Novel Pathogenic Sequence Variants in NR2E3 and Clinical Findings in Three Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Clinical Data
2.3. Molecular Genetic Analysis
3. Results
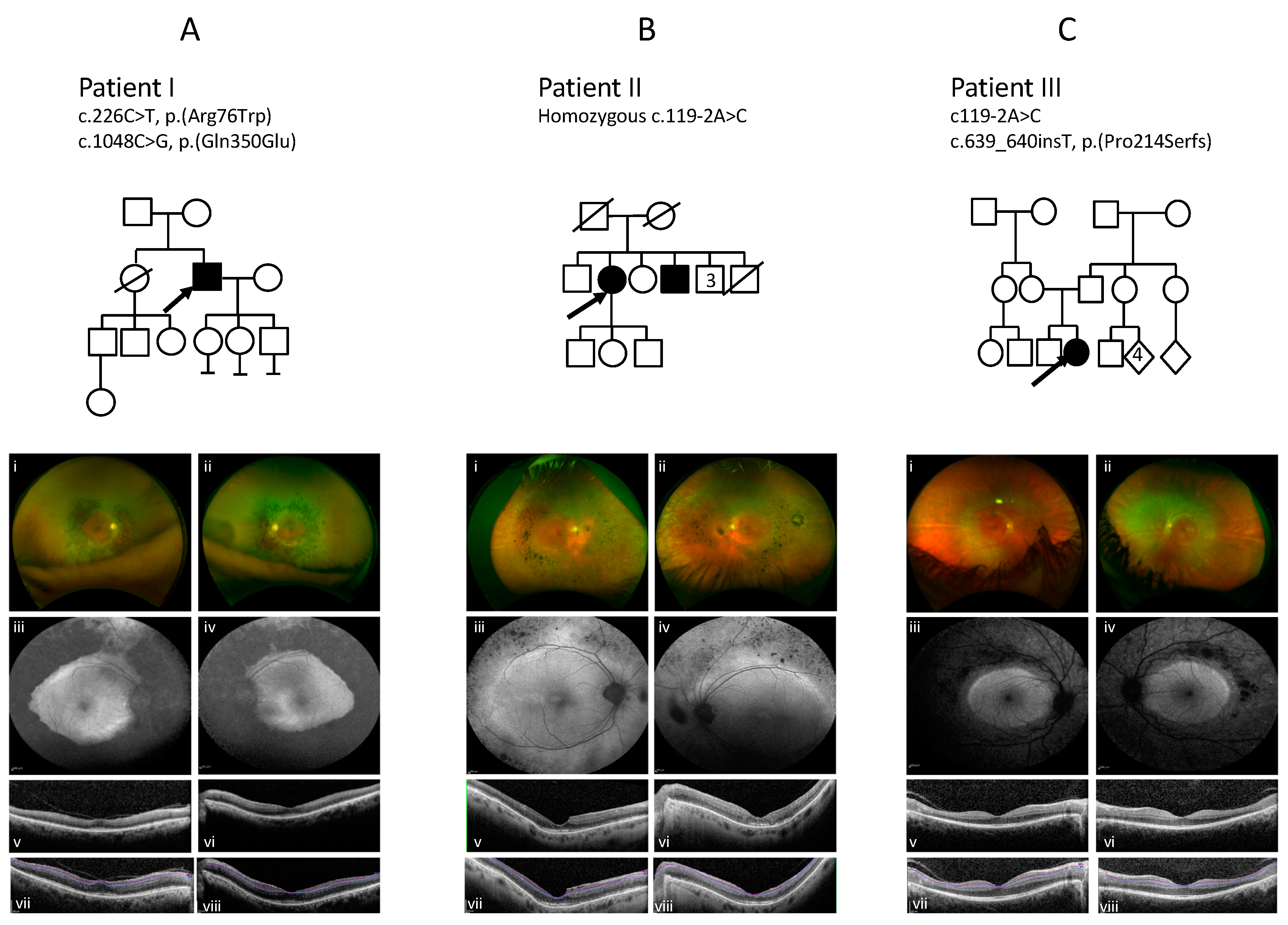
3.1. Patient I
3.2. Patient II
3.3. Patient III
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haider, N.B.; Jacobson, S.G.; Cideciyan, A.V.; Swiderski, R.; Streb, L.M.; Searby, C.; Beck, G.; Hockey, R.; Hanna, D.B.; Gorman, S.; et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 2000, 24, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Jacobson, S.G.; Foerster, M.H.; Kellner, U.; Weleber, R.G. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am. J. Ophthalmol. 1990, 110, 124–134. [Google Scholar] [CrossRef]
- Audo, I.; Michaelides, M.; Robson, A.G.; Hawlina, M.; Vaclavik, V.; Sandbach, J.M.; Neveu, M.M.; Hogg, C.R.; Hunt, D.M.; Moore, A.T.; et al. Phenotypic variation in enhanced S-cone syndrome. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2082–2093. [Google Scholar] [CrossRef]
- Gelman, R.; Greenberg, J.P.; Duncker, T.; Nguyen, H.V.; Yannuzzi, L.A.; Tsang, S.H. Hyperautofluorescent macular ring in a series of patients with enhanced S-cone syndrome. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 592–595. [Google Scholar] [CrossRef] [Green Version]
- Milam, A.H.; Rose, L.; Cideciyan, A.V.; Barakat, M.R.; Tang, W.X.; Gupta, N.; Aleman, T.S.; Wright, A.F.; Stone, E.M.; Sheffield, V.C.; et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, M.J.; Scavelli, K.T.; Uyhazi, K.E.; Bedoukian, E.C.; Serrano, L.W.; Edelstein, I.D.; Vergilio, G.; Cooper, R.F.; Morgan, J.I.W.; Kumar, P.; et al. Enhanced S-Cone Syndrome: Visual Function, Cross-Sectional Imaging, and Cellular Structure with Adaptive Optics Ophthalmoscopy. Retin. Cases Brief Rep. 2019. [Google Scholar] [CrossRef]
- Hood, D.C.; Cideciyan, A.V.; Roman, A.J.; Jacobson, S.G. Enhanced S cone syndrome: Evidence for an abnormally large number of S cones. Vis. Res. 1995, 35, 1473–1481. [Google Scholar] [CrossRef] [Green Version]
- Pachydaki, S.I.; Klaver, C.C.; Barbazetto, I.A.; Roy, M.S.; Gouras, P.; Allikmets, R.; Yannuzzi, L.A. Phenotypic features of patients with NR2E3 mutations. Arch. Ophthalmol. 2009, 127, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Roman, A.J.; Jacobson, S.G. S cone-driven but not S cone-type electroretinograms in the enhanced S cone syndrome. Exp. Eye Res. 1991, 53, 685–690. [Google Scholar] [CrossRef]
- Hull, S.; Arno, G.; Sergouniotis, P.I.; Tiffin, P.; Borman, A.D.; Chandra, A.; Robson, A.G.; Holder, G.E.; Webster, A.R.; Moore, A.T. Clinical and molecular characterization of enhanced S-cone syndrome in children. JAMA Ophthalmol. 2014, 132, 1341–1349. [Google Scholar] [CrossRef] [Green Version]
- Favre, M. Two cases of hyaloid-retinal degeneration. Ophthalmologica 1958, 135, 604–609. [Google Scholar] [CrossRef] [PubMed]
- To, K.W.; Adamian, M.; Jakobiec, F.A.; Berson, E.L. Clinical and histopathologic findings in clumped pigmentary retinal degeneration. Arch. Ophthalmol. 1996, 114, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Batioglu, F. Goldmann-Favre vitreoretinal degeneration. Eur. J. Ophthalmol. 2003, 13, 307–310. [Google Scholar] [CrossRef]
- Fishman, G.A.; Jampol, L.M.; Goldberg, M.F. Diagnostic features of the Favre-Goldmann syndrome. Br. J. Ophthalmol. 1976, 60, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Chavala, S.H.; Sari, A.; Lewis, H.; Pauer, G.J.; Simpson, E.; Hagstrom, S.A.; Traboulsi, E.I. An Arg311Gln NR2E3 mutation in a family with classic Goldmann-Favre syndrome. Br. J. Ophthalmol. 2005, 89, 1065–1066. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Hayashi, T.; Kozaki, K.; Kubo, A.; Omoto, S.; Watanabe, A.; Toda, K.; Takeuchi, T.; Gekka, T.; Kitahara, K. Enhanced S-cone syndrome in a Japanese family with a nonsense NR2E3 mutation (Q350X). Acta Ophthalmol. Scand. 2004, 82, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Udar, N.; Small, K.; Chalukya, M.; Silva-Garcia, R.; Marmor, M. Developmental or degenerative--NR2E3 gene mutations in two patients with enhanced S cone syndrome. Mol. Vis. 2011, 17, 519–525. [Google Scholar]
- Cima, I.; Brecelj, J.; Sustar, M.; Coppieters, F.; Leroy, B.P.; De Baere, E.; Hawlina, M. Enhanced S-cone syndrome with preserved macular structure and severely depressed retinal function. Doc. Ophthalmol. Adv. Ophthalmol. 2012, 125, 161–168. [Google Scholar] [CrossRef]
- Collison, F.T.; Park, J.C.; Fishman, G.A.; Stone, E.M.; McAnany, J.J. Two-color pupillometry in enhanced S-cone syndrome caused by NR2E3 mutations. Doc. Ophthalmol. Adv. Ophthalmol. 2016, 132, 157–166. [Google Scholar] [CrossRef]
- Kuniyoshi, K.; Hayashi, T.; Sakuramoto, H.; Mishima, H.; Tsuneoka, H.; Tsunoda, K.; Iwata, T.; Shimomura, Y. New truncation mutation of the NR2E3 gene in a Japanese patient with enhanced S-cone syndrome. Jpn. J. Ophthalmol. 2016, 60, 476–485. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takezawa, S.; Hara, K.; Yu, R.T.; Umesono, Y.; Agata, K.; Taniwaki, M.; Yasuda, K.; Umesono, K. Identification of a photoreceptor cell-specific nuclear receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 4814–4819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollema, N.; Haider, N.B. Focus on molecules: Nuclear hormone receptor Nr2e3: Impact on retinal development and disease. Exp. Eye Res. 2010, 91, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Bumsted O’Brien, K.M.; Cheng, H.; Jiang, Y.; Schulte, D.; Swaroop, A.; Hendrickson, A.E. Expression of photoreceptor-specific nuclear receptor NR2E3 in rod photoreceptors of fetal human retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2807–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Khanna, H.; Oh, E.C.; Hicks, D.; Mitton, K.P.; Swaroop, A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum. Mol. Genet. 2004, 13, 1563–1575. [Google Scholar] [CrossRef]
- Chen, J.; Rattner, A.; Nathans, J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J. Neurosci. 2005, 25, 118–129. [Google Scholar] [CrossRef]
- Peng, G.H.; Ahmad, O.; Ahmad, F.; Liu, J.; Chen, S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum. Mol. Genet. 2005, 14, 747–764. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.C.; Cheng, H.; Hao, H.; Jia, L.; Khan, N.W.; Swaroop, A. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res. 2008, 1236, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Haider, N.B.; Mollema, N.; Gaule, M.; Yuan, Y.; Sachs, A.J.; Nystuen, A.M.; Naggert, J.K.; Nishina, P.M. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp. Eye Res. 2009, 89, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Cehajic-Kapetanovic, J.; Cottriall, C.L.; Jolly, J.K.; Shanks, M.; Clouston, P.; Charbel Issa, P.; MacLaren, R.E. Electrophysiological verification of enhanced S-cone syndrome caused by a novel c.755T>C NR2E3 missense variant. Ophthalmic Genet. 2018. [Google Scholar] [CrossRef]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. Adv. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef]
- Shah, M.; Shanks, M.; Packham, E.; Williams, J.; Haysmoore, J.; MacLaren, R.E.; Nemeth, A.H.; Clouston, P.; Downes, S.M. Next generation sequencing using phenotype-based panels for genetic testing in inherited retinal diseases. Ophthalmic Genet. 2020, 41, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [Green Version]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Murro, V.; Mucciolo, D.P.; Sodi, A.; Passerini, I.; Giorgio, D.; Virgili, G.; Rizzo, S. Novel clinical findings in autosomal recessive NR2E3-related retinal dystrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2019, 257, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, N.B.; Piriev, N.I.; Chang, B.; Rapoport, A.L.; Hawes, N.L.; Nishina, P.M.; Nusinowitz, S.; Heckenlively, J.R.; Roderick, T.H.; Kozak, C.A.; et al. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 5551–5556. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Han, S.; Qu, Z.; Liu, F.; Li, J.; Yu, S.; Reilly, J.; Tu, J.; Liu, X.; Lu, Z.; et al. Knockout of Nr2e3 prevents rod photoreceptor differentiation and leads to selective L-/M-cone photoreceptor degeneration in zebrafish. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Sharon, D.; Sandberg, M.A.; Caruso, R.C.; Berson, E.L.; Dryja, T.P. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch. Ophthalmol. 2003, 121, 1316–1323. [Google Scholar] [CrossRef] [Green Version]
- Kuniyoshi, K.; Hayashi, T.; Sakuramoto, H.; Nakao, A.; Sato, T.; Utsumi, T.; Tsuneoka, H.; Shimomura, Y. Novel mutations in enhanced S-cone syndrome. Ophthalmology 2013, 120, 431.e1–431.e6. [Google Scholar] [CrossRef]
- Park, S.P.; Hong, I.H.; Tsang, S.H.; Lee, W.; Horowitz, J.; Yzer, S.; Allikmets, R.; Chang, S. Disruption of the human cone photoreceptor mosaic from a defect in NR2E3 transcription factor function in young adults. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2013, 251, 2299–2309. [Google Scholar] [CrossRef]
- Iannaccone, A.; Fung, K.H.; Eyestone, M.E.; Stone, E.M. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am. J. Ophthalmol. 2009, 147, 307–312.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassiman, C.; Spileers, W.; De Baere, E.; de Ravel, T.; Casteels, I. Peculiar fundus abnormalities and pathognomonic electrophysiological findings in a 14-month-old boy with NR2E3 mutations. Ophthalmic Genet. 2013, 34, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Escher, P.; Gouras, P.; Roduit, R.; Tiab, L.; Bolay, S.; Delarive, T.; Chen, S.; Tsai, C.C.; Hayashi, M.; Zernant, J.; et al. Mutations in NR2E3 can cause dominant or recessive retinal degenerations in the same family. Hum. Mutat. 2009, 30, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandah, D.; Merin, S.; Ashhab, M.; Banin, E.; Sharon, D. The spectrum of retinal diseases caused by NR2E3 mutations in Israeli and Palestinian patients. Arch. Ophthalmol. 2009, 127, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.; Solans, T.; Gamundi, M.J.; Hernan, I.; de Jorge, L.; Carballo, M.; Navarro, R.; Tizzano, E.; Ayuso, C.; Baiget, M. Analysis of the involvement of the NR2E3 gene in autosomal recessive retinal dystrophies. Clin. Genet. 2008, 73, 360–366. [Google Scholar] [CrossRef]
- Bohrer, L.R.; Wiley, L.A.; Burnight, E.R.; Cooke, J.A.; Giacalone, J.C.; Anfinson, K.R.; Andorf, J.L.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Correction of NR2E3 Associated Enhanced S-cone Syndrome Patient-specific iPSCs using CRISPR-Cas9. Genes 2019, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Kanda, A.; Swaroop, A. A comprehensive analysis of sequence variants and putative disease-causing mutations in photoreceptor-specific nuclear receptor NR2E3. Mol. Vis. 2009, 15, 2174–2184. [Google Scholar]
- Wright, A.F.; Reddick, A.C.; Schwartz, S.B.; Ferguson, J.S.; Aleman, T.S.; Kellner, U.; Jurklies, B.; Schuster, A.; Zrenner, E.; Wissinger, B.; et al. Mutation analysis of NR2E3 and NRL genes in Enhanced S Cone Syndrome. Hum. Mutat. 2004, 24, 439. [Google Scholar] [CrossRef]
- Cruz, N.M.; Yuan, Y.; Leehy, B.D.; Baid, R.; Kompella, U.; DeAngelis, M.M.; Escher, P.; Haider, N.B. Modifier genes as therapeutics: The nuclear hormone receptor Rev Erb α (Nr1d1) rescues Nr2e3 associated retinal disease. PLoS ONE 2014, 9, e87942. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Datta, S.; Brabbit, E.; Love, Z.; Woytowicz, V.; Flattery, K.; Capri, J.; Yao, K.; Wu, S.; Imboden, M.; et al. Nr2e3 is a genetic modifier that rescues retinal degeneration and promotes homeostasis in multiple models of retinitis pigmentosa. Gene Ther. 2020. [Google Scholar] [CrossRef] [Green Version]
- Neveling, K.; Collin, R.W.; Gilissen, C.; van Huet, R.A.; Visser, L.; Kwint, M.P.; Gijsen, S.J.; Zonneveld, M.N.; Wieskamp, N.; de Ligt, J.; et al. Next-generation genetic testing for retinitis pigmentosa. Hum. Mutat. 2012, 33, 963–972. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, E.R.; Robson, A.G.; Arno, G.; Boon, C.; Webster, A.A.; Michaelides, M. Enhanced S-cone syndrome: Spectrum of clinical, imaging, electrophysiological and genetic findings in a retrospective case series of 56 patients. Ophthalmol. Retin. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.L.; Goldberg, J.L.; Hartley, K.L.; Stone, E.M.; Liu, M. Atypical mild enhanced S-cone syndrome with novel compound heterozygosity of the NR2E3 gene. Am. J. Ophthalmol. 2007, 144, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Beryozkin, A.; Zelinger, L.; Bandah-Rozenfeld, D.; Shevach, E.; Harel, A.; Storm, T.; Sagi, M.; Eli, D.; Merin, S.; Banin, E.; et al. Identification of mutations causing inherited retinal degenerations in the israeli and palestinian populations using homozygosity mapping. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Alpen, D.; Tran, H.V.; Guex, N.; Venturini, G.; Munier, F.L.; Schorderet, D.F.; Haider, N.B.; Escher, P. Differential dimerization of variants linked to enhanced S-cone sensitivity syndrome (ESCS) located in the NR2E3 ligand-binding domain. Hum. Mutat. 2015, 36, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Littink, K.W.; Stappers, P.T.Y.; Riemslag, F.C.C.; Talsma, H.E.; van Genderen, M.M.; Cremers, F.P.M.; Collin, R.W.J.; van den Born, L.I. Autosomal Recessive NRL Mutations in Patients with Enhanced S-Cone Syndrome. Genes 2018, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, D.; Votruba, M. A novel NR2E3 gene mutation in autosomal recessive retinitis pigmentosa with cystic maculopathy. Acta Ophthalmol. 2018, 96, e535–e536. [Google Scholar] [CrossRef] [Green Version]
- Termuhlen, J.; Alex, A.F.; Glockle, N.; Kellner, U.; Fiedler, B.; Eter, N.; Uhlig, C.E. A new mutation in enhanced S-cone syndrome. Acta Ophthalmol. 2018, 96, e539–e540. [Google Scholar] [CrossRef]
- Ellingford, J.M.; Barton, S.; Bhaskar, S.; O’Sullivan, J.; Williams, S.G.; Lamb, J.A.; Panda, B.; Sergouniotis, P.I.; Gillespie, R.L.; Daiger, S.P.; et al. Molecular findings from 537 individuals with inherited retinal disease. J. Med Genet. 2016, 53, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Stone, E.M.; Andorf, J.L.; Whitmore, S.S.; DeLuca, A.P.; Giacalone, J.C.; Streb, L.M.; Braun, T.A.; Mullins, R.F.; Scheetz, T.E.; Sheffield, V.C.; et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology 2017, 124, 1314–1331. [Google Scholar] [CrossRef]
- Lingao, M.D.; Ganesh, A.; Karthikeyan, A.S.; Al Zuhaibi, S.; Al-Hosni, A.; Al Khayat, A.; Capasso, J.; Trumler, A.A.; Stroh, E.; Al Shekaili, H.; et al. Macular cystoid spaces in patients with retinal dystrophy. Ophthalmic Genet. 2016, 37, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Dockery, A.; Stephenson, K.; Keegan, D.; Wynne, N.; Silvestri, G.; Humphries, P.; Kenna, P.F.; Carrigan, M.; Farrar, G.J. Target 5000: Target Capture Sequencing for Inherited Retinal Degenerations. Genes 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Huet, R.A.; Pierrache, L.H.; Meester-Smoor, M.A.; Klaver, C.C.; van den Born, L.I.; Hoyng, C.B.; de Wijs, I.J.; Collin, R.W.; Hoefsloot, L.H.; Klevering, B.J. The efficacy of microarray screening for autosomal recessive retinitis pigmentosa in routine clinical practice. Mol. Vis. 2015, 21, 461–476. [Google Scholar]
- Khan, A.O.; Aldahmesh, M.A.; Al-Harthi, E.; Alkuraya, F.S. Helicoid subretinal fibrosis associated with a novel recessive NR2E3 mutation p.S44X. Arch. Ophthalmol. 2010, 128, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Kannabiran, C.; Singh, H.; Sahini, N.; Jalali, S.; Mohan, G. Mutations in TULP1, NR2E3, and MFRP genes in Indian families with autosomal recessive retinitis pigmentosa. Mol. Vis. 2012, 18, 1165–1174. [Google Scholar]
- Coppieters, F.; Leroy, B.P.; Beysen, D.; Hellemans, J.; De Bosscher, K.; Haegeman, G.; Robberecht, K.; Wuyts, W.; Coucke, P.J.; De Baere, E. Recurrent mutation in the first zinc finger of the orphan nuclear receptor NR2E3 causes autosomal dominant retinitis pigmentosa. Am. J. Hum. Genet. 2007, 81, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Gire, A.I.; Sullivan, L.S.; Bowne, S.J.; Birch, D.G.; Hughbanks-Wheaton, D.; Heckenlively, J.R.; Daiger, S.P. The Gly56Arg mutation in NR2E3 accounts for 1–2% of autosomal dominant retinitis pigmentosa. Mol. Vis. 2007, 13, 1970–1975. [Google Scholar] [PubMed]
- Yang, Y.; Zhang, X.; Chen, L.J.; Chiang, S.W.; Tam, P.O.; Lai, T.Y.; Chan, C.K.; Wang, N.; Lam, D.S.; Pang, C.P. Association of NR2E3 but not NRL mutations with retinitis pigmentosa in the Chinese population. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2229–2235. [Google Scholar] [CrossRef] [Green Version]
- Audo, I.; Bujakowska, K.M.; Leveillard, T.; Mohand-Said, S.; Lancelot, M.E.; Germain, A.; Antonio, A.; Michiels, C.; Saraiva, J.P.; Letexier, M.; et al. Development and application of a next-generation-sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. Orphanet J. Rare Dis. 2012, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, A.; Crippa, S.V.; Kardon, R.; Leon, L.; Hamel, C. Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5562–5569. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, L.S.; Bowne, S.J.; Reeves, M.J.; Blain, D.; Goetz, K.; Ndifor, V.; Vitez, S.; Wang, X.; Tumminia, S.J.; Daiger, S.P. Prevalence of mutations in eyeGENE probands with a diagnosis of autosomal dominant retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6255–6261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Kelly, F.; Garcia Hoyos, M.; Lopez Martinez, M.A.; Lopez-Molina, M.I.; Riveiro-Alvarez, R.; Fernandez-San Jose, P.; Avila-Fernandez, A.; Corton, M.; Millan, J.M.; Garcia Sandoval, B.; et al. Dominant Retinitis Pigmentosa, p.Gly56Arg Mutation in NR2E3: Phenotype in a Large Cohort of 24 Cases. PLoS ONE 2016, 11, e0149473. [Google Scholar] [CrossRef] [Green Version]
- Van Cauwenbergh, C.; Coppieters, F.; Roels, D.; De Jaegere, S.; Flipts, H.; De Zaeytijd, J.; Walraedt, S.; Claes, C.; Fransen, E.; Van Camp, G.; et al. Mutations in Splicing Factor Genes Are a Major Cause of Autosomal Dominant Retinitis Pigmentosa in Belgian Families. PLoS ONE 2017, 12, e0170038. [Google Scholar] [CrossRef]
- Smirnov, V.M.; Dhaenens, C.M.; Vincent-Delorme, C.; Defoort-Dhellemmes, S. Triple hyperautofluorescent retinal ring. Pathognomonic appearance of c.166G>A NR2E3-related inherited retinal degeneration. J. Fr. Ophtalmol. 2019, 42, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guan, L.; Shen, T.; Zhang, J.; Xiao, X.; Jiang, H.; Li, S.; Yang, J.; Jia, X.; Yin, Y.; et al. Mutations of 60 known causative genes in 157 families with retinitis pigmentosa based on exome sequencing. Hum. Genet. 2014, 133, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Zerbib, J.; Blanco Garavito, R.; Gerber, S.; Oubraham, H.; Sikorav, A.; Audo, I.; Kaplan, J.; Rozet, J.M.; Souied, E.H. Retinochoroidal Anastomosis Associated with Enhanced S-Cone Syndrome. Retin. Cases Brief Rep. 2019, 13, 295–299. [Google Scholar] [CrossRef]
- Maeda, A.; Yoshida, A.; Kawai, K.; Arai, Y.; Akiba, R.; Inaba, A.; Takagi, S.; Fujiki, R.; Hirami, Y.; Kurimoto, Y.; et al. Development of a molecular diagnostic test for Retinitis Pigmentosa in the Japanese population. Jpn. J. Ophthalmol. 2018, 62, 451–457. [Google Scholar] [CrossRef]
- Ge, Z.; Bowles, K.; Goetz, K.; Scholl, H.P.; Wang, F.; Wang, X.; Xu, S.; Wang, K.; Wang, H.; Chen, R. NGS-based Molecular diagnosis of 105 eyeGENE((R)) probands with Retinitis Pigmentosa. Sci. Rep. 2015, 5, 18287. [Google Scholar] [CrossRef] [Green Version]
- Roorda, A.; Sundquist, S.; Solovyev, A.; Ratnam, K.; Lujan, B.J.; Stone, E.M.; Duncan, J.L. Adaptive Optics Imaging Reveals Supernormal Cone Density in Enhanced S-Cone Syndrome. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2934. [Google Scholar]
- Rocha-Sousa, A.; Hayashi, T.; Gomes, N.L.; Penas, S.; Brandao, E.; Rocha, P.; Urashima, M.; Yamada, H.; Tsuneoka, H.; Falcao-Reis, F. A novel mutation (Cys83Tyr) in the second zinc finger of NR2E3 in enhanced S-cone syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2011, 249, 201–208. [Google Scholar] [CrossRef]
- Hayashi, T.; Gekka, T.; Goto-Omoto, S.; Takeuchi, T.; Kubo, A.; Kitahara, K. Novel NR2E3 mutations (R104Q, R334G) associated with a mild form of enhanced S-cone syndrome demonstrate compound heterozygosity. Ophthalmology 2005, 112, 2115. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, M.; Duignan, E.; Malone, C.P.; Stephenson, K.; Saad, T.; McDermott, C.; Green, A.; Keegan, D.; Humphries, P.; Kenna, P.F.; et al. Panel-Based Population Next-Generation Sequencing for Inherited Retinal Degenerations. Sci. Rep. 2016, 6, 33248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minnella, A.M.; Pagliei, V.; Savastano, M.C.; Federici, M.; Bertelli, M.; Maltese, P.E.; Placidi, G.; Corbo, G.; Falsini, B.; Caporossi, A. Swept source optical coherence tomography and optical coherence tomography angiography in pediatric enhanced S-cone syndrome: A case report. J. Med. Case Rep. 2018, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, B.; Marzouka, N.A.; Hebrard, M.; Manes, G.; Senechal, A.; Meunier, I.; Hamel, C.P. Homozygosity mapping in autosomal recessive retinitis pigmentosa families detects novel mutations. Mol. Vis. 2013, 19, 2487–2500. [Google Scholar]
- Jinda, W.; Taylor, T.D.; Suzuki, Y.; Thongnoppakhun, W.; Limwongse, C.; Lertrit, P.; Suriyaphol, P.; Trinavarat, A.; Atchaneeyasakul, L.O. Whole exome sequencing in Thai patients with retinitis pigmentosa reveals novel mutations in six genes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2259–2268. [Google Scholar] [CrossRef]
- Patel, N.; Aldahmesh, M.A.; Alkuraya, H.; Anazi, S.; Alsharif, H.; Khan, A.O.; Sunker, A.; Al-Mohsen, S.; Abboud, E.B.; Nowilaty, S.R.; et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet. Med. Off. J. Am. Coll. Med. Genet. 2016, 18, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Eisenberger, T.; Neuhaus, C.; Khan, A.O.; Decker, C.; Preising, M.N.; Friedburg, C.; Bieg, A.; Gliem, M.; Charbel Issa, P.; Holz, F.G.; et al. Increasing the yield in targeted next-generation sequencing by implicating CNV analysis, non-coding exons and the overall variant load: The example of retinal dystrophies. PLoS ONE 2013, 8, e78496. [Google Scholar] [CrossRef] [Green Version]
- Collin, R.W.; van den Born, L.I.; Klevering, B.J.; de Castro-Miro, M.; Littink, K.W.; Arimadyo, K.; Azam, M.; Yazar, V.; Zonneveld, M.N.; Paun, C.C.; et al. High-resolution homozygosity mapping is a powerful tool to detect novel mutations causative of autosomal recessive RP in the Dutch population. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2227–2239. [Google Scholar] [CrossRef] [Green Version]
- Gerber, S.; Rozet, J.M.; Takezawa, S.I.; dos Santos, L.C.; Lopes, L.; Gribouval, O.; Penet, C.; Perrault, I.; Ducroq, D.; Souied, E.; et al. The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the Crypto-Jews from Portugal (Marranos), survivors from the Spanish Inquisition. Hum. Genet. 2000, 107, 276–284. [Google Scholar] [CrossRef]
- Wang, N.K.; Fine, H.F.; Chang, S.; Chou, C.L.; Cella, W.; Tosi, J.; Lin, C.S.; Nagasaki, T.; Tsang, S.H. Cellular origin of fundus autofluorescence in patients and mice with a defective NR2E3 gene. Br. J. Ophthalmol. 2009, 93, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Habibi, I.; Chebil, A.; Falfoul, Y.; Allaman-Pillet, N.; Kort, F.; Schorderet, D.F.; El Matri, L. Identifying mutations in Tunisian families with retinal dystrophy. Sci. Rep. 2016, 6, 37455. [Google Scholar] [CrossRef] [Green Version]
- Neuhaus, C.; Eisenberger, T.; Decker, C.; Nagl, S.; Blank, C.; Pfister, M.; Kennerknecht, I.; Muller-Hofstede, C.; Charbel Issa, P.; Heller, R.; et al. Next-generation sequencing reveals the mutational landscape of clinically diagnosed Usher syndrome: Copy number variations, phenocopies, a predominant target for translational read-through, and PEX26 mutated in Heimler syndrome. Mol. Genet. Genom. Med. 2017, 5, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Abu-Safieh, L.; Alrashed, M.; Anazi, S.; Alkuraya, H.; Khan, A.O.; Al-Owain, M.; Al-Zahrani, J.; Al-Abdi, L.; Hashem, M.; Al-Tarimi, S.; et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013, 23, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Gil, N.; Mendez-Vidal, C.; Romero-Perez, L.; Gonzalez-del Pozo, M.; Rodriguez-de la Rua, E.; Dopazo, J.; Borrego, S.; Antinolo, G. Improving the management of Inherited Retinal Dystrophies by targeted sequencing of a population-specific gene panel. Sci. Rep. 2016, 6, 23910. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, C.; Aboshiha, J.; Henning, G.B.; Sergouniotis, P.I.; Michaelides, M.; Moore, A.T.; Webster, A.R.; Stockman, A. Vision in observers with enhanced S-cone syndrome: An excess of s-cones but connected mainly to conventional s-cone pathways. Investig. Ophthalmol. Vis. Sci. 2014, 55, 963–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemiatkowska, A.M.; Arimadyo, K.; Moruz, L.M.; Astuti, G.D.; de Castro-Miro, M.; Zonneveld, M.N.; Strom, T.M.; de Wijs, I.J.; Hoefsloot, L.H.; Faradz, S.M.; et al. Molecular genetic analysis of retinitis pigmentosa in Indonesia using genome-wide homozygosity mapping. Mol. Vis. 2011, 17, 3013–3024. [Google Scholar] [PubMed]
- Manayath, G.J.; Namburi, P.; Periasamy, S.; Kale, J.A.; Narendran, V.; Ganesh, A. A novel mutation in the NR2E3 gene associated with Goldmann-Favre syndrome and vasoproliferative tumor of the retina. Mol. Vis. 2014, 20, 724–731. [Google Scholar]
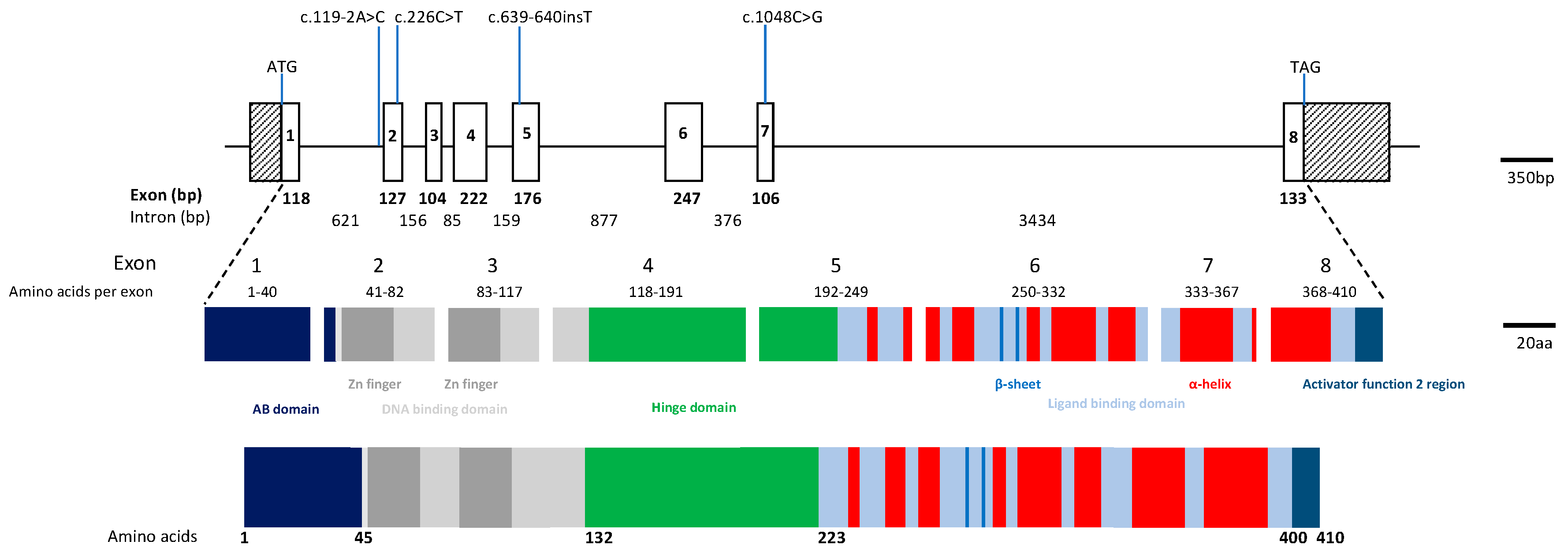

| Patient | Variant | Protein | Genotype | Position | Location | gnomAD MAF | PolyPhen2 | SIFT | Original Reference |
|---|---|---|---|---|---|---|---|---|---|
| I | c.226C > T | p.R76W | Het | Exon 2 | chr15: 72103930 | 0 | D | D | [1] |
| c.1048C > G | p.Q350E | Het | Exon 7 | chr15: 72106406 | 0 | D | D | Novel | |
| II | c.119-2A > C | Splicing | Hom | Intron 1 | chr15: 72103821 | 0.0005 | n/a | n/a | [1] |
| III | c.119-2A > C | Splicing | Het | Intron 1 | chr15: 72103821 | 0.0005 | n/a | n/a | [1] |
| c.639_640insT | p.P214SfsX39 | Het | Exon 5 | chr15: 72104744 | 0.000004 | n/a | n/a | Novel |
| Patient | Gender | Ethnic Origin | First Symptoms (and Age at Diagnosis) | Age of Last Examination (Years) | Symptoms at Last Review & Presence of Cataract | Best Corrected VA | Fundus | OCT | Autofluorescence | Visual Field | ERG | Variant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | M | Nepalese | Night blindness since childhood diagnosed (20) | 66 | Nyctalopia, blurred central vision, photopsia Cataract bilateral: posterior subcapsular | 6/18 RE 6/24 LE | Ring of dense pigment with atrophy surrounding and including the arcades extending to the mid periphery; sparing maculae and peripheral retinas | Central preservation of the retina with loss of the EZ temporal to macula, vitreomacular traction OD Thickened EZ/IZ | Annulus of decreased signal consistent with coalescing atrophy extending from and including the arcades to the mid-periphery and increased signal disturbance within both maculae | Severely constricted field to all isopters 12e below threshold in both eyes <5 degrees | Severe widespread rod, cone, macular and RPE dysfunction with residual S cone response | c.226C>T c.1048C>G |
| II | F | White Caucasian | Night blindness (lifelong) (40) | 69 | Abnormal colour vision, poor contrast, mild photosensitivity Cataracts bilateral | 6/36+1 RE 6/36 LE | Clumped pigmentary deposition extending from, and including the vascular arcades into the mid periphery, with far peripheral atrophy | Disrupted fovea, with thickened EZ and IZ | Increased AF at the maculae and at the arcades with decreased patchy AF in the areas of atrophy in the mid periphery | Partial ring scotoma to V4e stimulus with infratemporal sparing, central scotoma to III.4e in both eyes | PERG was almost extinguished. Both rods and cones were grossly affected, with a similarly reduced and delayed response to standard flash under both scotopic and photopic conditions The 30Hz flicker was markedly abnormal in the presence of a large S cone response. | c.119-2A>C Hom |
| III | F | White Caucasian | Nyctalopia, photophobia and photopsias (10) | 16 | Nyctalopia, photophobia, photopsias Cataract: no | 6/5 RE 6/6 LE | Band of atrophy and white punctate dots in mid-peripheral retina circumferential to the macula | Central preservation of the retina | Increased AF at both maculae with a brighter ring internal to arcades, patchy reduction in signal mapping to the atrophic patches | Ring scotoma to 1.4e stimulus with central 10 degree island. Preserved fields bilaterally to large targets | Relatively well preserved PERG Extinguished rod-specific dim flash responses and reduced and delayed responses to standard flash under both photopic and scotopic conditions. The response to the 30 Hz flicker stimulus was abnormal. | c.119-2A>C c.639_640insT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-khuzaei, S.; Broadgate, S.; Halford, S.; Jolly, J.K.; Shanks, M.; Clouston, P.; Downes, S.M. Novel Pathogenic Sequence Variants in NR2E3 and Clinical Findings in Three Patients. Genes 2020, 11, 1288. https://doi.org/10.3390/genes11111288
Al-khuzaei S, Broadgate S, Halford S, Jolly JK, Shanks M, Clouston P, Downes SM. Novel Pathogenic Sequence Variants in NR2E3 and Clinical Findings in Three Patients. Genes. 2020; 11(11):1288. https://doi.org/10.3390/genes11111288
Chicago/Turabian StyleAl-khuzaei, Saoud, Suzanne Broadgate, Stephanie Halford, Jasleen K. Jolly, Morag Shanks, Penny Clouston, and Susan M. Downes. 2020. "Novel Pathogenic Sequence Variants in NR2E3 and Clinical Findings in Three Patients" Genes 11, no. 11: 1288. https://doi.org/10.3390/genes11111288
APA StyleAl-khuzaei, S., Broadgate, S., Halford, S., Jolly, J. K., Shanks, M., Clouston, P., & Downes, S. M. (2020). Novel Pathogenic Sequence Variants in NR2E3 and Clinical Findings in Three Patients. Genes, 11(11), 1288. https://doi.org/10.3390/genes11111288





