Hp1-1 as a Genetic Marker Regulating Inflammation and the Possibility of Developing Diabetic Complications in Patients with Type 2 Diabetes—Cohort Studies
Abstract
:1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Study Population
2.3. Clinical and Biochemical Measurements
2.4. Haptoglobin Phenotyping
2.5. Assessment of Serum sCD163 Concentration
2.6. Assessment of Serum IL-10 Concentration
2.7. Assessment of Serum p53 Extracellular Secretion
2.8. Assessment of Serum HMGB1 Extracellular Secretion
2.9. Statistical Analyses
3. Results
3.1. Haptoglobin Phenotyping
3.2. Comparative Analysis Results According to the Criterion of the Haptoglobin Phenotype
3.3. Comparative Analysis Results According to the Criterion of the Presence or Absence of Cardiovascular Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SMRIP | specific markers regulating inflammatory processes |
| Hp | haptoglobin |
| Hp1-1 | haptoglobin phenotype 1-1 |
| Hp2-1 | haptoglobin phenotype 2-1 |
| Hp2-2 | haptoglobin phenotype 2-2 |
| HMGB1 | high mobility group box protein 1 |
| IL-10 | interleukin 10 |
| sCD163 | soluble form of CD163 receptor |
| RDW-SD | red blood cell distribution width |
References
- Diabetology, C. Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clin. Diabetol. 2020, 6, 7. [Google Scholar]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arter. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.P.; Purushothaman, K.R.; Levy, N.S.; Purushothaman, M.; Strauss, M.; Asleh, R.; Marsh, S.; Cohen, O.; Moestrup, S.K.; Moller, H.J.; et al. Downregulation of the Hemoglobin Scavenger Receptor in Individuals With Diabetes and the Hp 2-2 Genotype. Circ. Res. 2007, 101, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, R.L.; Yiamunaa, M.; Liu, S.; Liu, J.J.; Chan, C.; Choo, R.W.M.; Ang, K.; Sum, C.F.; Tavintharan, S.; Lim, S.C. Association of haptoglobin phenotype with incident acute myocardial infarction in Chinese patients with type 2 diabetes. Cardiovasc. Diabetol. 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langlois, M.R.; Delanghe, J.R. Biological and clinical significance of haptoglobin polymorphism in humans. Clin. Chem. 1996, 42, 1589–1600. [Google Scholar] [CrossRef] [Green Version]
- Levy, N.S.; Vardi, M.; Blum, S.; Miller-Lotan, R.; Afinbinder, Y.; Cleary, P.A.; Paterson, A.D.; Bharaj, B.; Snell-Bergeon, J.K.; Rewers, M.J.; et al. An enzyme linked immunosorbent assay (ELISA) for the determination of the human haptoglobin phenotype. Clin. Chem. Lab. Med. 2013, 51, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Filipek, A.; Czerwińska, M.E.; Kiss, A.K.; Wrzosek, M.; Naruszewicz, M. Oleacein enhances anti-inflammatory activity of human macrophages by increasing CD163 receptor expression. Phytomedicine 2015, 22, 1255–1261. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Dijkstra, C.D.; Berg, T.K.V.D. The macrophage scavenger receptor CD 163. Immunology 2005, 210, 153–160. [Google Scholar] [CrossRef]
- Philippidis, P.; Mason, J.; Evans, B.J.; Nadra, I.; Taylor, K.M.; Haskard, D.O.; Landis, R.C. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: Antiinflammatory monocyte- macrophage responses In Vitro, in resolving skin blisters In Vivo, and after cardiopulmonary bypass surgery. Circ. Res. 2004, 94, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Buechler, C.; Ritter, M.; Orso, E.; Langmann, T.; Klucken, J.; Schmitz, G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and anti-inflammatory stimuli. J. Leukoc. Biol. 2000, 67, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.H.; Zarev, P.V. Human monocyte CD163 expression inversely correlates with soluble CD 163 plasma levels. Cytom. Part B: Clin. Cytom. 2004, 63, 16–22. [Google Scholar] [CrossRef]
- Moller, H.J.; Peterslund, N.A.; Graversen, J.H.; Moestrup, S.K. Inentification of the hemoglobin scavenger receptor/CD 163 as a natural soluble protein in plasma. Blood 2002, 99, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Sulahian, T.H.; Pioli, P.A.; Wardwell, K.; Guyre, P.M. Cross-linking of FcgammaR triggers shedding of the hemoglobin-haptoglobin scavenger receptor CD163. J. Leukoc. Biol. 2004, 76, 271–277. [Google Scholar] [CrossRef]
- Matsushita, N.; Kashiwagi, M.; Wait, R.; Nagayoshi, R.; Nakamura, M.; Matsuda, T.; Hogger, P.; Guyre, P.M.; Nagase, H.; Matsuyama, T. Elevated levels of soluble CD163 insera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin. Exp. Immunol. 2002, 130, 156–161. [Google Scholar] [CrossRef]
- Willemsen, H.; Van Der Horst, I.; Nieuwland, W.; Slart, R.; Zeebregts, C.; De Boef, E.; Schuitemaker, J.; Zijlstra, F.; Tio, R. The diagnostic value of soluble CD163 in patients presenting with chest pain. Clin. Biochem. 2009, 42, 1662–1666. [Google Scholar] [CrossRef]
- Aristoteli, L.P.; Møller, H.J.; Bailey, B.; Moestrup, S.K.; Kritharides, L. The monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosis. Atherosclerosis 2006, 184, 342–347. [Google Scholar] [CrossRef]
- Mallat, Z.; Besnard, S.; Duriez, M.; Deleuze, V.; Emmanuel, F.; Bureau, M.F.; Soubrier, F.; Esposito, B.; Duez, H.; Fievet, C.; et al. Protective Role of Interleukin-10 in Atherosclerosis. Circ. Res. 1999, 85, e17–e24. [Google Scholar] [CrossRef]
- Lacraz, S.; Nicod, L.P.; Chicheportiche, R.; Welgus, H.G.; Dayer, J.M. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J. Clin. Investig. 1995, 96, 2304–2310. [Google Scholar] [CrossRef] [Green Version]
- Pérez Fernández, R.; Kaski, J.C. Interleukin-10 and Coronary Disease. Rev. Esp. Cardiol. 2002, 55, 738–750. [Google Scholar]
- Zhu, S.; Li, W.; Ward, M.F.; Sama, A.E.; Wang, H. High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation. Inflamm. Allergy-Drug Targets 2010, 9, 60–72. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.W.S.; Westra, J.; Limburg, P.C.; Bijl, M.; Kallenberg, C. HMGB1 in vascular diseases: Its role in vascular inflammation and atherosclerosis. Autoimmun. Rev. 2012, 11, 909–917. [Google Scholar] [CrossRef]
- Kung, C.-P.; Murphy, M.E. The role of the p53 tumor suppressor in metabolism and diabetes. J. Endocrinol. 2016, 231, R61–R75. [Google Scholar] [CrossRef] [Green Version]
- Kolovou, V.; Tsipis, A.; Mihas, C.; Katsiki, N.; Vartela, V.; Koutelou, M.; Manolopoulou, D.; Leondiadis, E.; Iakovou, I.; Mavrogieni, S.; et al. Tumor Protein p53 (TP53) Gene and Left Main Coronary Artery Disease. Angiology 2018, 69, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Filipek, A.; Czerwińska, M.E.; Kiss, A.K.; Polański, J.A.; Naruszewicz, M. Oleacein may inhibit destabilization of carotid plaques from hypertensive patients. Impact on high mobility group protein-1. Phytomedicine 2017, 32, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S. Serum sCD163 as a biomarker of adipose tissue inflammation in obstructive sleep apnoea patients: Limits and perspectives. Eur. Respir. J. 2017, 50, 1701182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Chavan, S.S.; Andersson, U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol. Med. 2015, 21, S6–S12. [Google Scholar] [CrossRef]
- Guetta, J.; Strauss, M.; Levy, N.S.; Levy, A.P. Haptoglobin genotype modulates the balance of Th1/Th2 cytokines produced by macrophages exposed to free hemoglobin. Atherosclerosis 2007, 191, 48–53. [Google Scholar] [CrossRef]
- Spitsina, E.V.; Yakunina, N.Y.; Chudakova, D.A.; Nikitin, A.G.; Svetlova, G.N.; Soluyanova, T.N.; Strokov, I.A.; Nosikov, V.V. Association of polymorphous markers Pro72Arg and C(-594)CC OF TP53 gene with diabetic polyneuropathy in patients with type 1 diabetes mellitus living in Moscow. Mol. Biol. 2007, 41, 901–905. [Google Scholar] [CrossRef]
- Kung, C.-P.; Leu, J.I.-J.; Basu, S.; Khaku, S.; Anokye-Danso, F.; Liu, Q.; George, D.L.; Ahima, R.S.; Murphy, M.E. The P72R Polymorphism of p53 Predisposes to Obesity and Metabolic Dysfunction. Cell Rep. 2016, 14, 2413–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itahana, Y.; Itahana, K. Emerging Roles of p53 Family Members in Glucose Metabolism. Int. J. Mol. Sci. 2018, 19, 776. [Google Scholar] [CrossRef] [Green Version]
- Tavana, O.; Puebla-Osorio, N.; Sang, M.; Zhu, C. Absence of p53-Dependent Apoptosis Combined With Nonhomologous End-Joining Deficiency Leads to a Severe Diabetic Phenotype in Mice. Diabetes 2009, 59, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Liu, J.; Liang, Y.; Wu, R.; Zhao, Y.; Hong, X.; Lin, M.; Yu, H.; Liu, L.; Levine, A.J.; et al. Tumour-associated mutant p53 drives the Warburg effect. Nat. Commun. 2013, 4, 2935. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, J.; Wu, R.; Liang, Y.; Lin, M.; Liu, J.; Chan, C.S.; Hu, W.; Feng, Z. Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD. Oncotarget 2014, 5, 5535–5546. [Google Scholar] [CrossRef]
- Li, Q.; Pang, L.; Shi, H.; Yang, W.; Liu, X.; Su, G.; Dong, Y. High glucose concentration induces retinal endothelial cell apoptosis by activating p53 signaling pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 2401–2407. [Google Scholar] [PubMed]
- Heo, K.-S.; Chang, E.; Le, N.-T.; Cushman, H.; Yeh, E.T.; Fujiwara, K.; Abe, J.-I. De-SUMOylation enzyme of sentrin/SUMO-specific protease 2 regulates disturbed flow-induced SUMOylation of ERK5 and p53 that leads to endothelial dysfunction and atherosclerosis. Circ. Res. 2013, 112, 911–923. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Che, Y.; Bai, L.; Zhang, J.; Fan, J.; Mao, H. High mobility group box 1 in diabetic nephropathy. Exp. Ther. Med. 2017, 14, 2431–2433. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhong, J.; Zhang, X.; Liu, Z.; Yang, Y.; Gong, Q.; Ren, B. The Role of HMGB1 in the Pathogenesis of Type 2 Diabetes. J. Diabetes Res. 2016. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, H.; Czura, C.J.; Tracey, K.J. The cytokine activity of HMGB1. J. Leukoc. Biol. 2005, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagiwara, S.; Iwasaka, H.; Hasegawa, A.; Koga, H.; Noguchi, T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model *. Crit. Care Med. 2008, 36, 2407–2413. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased Toll-Like Receptor (TLR) Activation and TLR Ligands in Recently Diagnosed Type 2 Diabetic Subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Škrha, J., Jr.; Kalousova, M.; Švarcová, J.; Muravska, A.; Kvasnička, J.; Landová, L.; Zima, T.; Škrha, J. Relationship of Soluble RAGE and RAGE Ligands HMGB1 and EN-RAGE to Endothelial Dysfunction in Type 1 and Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2012, 120, 277–281. [Google Scholar] [CrossRef]
- Kanellakis, P.; Agrotis, A.; Kyaw, T.S.; Koulis, C.; Ahrens, I.; Mori, S.; Takahashi, H.K.; Liu, K.; Peter, K.; Nishibori, M.; et al. High-Mobility Group Box Protein 1 Neutralization Reduces Development of Diet-Induced Atherosclerosis in Apolipoprotein E–Deficient Mice. Arter. Thromb. Vasc. Biol. 2011, 31, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Peter, K.; Bobik, A. HMGB1 signals danger in acute coronary syndrome: Emergence of a new risk marker for cardiovascular death? Atherosclerosis 2012, 221, 317–318. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Preedy, V.R. Diabetes: Oxidative Stress and Dietary Antioxidants; Elsevier: London, UK, 2014; Volume 16, p. 158. [Google Scholar]
- Wobeto, V.P.D.A.; Zaccariotto, T.R.; Sonati, M.F. Polymorphism of human haptoglobin and its clinical importance. Genet. Mol. Biol. 2008, 31, 602–620. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Feng, D.; Gu, D.; Zheng, R.; Esperat, C.; Gao, W. Differentially expressed haptoglobin as a potential biomarker for type 2 diabetic mellitus in Hispanic population. BioFactors 2017, 43, 424–433. [Google Scholar] [CrossRef]
- Shi, X.; Sun, L.; Wang, L.; Jin, F.; Sun, J.; Zhu, X.; Tang, L.; Qu, Y.; Yang, Z. Haptoglobin 2-2 Genotype Is Associated with Increased Risk of Type 2 Diabetes Mellitus in Northern Chinese. Genet. Test. Mol. Biomarkers 2012, 16, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, L.E.; Levy, A.P.; Chiuve, S.E.; Jensen, M.K.; Wang, H.; Shara, N.M.; Blum, S.; Howard, B.V.; Pai, J.K.; Mukamal, K.J.; et al. Haptoglobin Genotype Is a Consistent Marker of Coronary Heart Disease Risk Among Individuals With Elevated Glycosylated Hemoglobin. J. Am. Coll. Cardiol. 2013, 61, 728–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, A.; Hochberg, I.; Jablonski, K.; Resnick, H.; Lee, E.T.; Best, L.; Howard, B.V. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes. J. Am. Coll. Cardiol. 2002, 40, 1984–1990. [Google Scholar] [CrossRef] [Green Version]
- Mackellar, M.; Vigerust, D.J. Role of Haptoglobin in Health and Disease: A Focus on Diabetes. Clin. Diabetes 2016, 34, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bale, B.F.; Doneen, A.L.; Vigerust, D.J. Precision Healthcare of Type 2 Diabetic Patients Through Implementation of Haptoglobin Genotyping. Front. Cardiovasc. Med. 2018, 5, 141. [Google Scholar] [CrossRef]
- Costacou, T.; Levy, A.P. Haptoglobin Genotype and Its Role in Diabetic Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2012, 5, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, J.; Zhang, R.; Wang, T.; Yan, D.; He, Z.; Jiang, F.; Hu, C.; Jia, W. Mendelian randomization analysis to assess a causal efect of haptoglobin on macroangiopathy in Chinese type 2 diabetes patients. Cardiovasc. Diabetol. 2018, 17, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awadallah, S.; Hamad, M. The prevalence of type II diabetes mellitus is haptoglobin phenotype-independent. Cytobios 2000, 101, 145–150. [Google Scholar]
- Pechlaner, R.; Kiechl, S.; Willeit, P.; Demetz, E.; Haun, M.; Weger, S.; Oberhollenzer, F.; Kronenberg, F.; Bonora, E.; Weiss, G.; et al. Haptoglobin 2-2 Genotype is Not Associated With Cardiovascular Risk in Subjects With Elevated Glycohemoglobin—Results From the Bruneck Study. J. Am. Hear. Assoc. 2014, 3, e000732. [Google Scholar] [CrossRef] [Green Version]

| Variable | Group without Complications (N = 60) | Group with Complications (N = 34) | t(df = 92) | p-Value (1) | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Age(years) | 59.08 | 7.60 | 61.88 | 6.97 | −1.767 | 0.081 |
| Waist (cm) | 111.32 | 15.23 | 116.03 | 11.58 | −1.564 | 0.121 |
| HbA1c (%) | 9.35 | 1.62 | 9.41 | 1.39 | −0.188 | 0.851 |
| Phosphorus (mg/dL) | 3.63 | 0.65 | 3.79 | 0.58 | −1.186 | 0.239 |
| Neutrophils (%) | 56.21 | 7.83 | 55.59 | 6.79 | 0.390 | 0.698 |
| Lymphocytes (k/uL) | 2.31 | 0.69 | 2.38 | 0.57 | −0.496 | 0.621 |
| MCV (fl) | 89.81 | 3.78 | 91.21 | 4.62 | −1.599 | 0.113 |
| Platelets (k/uL) | 239.77 | 60.17 | 237.32 | 50.14 | 0.200 | 0.842 |
| Variable | Group without Complications (N = 60) | Group with Complications (N = 34) | z | p-Value (2) | ||
|---|---|---|---|---|---|---|
| Mdn | IQR | Mdn | IQR | |||
| BMI (kg/m2) | 32.0 | 8.5 | 35.0 | 8.0 | −1.502 | 0.13 |
| Duration of diabetes (years) | 10.0 | 10.0 | 15.0 | 10.0 | −2.994 | 0.003 |
| Fasting glucose (mg/dL) | 150.0 | 60.0 | 175.0 | 50.0 | −1.707 | 0.090 |
| Glycemia 2 h after a meal (mg/dL) | 205.0 | 90.0 | 250.0 | 100.0 | −2.090 | 0.039 |
| CRP (mg/L) | 2.2 | 2.6 | 2.7 | 3.5 | −0.709 | 0.478 |
| Ca (mmol/L) | 2.4 | 0.1 | 2.4 | 0.2 | −0.559 | 0.576 |
| Vitamin D (ng/mL) | 16.8 | 9.9 | 18.5 | 9.1 | 0.354 | 0.722 |
| Daily dose of metformin (mg) | 2550.0 | 1225.0 | 2550.0 | 1500.0 | 0.562 | 0.586 |
| Leukocytes (tys./uL) | 7.1 | 2.4 | 7.6 | 1.8 | −0.826 | 0.408 |
| Neutrophils (tys./uL) | 3.9 | 2.0 | 3.9 | 1.2 | −0.378 | 0.704 |
| Lymphocytes (%) | 30.5 | 9.8 | 31.6 | 8.2 | −0.956 | 0.341 |
| Hemoglobin (g/dL) | 14.6 | 1.9 | 13.6 | 2.3 | 1.583 | 0.113 |
| Erythrocytes (mln/uL) | 4.9 | 0.6 | 4.8 | 0.8 | 0.775 | 0.440 |
| Hematocrit (%) | 44.3 | 5.7 | 42.7 | 6.5 | 1.165 | 0.244 |
| RDW-SD | 42.0 | 3.3 | 44.3 | 5.2 | −2.062 | 0.038 |
| RDW (%) | 12.9 | 1.1 | 13.1 | 1.0 | −1.611 | 0.108 |
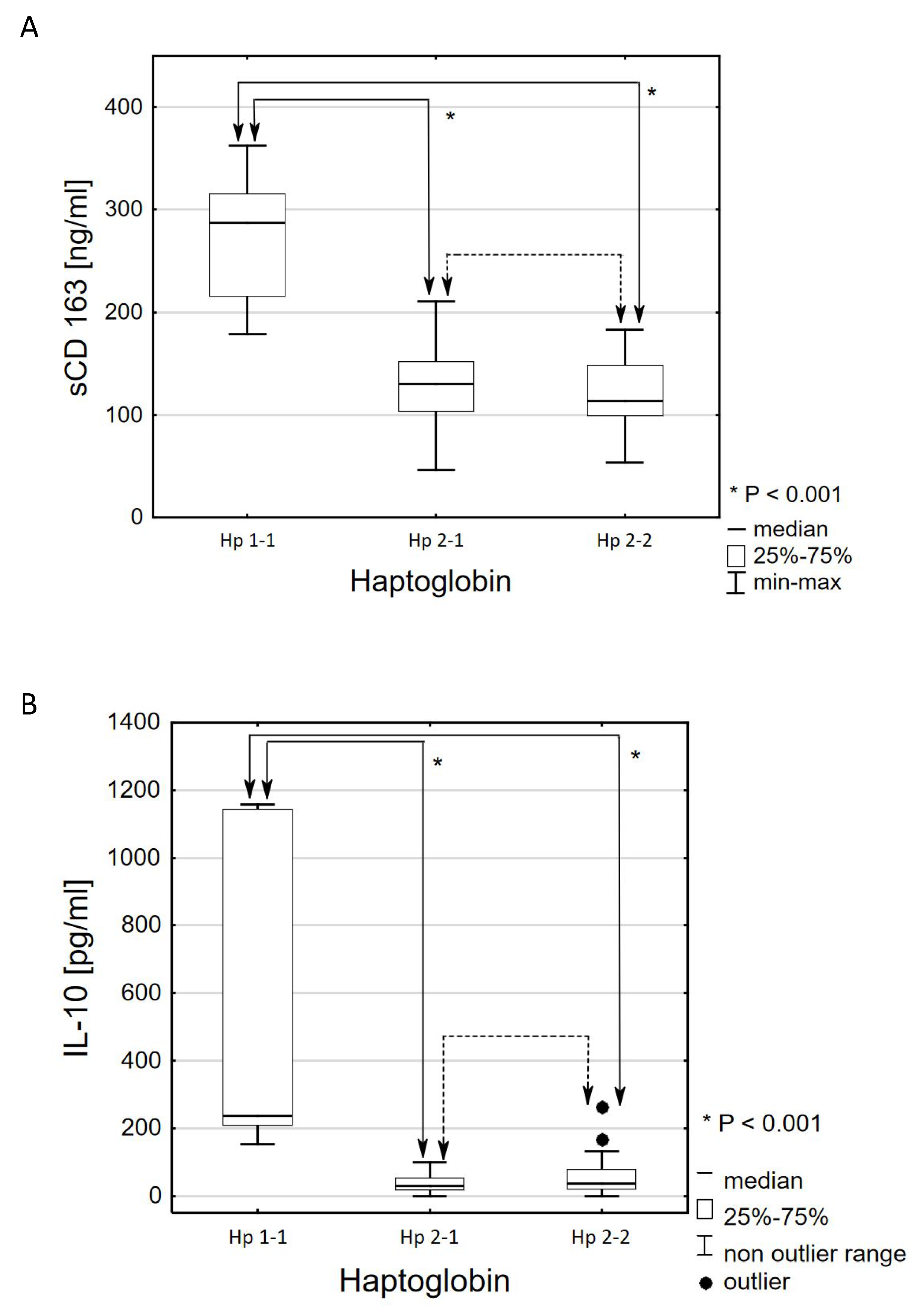
| Il−10 (pg/mL) | 31.9 | 43.7 | 46.1 | 66.0 | −1.319 | 0.188 |
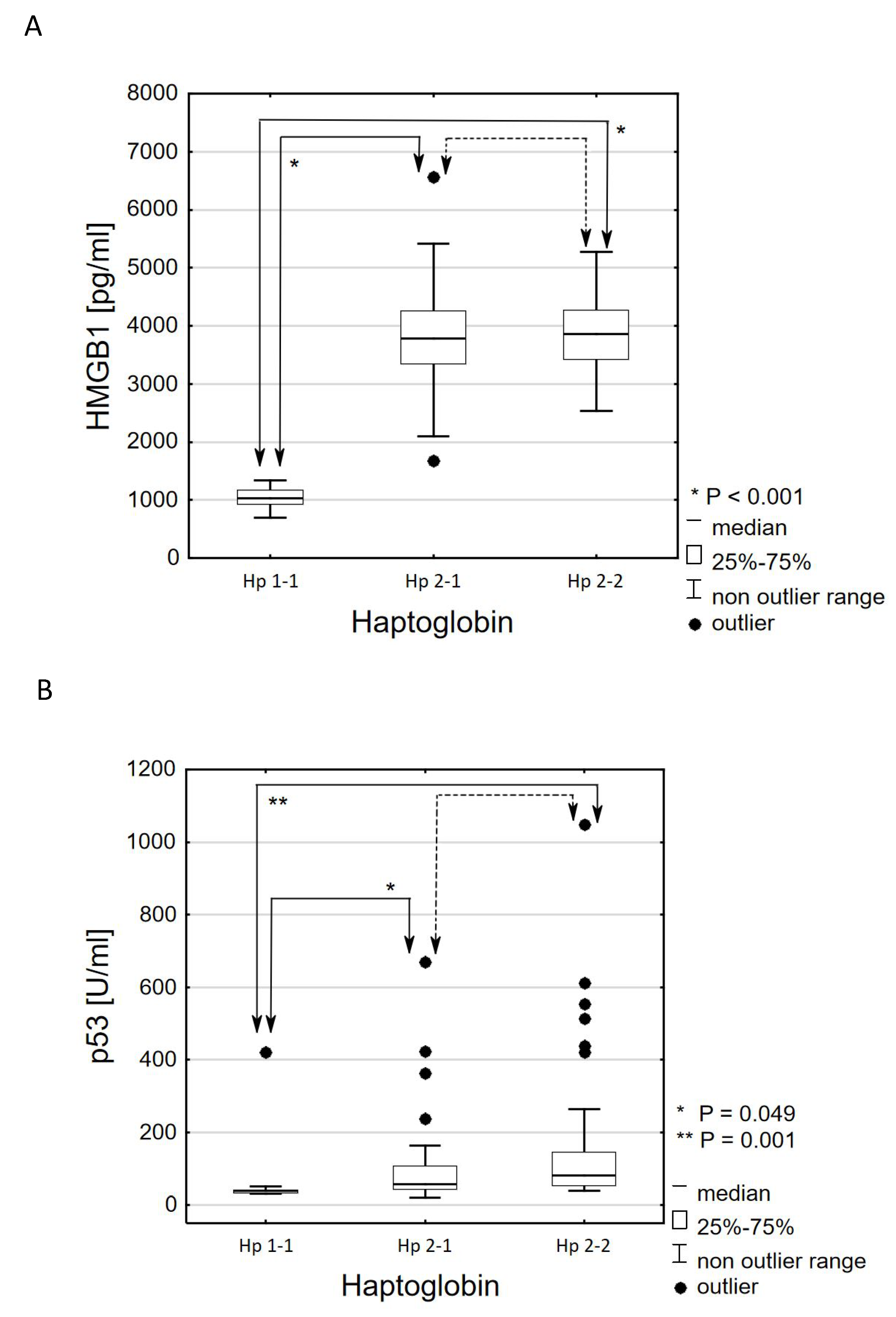
| Protein p53 (U/mL) | 62.4 | 66.8 | 60.9 | 110.2 | −0.574 | 0.565 |
| sCD163 (ng/mL) | 118.0 | 58.3 | 135.1 | 62.1 | −1.098 | 0.274 |
| HMGB1 (pg/mL) | 3811.5 | 834.1 | 3416.2 | 1346.8 | 1.432 | 0.152 |
| Variable | Variants | Group without Complications (N = 60) | Group with Complications (N = 34) | p-Value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Sex | Men | 37 | 61.7 | 19 | 55.9 | 0.664 (3) |
| Women | 23 | 38.3 | 15 | 44.1 | ||
| Nicotine addiction | No | 32 | 53.3 | 12 | 35.3 | 0.132 (3) |
| Yes | 28 | 46.7 | 22 | 64.7 | ||
| Retinopathy | No | 52 | 86.7 | 27 | 79.4 | 0.389 (3) |
| Yes | 8 | 13.3 | 7 | 20.6 | ||
| Additional treatment | Insulin | 40 | 66.7 | 27 | 79.4 | 0.239 (3) |
| Oral medications | 20 | 33.3 | 7 | 20.6 | ||
| Statins or fibrates | No | 20 | 33.3 | 9 | 26.5 | 0.643 (3) |
| Yes | 40 | 66.7 | 25 | 73.5 | ||
| Haptoglobin | Hp1-1 | 5 | 8.3 | 4 | 11.8 | 0.569 (4) |
| Hp2-1 | 26 | 43.3 | 11 | 32.4 | ||
| Hp2-2 | 29 | 48.3 | 19 | 55.9 | ||
| Variable | Group without Complications | Group with Complications | z | p -Value (5) | η2(6) | ||
|---|---|---|---|---|---|---|---|
| Mdn | IQR | Mdn | IQR | ||||
| Group not taking lipid-lowering drugs (3 vs. 11 vs. 15) | |||||||
| HDL (mg/dL) | 46.5 | 11.5 | 42.0 | 8.0 | 1.982 | 0.044 | 0.135 |
| LDL (mg/dL) | 86.0 | 41.0 | 134.0 | 59.0 | -2.641 | 0.006 | 0.241 |
| Total cholesterol | 160.5 | 42.5 | 226.0 | 62.0 | −2.381 | 0.015 | 0.195 |
| TG (mg/dL) | 108.5 | 73.5 | 190.0 | 255.0 | −2.664 | 0.006 | 0.245 |
| Group taking lipid-lowering drugs (40 vs. 25) | |||||||
| HDL (mg/dL) | 41.5 | 16.5 | 43.0 | 10.0 | −0.270 | 0.784 | 0.001 |
| LDL (mg/dL) | 108.5 | 58.0 | 64.0 | 41.0 | 3.291 | 0.001 | 0.167 |
| Total cholesterol | 186.0 | 60.5 | 135.0 | 40.0 | 3.506 | 0.000 | 0.189 |
| TG (mg/dL) | 154.0 | 134.5 | 148.0 | 74.0 | 0.930 | 0.351 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stempkowska, A.; Walicka, M.; Franek, E.; Naruszewicz, M.; Panczyk, M.; Sanchak, Y.; Filipek, A. Hp1-1 as a Genetic Marker Regulating Inflammation and the Possibility of Developing Diabetic Complications in Patients with Type 2 Diabetes—Cohort Studies. Genes 2020, 11, 1253. https://doi.org/10.3390/genes11111253
Stempkowska A, Walicka M, Franek E, Naruszewicz M, Panczyk M, Sanchak Y, Filipek A. Hp1-1 as a Genetic Marker Regulating Inflammation and the Possibility of Developing Diabetic Complications in Patients with Type 2 Diabetes—Cohort Studies. Genes. 2020; 11(11):1253. https://doi.org/10.3390/genes11111253
Chicago/Turabian StyleStempkowska, Anna, Magdalena Walicka, Edward Franek, Marek Naruszewicz, Mariusz Panczyk, Yaroslav Sanchak, and Agnieszka Filipek. 2020. "Hp1-1 as a Genetic Marker Regulating Inflammation and the Possibility of Developing Diabetic Complications in Patients with Type 2 Diabetes—Cohort Studies" Genes 11, no. 11: 1253. https://doi.org/10.3390/genes11111253
APA StyleStempkowska, A., Walicka, M., Franek, E., Naruszewicz, M., Panczyk, M., Sanchak, Y., & Filipek, A. (2020). Hp1-1 as a Genetic Marker Regulating Inflammation and the Possibility of Developing Diabetic Complications in Patients with Type 2 Diabetes—Cohort Studies. Genes, 11(11), 1253. https://doi.org/10.3390/genes11111253







