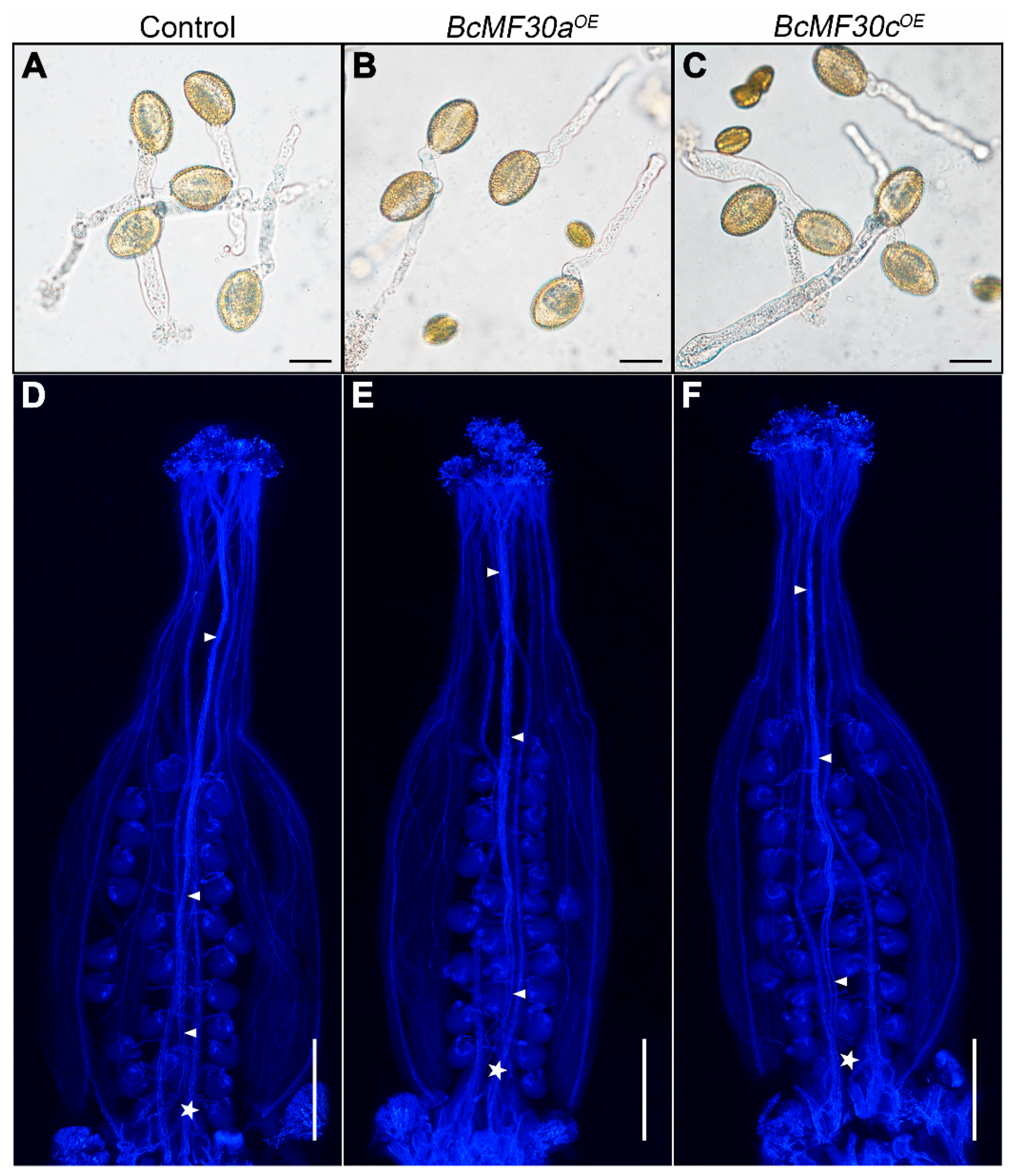
3.3. The Degradation of Microspore Contents Starts from the Late Uninucleate Stage in BcMF30aOE and BcMF30cOE Transgenic Plants
3.4. In Vitro and In Vivo Pollen Germination Tests in BcMF30aOE and BcMF30cOE Transgenic Plants
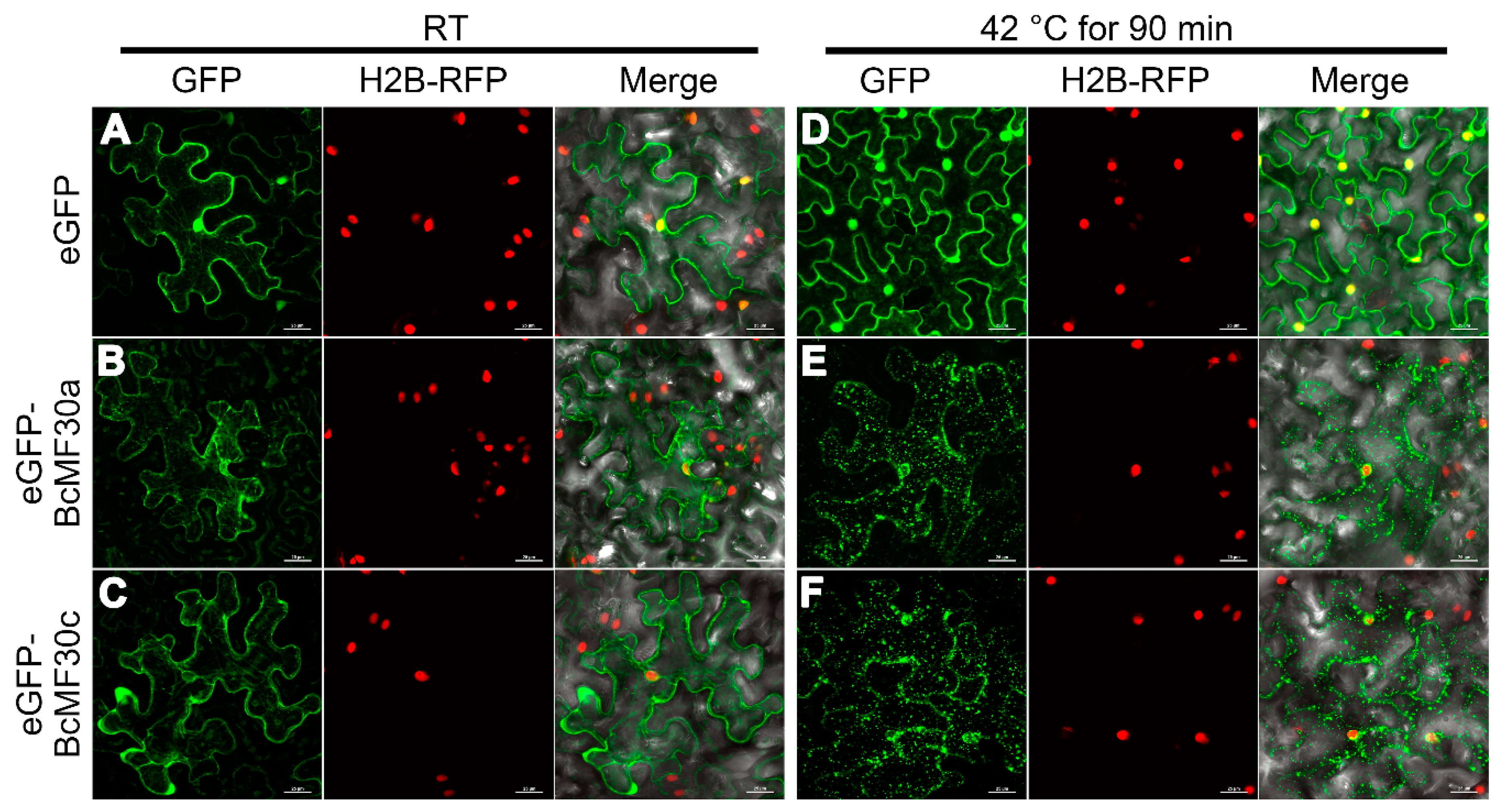
3.5. BcMF30a and BcMF30c Can Localize to Cytoplasmic Foci
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Honys, D.; Twell, D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 2004, 5, R85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Østergaard, L.; Yanofsky, M.F. Establishing gene function by mutagenesis in Arabidopsis thaliana. Plant J. 2004, 39, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Twell, D. Male gametophyte development. In Plant Developmental Biology-Biotechnological Pespectives, 1st ed.; Pua, E.C., Davey, M.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1, pp. 225–244. [Google Scholar]
- Kim, M.-J.; Kim, M.; Lee, M.R.; Park, S.K.; Kim, J. Lateral Organ Boundaries Domain (LBD) 10 interacts with Sidecar Pollen/Lbd27 to control pollen development in Arabidopsis. Plant J. 2015, 81, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-A.; Hoai, T.N.T.; Park, H.-J.; Zhao, M.; Twell, D.; Honys, D.; Park, S.-K. MYB81, a microspore-specific GAMYB transcription factor, promotes pollen mitosis I and cell lineage formation in Arabidopsis. Plant J. 2020, 101, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-T.; Li, E.; Guo, Y.-K.; Yu, S.-X.; Wan, Z.-Y.; Ma, T.; Li, S.; Hirano, T.; Sato, M.H.; Zhang, Y. Arabidopsis VAC14 is Critical for Pollen Development through Mediating Vacuolar Organization. Plant Physiol. 2018, 177, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Gu, H.; Liu, J.; Qu, L.-J. Four closely-related RING-type E3 ligases, APD1-4, are involved in pollen mitosis II regulation in Arabidopsis. J. Integr. Plant Biol. 2012, 54, 814–827. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, Y.; Xu, X.; Yang, Z.-N. A genetic pathway for tapetum development and function in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 892–900. [Google Scholar] [CrossRef]
- Quilichini, T.D.; Samuels, A.L.; Douglas, C.J. ABCG26-mediated polyketide trafficking and hydroxycinnamoyl spermidines contribute to pollen wall exine formation in Arabidopsis. Plant Cell 2014, 26, 4483–4498. [Google Scholar] [CrossRef] [Green Version]
- Kaul, S.; Koo, H.L.; Jenkins, J.; Rizzo, M.; Rooney, T.; Tallon, L.J. The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar]
- Goff, S.A.; Ricke, D.; Lan, T.-H.; Presting, G.; Wang, R.; Dunn, M.; Glazebrook, J.; Sessions, A.; Oeller, P.; Varma, H.; et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 2002, 296, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Zhu, E.; You, C.; Wang, S.; Cui, J.; Niu, B.; Wang, Y.; Qi, J.; Ma, H.; Chang, F. The DYT1-interacting proteins bHLH010, bHLH089 and bHLH091 are redundantly required for Arabidopsis anther development and transcriptome. Plant J. 2015, 83, 976–990. [Google Scholar] [CrossRef]
- Kondou, Y.; Higuchi, M.; Matsui, M. High-throughput characterization of plant gene functions by using gain-of-function technology. Annu. Rev. Plant Biol. 2010, 61, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ge, X.; Ma, H. The rice OsDIL gene plays a role in drought tolerance at vegetative and reproductive stages. Plant Mol. Biol. 2013, 82, 239–253. [Google Scholar] [CrossRef]
- Shen, X.; Hu, Z.; Xiang, X.; Xu, L.; Cao, J. Overexpression of a stamen-specific R2R3-MYB gene BcMF28 causes aberrant stamen development in transgenic Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 518, 726–731. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Li, L.; Lin, J.; Zheng, C.; Zhang, L. Overexpression of PwTUA1, a pollen-specific tubulin gene, increases pollen tube elongation by altering the distribution of alpha-tubulin and promoting vesicle transport. J. Exp. Bot. 2009, 60, 2737–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, S.; Zhou, L.-Z.; Fox, E.; Pao, J.; Sun, W.; Zhou, C.; McCormick, S. Overexpression of Arabidopsis thaliana PTEN caused accumulation of autophagic bodies in pollen tubes by disrupting phosphatidylinositol 3-phosphate dynamics. Plant J. 2011, 68, 1081–1092. [Google Scholar] [CrossRef]
- Gui, C.-P.; Dong, X.; Liu, H.-K.; Huang, W.-J.; Zhang, D.; Wang, S.-J.; Barberini, M.L.; Gao, X.-Y.; Muschietti, J.; McCormick, S.; et al. Overexpression of the tomato pollen receptor kinase LePRK1 rewires pollen tube growth to a blebbing mode. Plant Cell 2014, 26, 3538–3555. [Google Scholar] [CrossRef] [Green Version]
- Bowers, J.E.; Chapman, B.A.; Rong, J.; Paterson, A.H. Ecological and immunological determinants of influenza evolution. Nature 2003, 422, 433–438. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.-H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Guo, Y.; Wu, C.; Yang, G.; Li, Y.; Zheng, C. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genom. 2008, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Xiong, X.; Liu, W.; Liu, T.; Yu, Y.; Cao, J. BcMF30a and BcMF30c, Two Novel Non-Tandem CCCH Zinc-Finger Proteins, Function in Pollen Development and Pollen Germination in Brassica campestris ssp. chinensis. Int. J. Mol. Sci. 2020, 21, 6428. [Google Scholar] [CrossRef]
- Mudunkothge, J.M.; Mudunkothge, B.A. The GUS reporter system in flower development studies. In Flower Development: Methods and Protocols, 1st ed.; Riechmann, J.L., Wellmer, F., Eds.; Springer: New York, NY, USA, 2014; Volume 1110, pp. 295–304. [Google Scholar]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.N.; Hsu, Y.-C.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Yu, X.; Cao, J.; Ye, W.; Wang, Y. Construction of an antisense CYP86MF gene plasmid vector and production of a male-sterile Chinese cabbage transformant by the pollen-tube method. J. Hortic. Sci. Biotechnol. 2004, 79, 833–839. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential staining of aborted and non-aborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Dong, H.; Zhang, F.; Qiu, L.; Wang, F.; Cao, J.; Huang, L. BcMF8, a putative arabinogalactan protein-encoding gene, contributes to pollen wall development, aperture formation and pollen tube growth in Brassica campestris. Ann. Bot. 2014, 113, 777–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Liu, T.; Xiong, X.; Liu, W.; Yu, Y.; Cao, J. AtC3H18L is a stop-codon read-through gene and encodes a novel non-tandem CCCH zinc-finger protein that can form cytoplasmic foci similar to mRNP granules. Biochem. Biophys. Res. Commun. 2020, 528, 140–145. [Google Scholar] [CrossRef]
- Pomeranz, M.; Lin, P.-C.; Finer, J.; Jang, J.-C. AtTZF gene family localizes to cytoplasmic foci. Plant Signal. Behav. 2010, 5, 190–192. [Google Scholar] [CrossRef]
- Bogamuwa, S.; Jang, J.-C. The Arabidopsis tandem CCCH zinc finger proteins AtTZF4, 5 and 6 are involved in light-, abscisic acid- and gibberellic acid-mediated regulation of seed germination. Plant Cell Environ. 2013, 36, 1507–1519. [Google Scholar] [CrossRef]
- Guzikowski, A.R.; Chen, Y.S.; Zid, B.M. Stress-induced mRNP granules: Form and function of processing bodies and stress granules. Wiley Interdiscip. Rev. RNA 2019, 10, e1524. [Google Scholar] [CrossRef]
- Lu, P.; Chai, M.; Yang, J.; Ning, G.; Wang, G.; Ma, H. The Arabidopsis CALLOSE DEFECTIVE MICROSPORE1 gene is required for male fertility through regulating callose metabolism during microsporogenesis. Plant Physiol. 2014, 164, 1893–1904. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.-H.; Zhang, C.; Xu, X.-F.; Zhu, J.; Zhou, Q.; Ma, L.-J.; Niu, J.; Yang, Z.-N. Overexpression of AtTTP affects ARF17 expression and leads to male sterility in Arabidopsis. PLoS ONE 2015, 10, e0117317. [Google Scholar] [CrossRef] [Green Version]
- Chai, G.; Kong, Y.; Zhu, M.; Yu, L.; Qi, G.; Tang, X.; Wang, Z.; Cao, Y.; Yu, C.; Zhou, G. Arabidopsis C3H14 and C3H15 have overlapping roles in the regulation of secondary wall thickening and anther development. J. Exp. Bot. 2015, 66, 2595–2609. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, B.; Xu, M.; Jamil, M.; Wang, G. ABA-induced CCCH tandem zinc finger protein OsC3H47 decreases ABA sensitivity and promotes drought tolerance in Oryza sativa. Biochem. Biophys. Res. Commun. 2015, 464, 33–37. [Google Scholar] [CrossRef]
- Lin, P.-C.; Pomeranz, M.C.; Jikumaru, Y.; Kang, S.G.; Hah, C.; Fujioka, S.; Kamiya, Y.; Jang, J.-C. The Arabidopsis tandem zinc finger protein AtTZF1 affects ABA- and GA-mediated growth, stress and gene expression responses. Plant J. 2011, 65, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Yamaguchi, S.; Lim, S.; Oh, E.; Park, J.; Hanada, A.; Kamiya, Y.; Choi, G. SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 2008, 20, 1260–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Yang, X.; Wang, L.; Xu, J.; Zhang, X. GhTZF1 regulates drought stress responses and delays leaf senescence by inhibiting reactive oxygen species accumulation in transgenic Arabidopsis. Plant Mol. Biol. 2014, 85, 163–177. [Google Scholar] [CrossRef]
- Jan, A.; Maruyama, K.; Todaka, D.; Kidokoro, S.; Abo, M.; Yoshimura, E.; Shinozaki, K.; Nakashima, K.; Yamaguchi-Shinozaki, K. OsTZF1, a CCCH-tandem zinc finger protein, confers delayed senescence and stress tolerance in rice by regulating stress-related genes. Plant Physiol. 2013, 161, 1202–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-j.; Jung, H.J.; Kang, H.; Kim, S.Y. Arabidopsis zinc finger proteins AtC3H49/AtTZF3 and AtC3H20/AtTZF2 are involved in ABA and JA responses. Plant Cell Physiol. 2012, 53, 673–686. [Google Scholar] [CrossRef]
- Huang, P.; Ju, H.-W.; Min, J.-H.; Zhang, X.; Chung, J.-S.; Cheong, H.-S.; Kim, C.S. Molecular and physiological characterization of the Arabidopsis thaliana Oxidation-related Zinc Finger 2, a plasma membrane protein involved in ABA and salt stress response through the ABI2-mediated signaling pathway. Plant Cell Physiol. 2012, 53, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Kong, Z.; Li, M.; Yang, W.; Xu, W.; Xue, Y. A novel nuclear-localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice. Plant Physiol. 2006, 141, 1376–1388. [Google Scholar] [CrossRef] [Green Version]
- Seok, H.-Y.; Nguyen, L.V.; Park, H.-Y.; Tarte, V.N.; Ha, J.; Lee, S.-Y.; Moon, Y.-H. Arabidopsis non-TZF gene AtC3H17 functions as a positive regulator in salt stress response. Biochem. Biophys. Res. Commun. 2018, 498, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, H.; Wang, Y.; Han, G.; Sui, N. Overexpression of CCCH zinc finger protein gene delays flowering time and enhances salt tolerance in Arabidopsis by increasing fatty acid unsaturation. Acta Physiol. Plant 2018, 40, 1842. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Xu, Y.; Li, H.; Wu, X.; Xie, Q.; Li, C. The CCCH-type zinc finger proteins AtSZF1 and AtSZF2 regulate salt stress responses in Arabidopsis. Plant Cell Physiol. 2007, 48, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Eschen-Lippold, L.; Athmer, B.; Baruah, M.; Brode, M.; Maldonado-Bonilla, L.D.; Hoehenwarter, W.; Hause, G.; Scheel, D.; Lee, J. Phosphorylation-dependent control of an RNA granule-localized protein that fine-tunes defence gene expression at a post-transcriptional level. Plant J. 2020, 101, 1023–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Bonilla, L.D.; Eschen-Lippold, L.; Gago-Zachert, S.; Tabassum, N.; Bauer, N.; Scheel, D.; Lee, J. The Arabidopsis tandem zinc finger 9 protein binds RNA and mediates pathogen-associated molecular pattern-triggered immune responses. Plant Cell Physiol. 2014, 55, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-H.; Yu, Y.-P.; Wang, D.; Wu, C.-A.; Yang, G.-D.; Huang, J.-G.; Zheng, C.-C. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol. 2009, 183, 62–75. [Google Scholar] [CrossRef]
- Huang, P.; Chung, M.-S.; Ju, H.-W.; Na, H.-S.; Lee, D.J.; Cheong, H.-S.; Kim, C.S. Physiological characterization of the Arabidopsis thaliana oxidation-related zinc finger 1, a plasma membrane protein involved in oxidative stress. J. Plant Res. 2011, 124, 699–705. [Google Scholar] [CrossRef]
- Kim, W.-C.; Kim, J.-Y.; Ko, J.-H.; Kang, H.; Kim, J.; Han, K.-H. AtC3H14, a plant-specific tandem CCCH zinc-finger protein, binds to its target mRNAs in a sequence-specific manner and affects cell elongation in Arabidopsis thaliana. Plant J. 2014, 80, 772–784. [Google Scholar] [CrossRef]
- Pomeranz, M.C.; Hah, C.; Lin, P.-C.; Kang, S.G.; Finer, J.J.; Blackshear, P.J.; Jang, J.-C. The Arabidopsis tandem zinc finger protein AtTZF1 traffics between the nucleus and cytoplasmic foci and binds both DNA and RNA. Plant Physiol. 2010, 152, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Kedersha, N.; Ivanov, P.; Anderson, P. Stress granules and cell signaling: More than just a passing phase? Trends Biochem. Sci. 2013, 38, 494–506. [Google Scholar] [CrossRef] [Green Version]
- Parker, R.; Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 2007, 25, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Anderson, P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007, 431, 61–81. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Liu, T.; Xiong, X.; Liu, W.; Yu, Y.; Cao, J. Overexpression of Two CCCH-type Zinc-Finger Protein Genes Leads to Pollen Abortion in Brassica campestris ssp. chinensis. Genes 2020, 11, 1287. https://doi.org/10.3390/genes11111287
Xu L, Liu T, Xiong X, Liu W, Yu Y, Cao J. Overexpression of Two CCCH-type Zinc-Finger Protein Genes Leads to Pollen Abortion in Brassica campestris ssp. chinensis. Genes. 2020; 11(11):1287. https://doi.org/10.3390/genes11111287
Chicago/Turabian StyleXu, Liai, Tingting Liu, Xingpeng Xiong, Weimiao Liu, Youjian Yu, and Jiashu Cao. 2020. "Overexpression of Two CCCH-type Zinc-Finger Protein Genes Leads to Pollen Abortion in Brassica campestris ssp. chinensis" Genes 11, no. 11: 1287. https://doi.org/10.3390/genes11111287
APA StyleXu, L., Liu, T., Xiong, X., Liu, W., Yu, Y., & Cao, J. (2020). Overexpression of Two CCCH-type Zinc-Finger Protein Genes Leads to Pollen Abortion in Brassica campestris ssp. chinensis. Genes, 11(11), 1287. https://doi.org/10.3390/genes11111287




