Fenugreek Stimulates the Expression of Genes Involved in Milk Synthesis and Milk Flow through Modulation of Insulin/GH/IGF-1 Axis and Oxytocin Secretion
Abstract
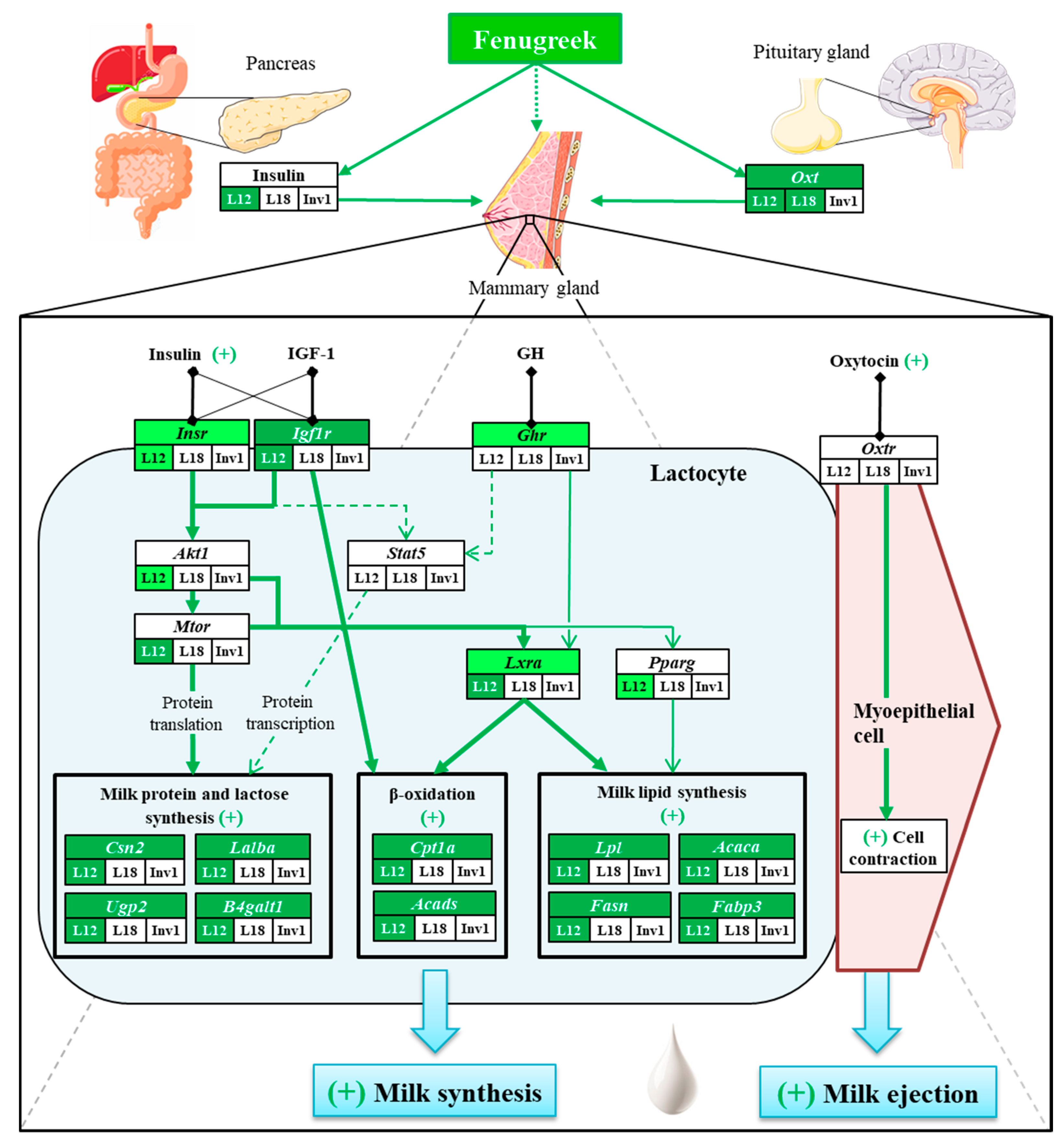
1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.1.1. Housing and Diets
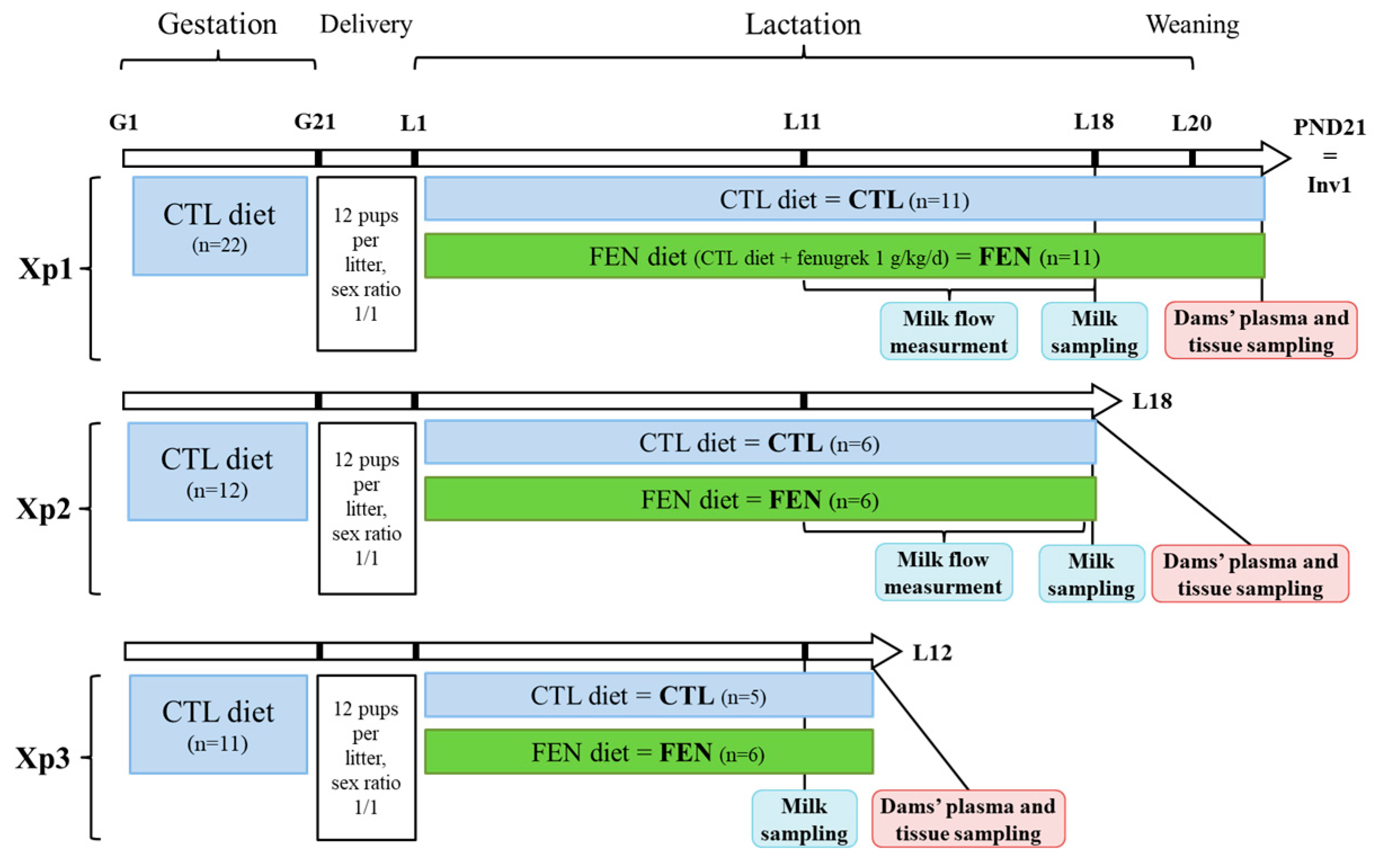
2.1.2. Experimental Design
2.1.3. Dam-Litter Dyad Follow-Up and Milk Flow Measurement
2.1.4. Milk, Plasma and Tissue Sampling
2.2. Determination of Gene Expression in Mammary and Pituitary Glands Using RT-qPCR
2.2.1. RNA Extraction and Purification
2.2.2. Reverse Transcription (RT)
2.2.3. Primers Design and Validation
2.2.4. Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) of Lactaogenic Hormones in Dam Plasma
2.4. Statistical Analysis
3. Results
3.1. Fenugreek Supplementation Enhances Lactation Performances in a Model of Rat Nursing a Large Litter
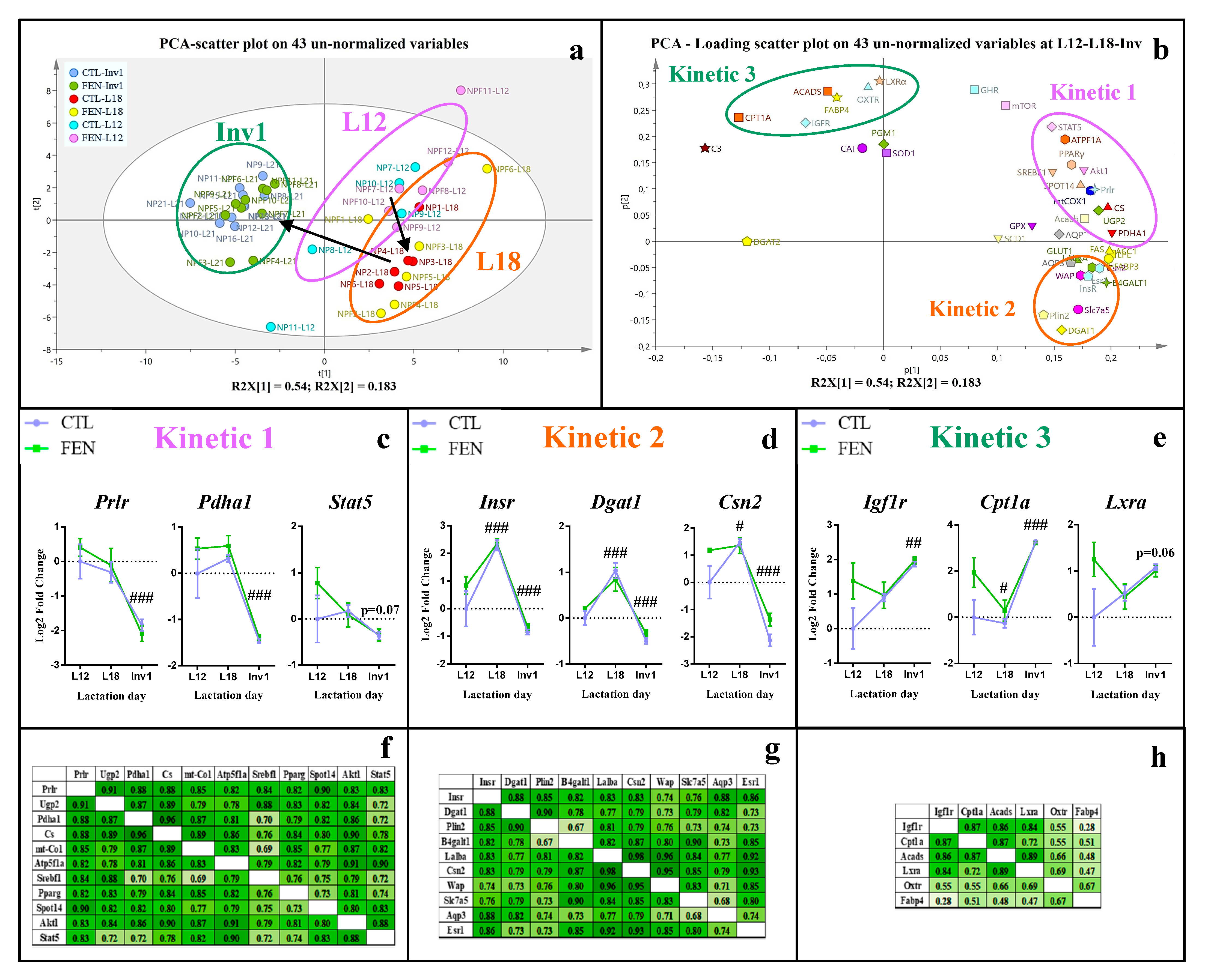
3.2. Different Patterns of Gene Expression in the Mammary and Pituitary Glands and of Plasma Hormone Concentration throughout Lactation and Involution
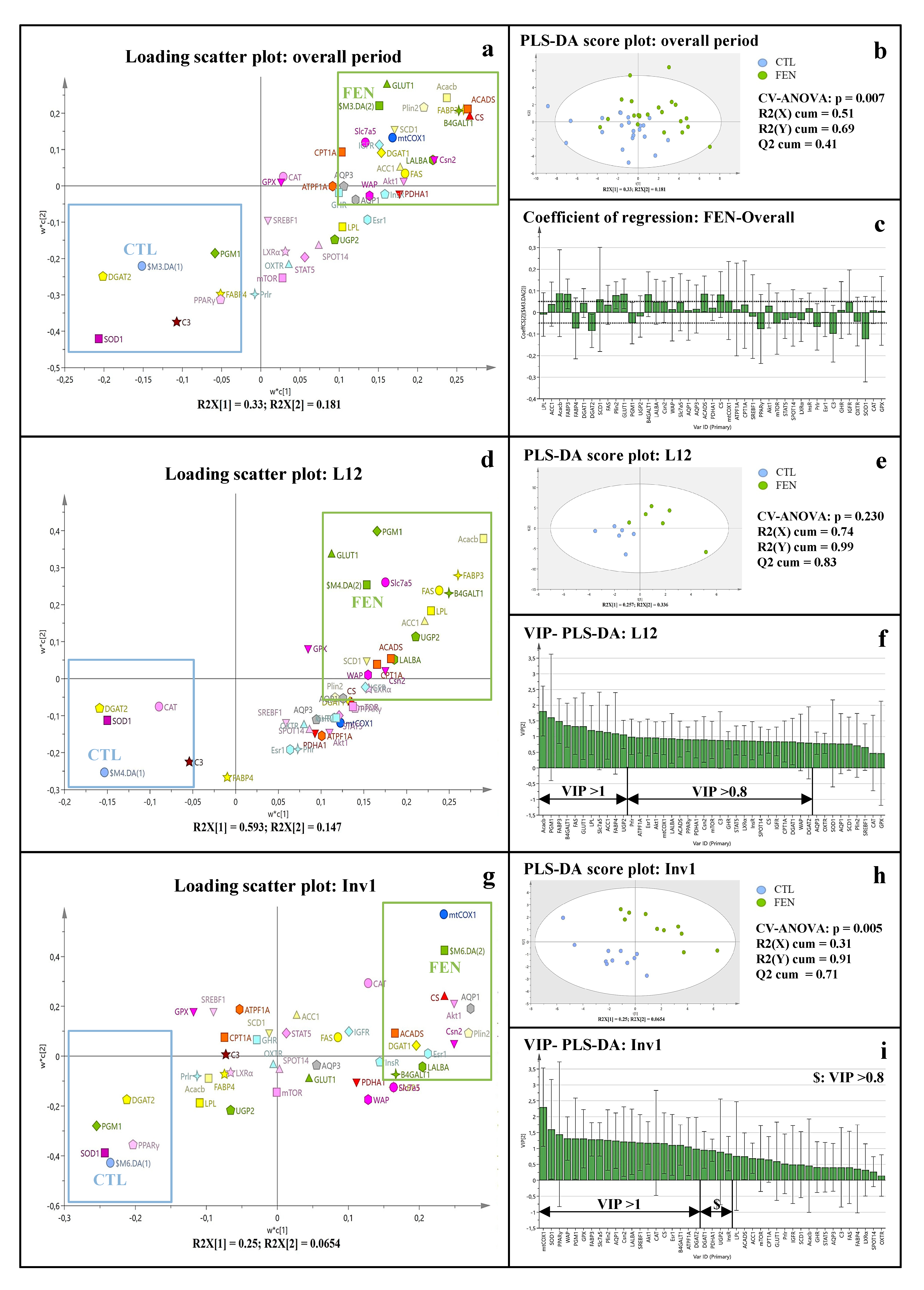
3.3. Fenugreek Supplementation Impacts Mammary Gland Metabolic Pathways Differently during Lactation and at Involution
3.3.1. Fenugreek Supplementation Increases the Expression of Mammary Genes Involved in Lipid, Lactose and Protein Synthesis
3.3.2. Fenugreek Supplementation Increases Expression of Genes Involved in Fuel Metabolism, Particularly Fatty Acid β-Oxidation
3.3.3. Fenugreek Increases the Expression of Regulatory Factors Involved in Protein and Lipid Metabolism
3.3.4. Fenugreek Upregulates the Expression of Insulin, GH and IGF-1 Receptors
3.4. Fenugreek Stimulates Oxytocin Expression at the Pituitary Level
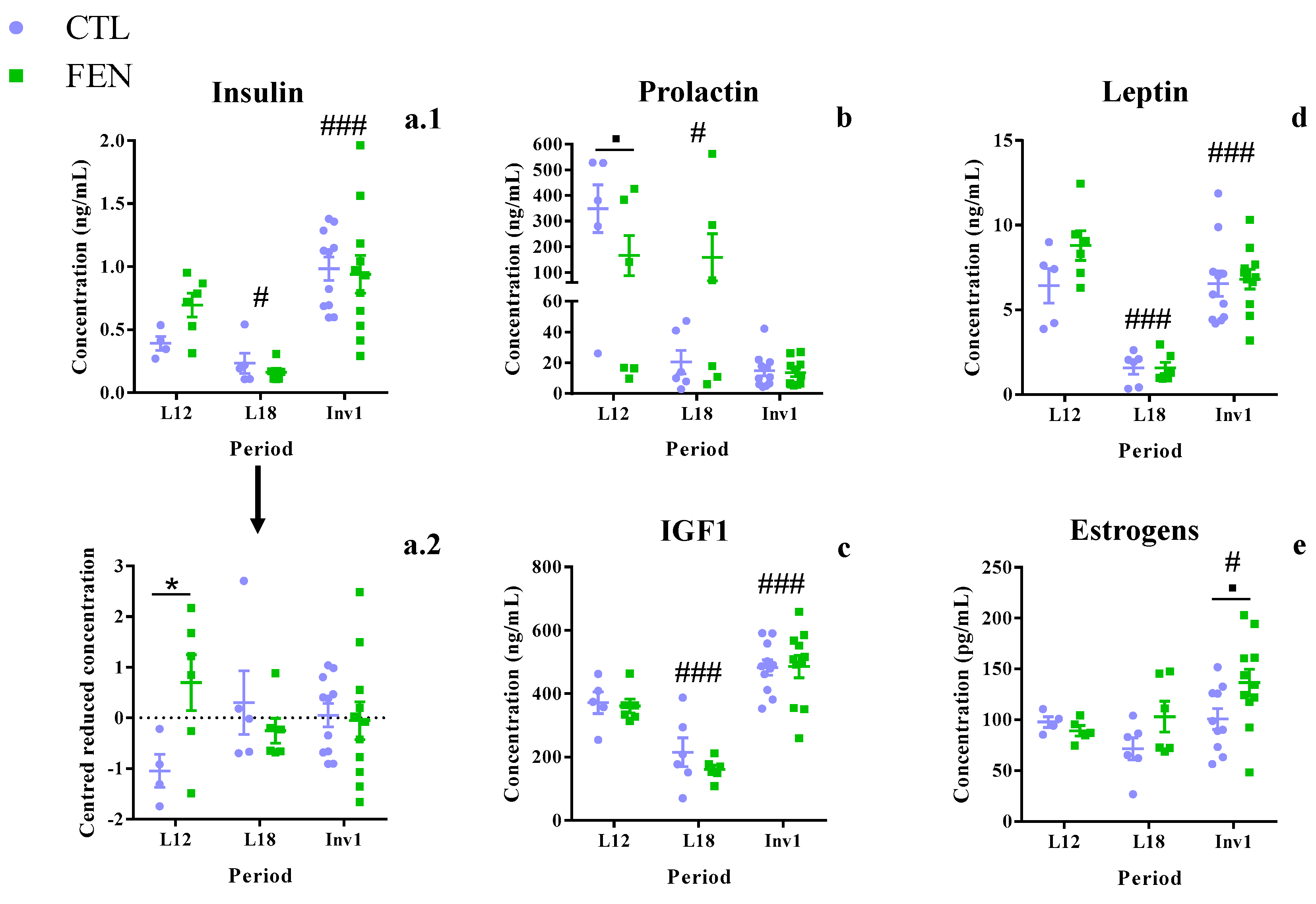
3.5. Fenugreek Supplementation Increases Plasma Insulin Concentration
4. Discussion
4.1. The Time Course of the Gene Expression in Mammary Gland and Lactogenic Hormones Suggests Peak Lactation Is Closer to L18
4.2. Fenugreek Supplementation Stimulates Milk Macronutrient Synthesis
4.3. Fenugreek Supplementation Advances Peak Lactation and Maintains Milk Production
4.4. Fenugreek Stimulates Milk Production Mainly by Modulating Insulin and Oxytocin Secretion
4.5. Fenugreek Stimulates Estrogenic Activity at the End of Lactation and Increased Energy Availability for Milk Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A. List of the Primers’ Sequences
| Gene Name | Gene Symbol | Function | Accession Number | Primer Sequences (5′ to 3′) | Amplicon (pb) | Efficiency (%) |
|---|---|---|---|---|---|---|
| Lipoprotein lipase | Lpl | Triglyceride hydrolysis and mediation of lipoprotein uptakes | NM_012598 | F: AACTGCCACTTCAACCACAGC R: CATACATTCCTGTCACCGTCCA | 70 | 95.8 |
| Fatty acid synthase | Fasn | Fatty acid biosynthesis, mainly palmitate and long chain fatty acid synthesis | NM_017332 | F: CGCCGTGGTGCTGGAGATTG R: CTTGCCGAGGTTGGTGAGGAAG | 142 | 98.3 |
| Acetyl-CoA carboxylase α | Acaca, Acc1 | Catalyze the rate limiting step in fatty acid biosynthesis: malonyl-CoA synthesis | NM_022193 | F: CGATTCCCATCCGCCTCTTCC R: GGTCCCTGCTTGTCTCCATACG | 127 | 92.3 |
| Acetyl-CoA carboxylase β | Acacb | Fatty acid biosynthesis, involved in inhibition of fatty acid oxidation | NM_053922 | F: GACGCCAGCAACATCACTTCG R: GCCTCTCGGTCCACCTTACG | 107 | 85.5 |
| Fatty acid binding protein 3 | Fabp3 | Uptake, intracellular metabolism and transport of fatty acids | NM_024162 | F: ACAGGAAGGTCAAGTCGGTC R: ATGGGTGAGAGTCAGGATGAG | 127 | 93.3 |
| Fatty acid binding protein 4 | Fabp4 | Lipid binding protein involved in fatty acid trafficking, main adipocyte FABP | NM_053365 | F: TGTGGGGACCTGGAAACTCGT R: CGAAGCCAACTCCCACTTCTTT | 71 | 92.9 |
| Stearoyl-CoA desaturase | Scd, Scd1 | Fatty acid desaturation, biosynthesis of oleic acid. | NM_139192 | F: GTTGGGTGCCTTATCGCTTTCC R: CTCCAGCCAGCCTCTTGTCTA | 114 | 97.8 |
| Diacylglycerol O-acyltransferase 1 | Dgat1 | Catalyzes the synthesis of triglyceride from diglyceride and acyl-CoA | NM_053437 | F: GGCATCATACTCCATCATCTTC R: CCCACTGACCTTCTTCCC | 115 | 97.1 |
| Diacylglycerol O-acyltransferase 2 | Dgat2 | Catalyzes the synthesis of triglyceride from diglyceride and acyl-CoA | NM_001012345 | F: GGTCATCTCAGTCCTACAG R: CTATCAGCCAGCAGTCAG | 101 | 88.6 |
| Perilipin 2 | Plin2 | Coating of intracellular lipid droplets and milk fat globules, involved in milk lipid secretion. | NM_001007144 | F: CTTCTTCATTGACCTGCGAC R: CACCCTTACCTTAAGTTTCTCCT | 108 | 97.1 |
| Solute carrier family 2, facilitated glucose transporter member 1 | Slc2a1, Glut1 | One of major glucose transporters, located in the cell membrane | NM_138827 | F: CCAATATGTGGAGCAACTGTG R: GGAAGCGATCTCATCGAAGG | 129 | 108.2 |
| Phosphoglucomutase 1 | Pgm1 | Interconvert glucose-1-phosphate and glucose-6-phosphate | NM_017033 | F: CCCTTCACAGTGGAGATCGT R: GTCGATGCGGATCTTCAGTC | 122 | 96.1 |
| UDP-glucose pyrophosphorylase 2 | Ugp2 | Convert glucose-1-phosphate to UDP-glucose | NM_001024743 | F: CGGAAGATTCGATTCAACCC R: GTTCCCAAACCACCATTGAG | 111 | 93.0 |
| β-1,4- galactosyltransferase 1 | B4galt1 | Catalytic subunit of lactose synthase complex, convert UDP-galactose and glucose into lactose | NM_053287 | F: CGCTTTGTGTTCAGTGATGTGG R: AACGTAAGGCAGGCTAAACC | 122 | 100.7 |
| Lacatlbumin α | Lalba | Regulatory subunit of lactose synthase complex, one of the main milk proteins | NM_012594 | F: CTTGCTTGAATGGACCTGTG R: GCTACTCTTGCACCAATTTCTG | 126 | 92.7 |
| Casein β | Csn2 | Principal milk protein and primary source of essential amino acids for sucking young | NM_017120 | F: AAACATCCAGCCTATTGCTC R: CATCTGTTTGTGCTTGGGAA | 118 | 96.6 |
| Whey acidic protein | Wap | Whey protein associated with mammary gland, a major constituent of milk | NM_053751 | F: CATGTCTTCAACTCAGTTCAGTCC R: CATGTCATTCTGGGCACACTC | 104 | 95.8 |
| Solute carrier family 7 member 5 | Slc7a5 | Amino acid transporter, mediate the uptake of large neutral amino acids (branch-chain, aromatic) | NM_017353 | F: GGGAAAGGACATAGGACAAGG R: TCAGATAGTTCCATCCTCCGT | 136 | 94.5 |
| Aquaporin 1 | Aqp1 | Integral membrane protein, allow the passive transport of water along osmotic gradient. | NM_012778 | F: GGCATTGAGATCATTGGCAC R: GTGTAGTCAATGGCCAGCAG | 142 | 97.8 |
| Aquaporin 3 | Aqp3 | Passive water transporter located in the basolateral cell membrane | NM_031703 | F: GGGCTCTACTATGATGCAATCTG R: GCTGCTGTGCCTATGAACTG | 148 | 95.5 |
| Carnitine palmitoyltransferase 1A | Cpt1a | Initiate β-oxidation by translocating long chain fatty acids across mitochondrial inner membrane | NM_031559 | F: TGCCTGCCAGTTCCATTAAGC R: GTCTCACTCCTCTTGCCAACAG | 143 | 90.1 |
| Acyl-CoA dehydrogenase short chain | Acads | Catalyze the first step of mitochondrial fatty acid β-oxidation | NM_022512 | F: CGGCAGAACAAGGGTATCAG R: AAGATGAGGTTAGCTGTAGATGAG | 115 | 99.1 |
| Pyruvate dehydrogenase E1 subunit α 1 | Pdha1 | Subunit of the pyruvate dehydrogenase complex that convert pyruvate to acetyl-CoA and CO2 | NM_001004072 | F: CACGGACCATCTCATCACTG R: CTCCTCTTCGTCCTGTTAATTCTG | 100 | 91.2 |
| Citrate synthase | Cs | Krebs cycle enzyme that catalyzes the synthesis of citrate from oxaloacetate and acetyl-CoA | NM_130755 | F: GCTATAGTATCCCTGAGTGCCA R: AGAGCCAAGAGACCTGTTCC | 128 | 91.1 |
| Cytochrome c oxidase I, mitochondrial | Mtco1, mtCox1 | Subunit of the cytochrome c oxidase, catalyzes reduction of oxygen to water for oxidative phosphorylation | YP_665631 | F: GCCTAGATGTAGACACCCGAG R: TATTTCCTCCATGTAGTGTAGCGA | 107 | 97.0 |
| ATP synthase F1 subunit α | Atp5f1a | Subunit of the mitochondrial ATP synthase, catalyzes the ATP synthesis during oxidative phosphorylation | NM_023093 | F: AGCGTTTCAATGATGGGACTG R: GTACTTCATGGCATCTGCGTC | 124 | 94.3 |
| Sterol regulatory element binding transcription factor 1 | Srebf1 | Nuclear transcription factor that bind to the sterol regulatory element 1, regulates the transcription of genes important for sterol biosynthesis and lipogenesis | NM_001276707 | F: GCAGCTGATGGAGACAGGGA R: CGACAGCGTCAGAACAGCTA | 76 | 99.3 |
| Peroxisome proliferator activated receptor γ (PPARγ) | Pparg | Transcription factor that regulate expression of genes involved in lipid metabolism | NM_001145366 | F: CCGTTCACAAGAGCTGACCC R: TCGCACTTTGGTATTCTTGGAGC | 73 | 94.7 |
| Thyroid hormone responsive | Thrsp, Spot14 | Play a role in regulation of lipogenesis and specially triglycerides with medium-chain fatty acids. | NM_012703 | F: CACATCCTTACCCACCTGAC R: CGTGTAAAGTGATCTTCCATAGAG | 109 | 88.5 |
| Nuclear receptor subfamily 1, group H, member 3 (LXRα) | Nr1h3, Lxra | Nuclear oxysterol receptor that regulate inflammation and homeostasis of lipids and cholesterol | NM_031627 | F: CAGAGCCTACAGAACTTCGT R: CAGCTCAGCACATTGTAATGG | 123 | 101.0 |
| AKT serine/threonine kinase 1 (AKT) | Akt1 | Key signaling pathway protein notably linked to mTOR signaling, regulate metabolism, proliferation | NM_033230 | F: CTACTATGCCATGAAGATCCTC R: CTACTATGCCATGAAGATCCTC | 124 | 93.9 |
| Mechanistic target of rapamycin kinase (mTOR) | Mtor | Central regulator of cell metabolism, growth and survival, mediate nutrient and energy signal, key regulator of mRNA translation | NM_019906 | F: ACCAATTATACTCGCTCCCTG R: CATAGCAACCTCAAAGCAGTC | 147 | 90.9 |
| Signal transducer and activator of transcription 5A (STAT5) | Stat5a, Stat5 | Involved in signal transduction and transcription activation in response to cytokine, growth factors and prolactin | NM_017064 | F: AGAACACCCGCAATGATTACAG R: GTGACATGTTTCTGAAGTGGG | 129 | 97.8 |
| Prolactin receptor | Prlr | Prolactin binding and signaling mainly through JAK2/STAT5 and Akt/mTOR pathways | NM_001034111 | F: GAAATGCCAAATGACTTCACCT R: TATAGCCCTTCAAAGCCACTG | 111 | 91.5 |
| Insulin receptor | Insr | Insulin binding and signaling mainly through AKT/mTOR and MAPK pathways | NM_017071 | F: TTCATTCAGGAAGACCTTCGA R: CAGGCCAGAGATGACAAGTGAC | 259 | 95.4 |
| Oxytocin receptor | Oxtr | G-protein coupled receptor binding oxytocin, activate PIP3-Ca2+ pathway | NM_012871 | F: TTCATTCAGGAAGACCTTCGA R: GAGTTCGTGGAAGAGGTGAC | 146 | 88.3 |
| Growth hormone receptor | Ghr | Growth hormone binding and signaling mainly through JAK2/STAT5 pathway | NM_017094 | F: TTCTTCGTGCAGATGTGGAG R: ATGGAAACCGGAAATCTTCTTCAC | 115 | 102.1 |
| Insulin-like growth factor 1 receptor | Igf1r | IGF-1 binding and signaling mainly through AKT/mTOR and MAPK pathways. | NM_052807 | F: CCATAGAAAGAGGAATAACAGCAG R: TACCTCCCATTCATCAGGCA | 108 | 88.9 |
| Estrogen receptor 1 | Esr1, Esrα | Nuclear receptor binding estrogens, act as a regulator of DNA transcription | NM_012689 | F: CCACGATCAAGTTCACCTTCTG R: CACATTTACCTTGATTCCTGTCCA | 136 | 86.8 (MG) 92.1 (PG) |
| Complement C3 | C3 | Involved in innate immune response; central role in activating the complement system | NM_000064 | F: CTTCTTCATTGACCTGCGAC R: CACCCTTACCTTAAGTTTCTCCT | 122 | 94.6 (MG) 98.4 (PG) |
| Superoxide dismutase 1 | Sod1 | Convert superoxide to hydrogen peroxide and oxygen, involved in response to oxidative stress | NM_017050 | F: TACACAAGGCTGTACCACTG R: CACACGATCTTCAATGGACAC | 150 | 101.4 |
| Catalase | Cat | Key antioxidant enzyme that convert hydrogen peroxide to water and oxygen, limit oxidative stress | NM_012520 | F: CATAGCCAGAAGAGAAACCC R:GAACAAGAAAGTAACCTGATGGAG | 104 | 93.7 |
| Glutathione peroxidase 1 | Gpx1 | Catalyze the reduction of hydroperoxides and H2O2 by glutathione to protect cells against oxidative damages | NM_030826 | F: GTTCGGACATCAGGAGAATGG R: GAAGGTAAAGAGCGGGTGAG | 144 | 95.7 |
| Prolactin | Prl | Pituitary hormone that primarily promote lactation. Play a role as growth regulator and in cell survival | NM_012629 | F: AAACAGTATGTCCAAGATCGTGAG R: ACTTCCGGAGGGACTTTCTG | 112 | 99.8 |
| Gh | Gh1 | Pituitary hormone playing major role in growth control. Stimulate IGF1-1 secretion by the liver | NM_001034848 | F: ACCTACAAAGAGTTCGAGCGT R: GAAGCAATTCCATGTCAGTTCTC | 147 | 94.2 |
| Oxytocin/neurophysin I prepropeptide | Oxt | Hormone involved in contraction of smooth muscle during parturition and lactation | NM_012996 | F: GGATATGCGCAAGTGTCTTCC R: GAGGGCAGGTAGTTCTCCTC | 140 | 106.8 |
| Dopamine receptor D2 | Drd2 | G-protein coupled receptor of dopamine which inhibit adenylyl-cyclase and notably prolactin secretion | NM_012547 | F: CCACTCAAGGGCAACTGTACC R: ATGGGGCTATACCGGGTCC | 179 | 91.5 |
| Thyrotropin releasing hormone receptor | Trhr | G-protein coupled receptor of TRH that activate PIP3-Ca2+ pathway, promote release of prolactin | NM_013047 | F: CAGACCGCTTTAGCACAGAG R: ACTGGGTCCATTCTTCTCGG | 112 | 90.1 |
| Vasoactive intestinal peptide receptor 2 | Vipr2 | G-protein coupled receptor of VIP which activate adenylyl-cyclase and notably prolactin secretion | NM_017238 | F: GCAGCCAAATGGAGAATCAC R: TTTGCTTATGTTTCCTGGTCTG | 145 | 104.0 |
| Growth hormone releasing hormone receptor | Ghrhr | G-protein coupled receptor of GHRH that activate adenylyl-cyclase and stimulate GH transcription and secretion | NM_012850 | F: GCTGTTTGCTACTTTCATCCT R: CCTTGCACAGAATAGTGGAC | 109 | 97.9 |
| Adrogen receptor | Ar | Steroid hormone activated transcription factor, involved in cell proliferation and differentiation | NM_012502 | F: CTCTGCCTCTGAAGTGTGGT R: TACTGTCCAAACGCATGTCC | 136 | 91.5 |
| Actin β | Actb | Highly conserved proteins that are involved in cell motility, structure, integrity and signaling | NM_031144 | F: CTATCGGCAATGAGCGGTTCC R: GCACTGTGTTGGCATAGAGGTC | 150 | 93.2 (PG) |
| Ubiquitously expressed, prefoldin-like chaperone | Uxt | Cofactor that modulates AR-dependent transcription, and also plays a critical role in TNF-induced apoptosis | NM_001006982 | F: ATTGACCGAAAGAGTTCCCT R: GTAGTTCTCTAAGTCCCTCTAGCA | 108 | 91.3 (MG) 87.2 (PG) |
| Ribosomal protein S9 | Rps9 | One of 40S ribosomal subunit protein | NM_031108 | F: GCTAAAGTTGATTGGAGAGTATGG R: AGAGCGTTGCCTTCAAACAG | 146 | 99.0 (MG) 89.5 (PG) |
| Valosin-containing protein | Vcp | ATP binding protein involved in vesicle transport and fusion and endoplasmic reticulum function | NM_053864 | F: TCAAGCGAGAGGATGAGGAG R: TCACACCAATTGCCTTAAAGAG | 140 | 92.3 |
References
- World Health Organization. Indicators for Assessing Infant and Young Child Feeding Practices; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Breastfeeding, S.O. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Giannì, M.L. Human milk: Composition and health benefits. Pediatr. Med. Chir. 2017, 39, 155. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; A França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Thulier, D.; Mercer, J. Variables Associated With Breastfeeding Duration. J. Obstet. Gynecol. Neonatal Nurs. 2009, 38, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L. Maternal Perceptions of Insufficient Milk Supply in Breastfeeding. J. Nurs. Scholarsh. 2008, 40, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.L.; Bettinelli, M.E.; Manfra, P.; Sorrentino, G.; Bezze, E.; Plevani, L.; Cavallaro, G.; Raffaeli, G.; Crippa, B.L.; Colombo, L.; et al. Breastfeeding Difficulties and Risk for Early Breastfeeding Cessation. Nutrients 2019, 11, 2266. [Google Scholar] [CrossRef]
- Hillervik-Lindquist, C. Studies on Perceived Breast Milk Insufficiency A Prospective Study in a Group of Swedish Women. Acta Paediatr. 1991, 80, 1–29. [Google Scholar] [CrossRef]
- Giugliani, E.R. Common problems during lactation and their management. Jornal de Pediatria 2004, 80, S147–S154. [Google Scholar] [CrossRef][Green Version]
- Bazzano, A.N.; Hofer, R.; Thibeau, S.; Gillispie, V.; Jacobs, M.; Theall, K.P. A Review of Herbal and Pharmaceutical Galactagogues for Breast-Feeding. Ochsner J. 2016, 16, 511–524. [Google Scholar]
- Sim, T.F.; Sherriff, J.L.; Hattingh, H.L.; Parsons, R.; Tee, L.B. The use of herbal medicines during breastfeeding: A population-based survey in Western Australia. BMC Complement. Altern. Med. 2013, 13, 317. [Google Scholar] [CrossRef]
- Abascal, K.; Yarnell, E. Botanical Galactagogues. Altern. Complement. Ther. 2008, 14, 288–294. [Google Scholar] [CrossRef]
- Forinash, A.B.; Yancey, A.M.; Barnes, K.N.; Myles, T.D. The Use of Galactogogues in the Breastfeeding Mother. Ann. Pharmacother. 2012, 46, 1392–1404. [Google Scholar] [CrossRef]
- Sevrin, T.; Alexandre-Gouabau, M.-C.; Darmaun, M.; Palvadeau, A.; André, A.; Nguyen, P.; Ouguerram, K.; Boquien, C.-Y. Use of water turnover method to measure mother’s milk flow in a rat model: Application to dams receiving a low protein diet during gestation and lactation. PLoS ONE 2017, 12, e0180550. [Google Scholar] [CrossRef]
- Sevrin, T.; Alexandre-Gouabau, M.-C.; Castellano, B.; Aguesse, A.; Ouguerram, K.; Nguyen, P.; Darmaun, D.; Boquien, C.-Y.; Gouabau, A. Impact of Fenugreek on Milk Production in Rodent Models of Lactation Challenge. Nutrients 2019, 11, 2571. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.C.; Tan, M.L.; Foong, W.C.; A Marasco, L.; Ho, J.J.; Ong, J.H. Oral galactagogues (natural therapies or drugs) for increasing breast milk production in mothers of non-hospitalised term infants. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Truchet, S.; Honvo-Houéto, E. Physiology of milk secretion. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 367–384. [Google Scholar] [CrossRef]
- E Grzeskowiak, L.; Wlodek, M.E.; Geddes, D.T. What Evidence Do We Have for Pharmaceutical Galactagogues in the Treatment of Lactation Insufficiency?-A Narrative Review. Nutrients 2019, 11, 974. [Google Scholar] [CrossRef]
- Osorio, J.S.; Lohakare, J.; Bionaz, M. Biosynthesis of milk fat, protein, and lactose: Roles of transcriptional and posttranscriptional regulation. Physiol. Genom. 2016, 48, 231–256. [Google Scholar] [CrossRef]
- Vernon, R.G. Lipid metabolism during lactation: A review of adipose tissue-liver interactions and the development of fatty liver. J. Dairy Res. 2005, 72, 460–469. [Google Scholar] [CrossRef]
- Hull, K.; Harvey, S.; Hull, K.L. Growth hormone: Roles in female reproduction. J. Endocrinol. 2001, 168, 1–23. [Google Scholar] [CrossRef]
- Anderson, S.M.; MacLean, P.S.; McManaman, J.L.; Neville, M.C. Lactation and its hormonal control. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Elsevier: New York, NY, USA, 2015; pp. 2055–2105. [Google Scholar]
- Bahmani, M.; Shirzad, H.; Mirhosseini, M.; Mesripour, A.; Rafieian-Kopaei, M. A Review on Ethnobotanical and Therapeutic Uses of Fenugreek (Trigonella foenum-graceum L). J. Evid.-Based Integr. Med. 2015, 21, 53–62. [Google Scholar] [CrossRef]
- Fuller, S.; Stephens, J.M. Diosgenin, 4-Hydroxyisoleucine, and Fiber from Fenugreek: Mechanisms of Actions and Potential Effects on Metabolic Syndrome. Adv. Nutr. 2015, 6, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Abdou, R.M.; Fathey, M. Evaluation of early postpartum fenugreek supplementation on expressed breast milk volume and prolactin levels variation. Egypt. Pediatr. Assoc. Gaz. 2018, 66, 57–60. [Google Scholar] [CrossRef]
- Anju, V.S.; Sreeharshan, S. In vitro estrogenic activities of fenugreek Trigonella foenum graecum seeds. Indian J. Med. Res. 2010, 131, 814–819. [Google Scholar]
- Al-Chalabii, I.M.S. Diosgenin effect on rats and mice ovaries. J. Fac. Med. Baghdad 2005, 47, 296–301. [Google Scholar]
- Kandhare, A.D.; Bandyopadhyay, D.; Thakurdesai, P.A. Low molecular weight galactomannans-based standardized fenugreek seed extract ameliorates high-fat diet-induced obesity in mice via modulation of FASn, IL-6, leptin, and TRIP-Br2. RSC Adv. 2018, 8, 32401–32416. [Google Scholar] [CrossRef]
- Shim, S.H.; Lee, E.J.; Kim, J.S.; Kang, S.S.; Ha, H.; Lee, H.Y.; Kim, C.; Lee, J.-H.; Son, K.H. Rat Growth-Hormone Release Stimulators from Fenugreek Seeds. Chem. Biodivers. 2008, 5, 1753–1761. [Google Scholar] [CrossRef]
- Koetsier, G.; Cantor, E. A Practicle Guide to Analysing Nucleic Acid Concentration and Purity with Microvolume Spectrophotometer; New England Biolabs Inc.: Ipswich, MA, USA, 2019. [Google Scholar]
- Rudolph, M.C.; McManaman, J.L.; Hunter, L.; Phang, T.; Neville, M.C. Functional Development of the Mammary Gland: Use of Expression Profiling and Trajectory Clustering to Reveal Changes in Gene Expression During Pregnancy, Lactation, and Involution. J. Mammary Gland Biol. Neoplasia 2003, 8, 287–307. [Google Scholar] [CrossRef]
- Rudolph, M.C.; McManaman, J.L.; Phang, T.; Russell, T.; Kominsky, D.J.; Serkova, N.J.; Stein, T.; Anderson, S.M.; Neville, M.C. Metabolic regulation in the lactating mammary gland: A lipid synthesizing machine. Physiol. Genom. 2007, 28, 323–336. [Google Scholar] [CrossRef]
- Marshall, O.J. PerlPrimer: Cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 2004, 20, 2471–2472. [Google Scholar] [CrossRef] [PubMed]
- Kasperczyk, A.; Machnik, G.; Dobrakowski, M.; Sypniewski, D.; Birkner, E.; Kasperczyk, S. Gene expression and activity of antioxidant enzymes in the blood cells of workers who were occupationally exposed to lead. Toxicology 2012, 301, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Identification of reference genes for quantitative real-time PCR in the bovine mammary gland during the lactation cycle. Physiol. Genom. 2007, 29, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sodhi, M.; Sharma, A.; Sharma, V.L.; Verma, P.; Swami, S.K.; Kumari, P.; Mukesh, M. Selection of suitable reference genes for normalization of quantitative RT-PCR (RT-qPCR) expression data across twelve tissues of riverine buffaloes (Bubalus bubalis). PLoS ONE 2018, 13, e0191558. [Google Scholar] [CrossRef] [PubMed]
- Agnoux, A.M.; Antignac, J.-P.; Boquien, C.-Y.; David, A.; Desnots, E.; Ferchaud-Roucher, V.; Darmaun, D.; Parnet, P.; Alexandre-Gouabau, M.-C. Perinatal protein restriction affects milk free amino acid and fatty acid profile in lactating rats: Potential role on pup growth and metabolic status. J. Nutr. Biochem. 2015, 26, 784–795. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical notes for clinical researchers:post-hocmultiple comparisons. Restor. Dent. Endod. 2015, 40, 172–176. [Google Scholar] [CrossRef]
- Hellemans, J.; Vandesompele, J. qPCR data analysis – unlocking the secret to successful results. In PCR Troubleshooting and Optimization: The Essential Guide; Caister Academic Press: Edinburgh, UK, 2011. [Google Scholar]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, J.C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]
- McManaman, J.L. Formation of milk lipids: A molecular perspective. Clin. Lipidol. 2009, 4, 391–401. [Google Scholar] [CrossRef]
- Modha, G.; Blanchard, A.; Iwasiow, B.; Mao, X.J.; Troup, S.; Adeyinka, A.; Watson, P.; Shiu, R.; Myal, Y. Developmental Changes in Insulin-like Growth Factor I Receptor Gene Expression in the Mouse Mammary Gland. Endocr. Res. 2004, 30, 127–140. [Google Scholar] [CrossRef]
- Oppi-Williams, C.; Suagee, J.; Corl, B. Regulation of lipid synthesis by liver X receptor α and sterol regulatory element-binding protein 1 in mammary epithelial cells. J. Dairy Sci. 2013, 96, 112–121. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Soloff, M.S. Oxytocin Receptors and Mammary Myoepithelial Cells. J. Dairy Sci. 1982, 65, 326–337. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene Networks Driving Bovine Mammary Protein Synthesis during the Lactation cycle. Bioinform. Biol. Insights 2011, 5, BBI-S7003. [Google Scholar] [CrossRef]
- Jones, R.G.; Ilic, V.; Williamson, D.H. Physiological significance of altered insulin metabolism in the conscious rat during lactation. Biochem. J. 1984, 220, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Brogan, R.S.; Mitchell, S.E.; Trayhurn, P.; Smith, M.S. Suppression of Leptin During Lactation: Contribution of the Suckling Stimulus Versus Milk Production 1. Endocrinology 1999, 140, 2621–2627. [Google Scholar] [CrossRef]
- Walsh, D.S.; Vesely, J.; Mahadevan, S. Relationship between Milk Production and Circulating Hormones in Dairy Cows. J. Dairy Sci. 1980, 63, 290–294. [Google Scholar] [CrossRef]
- Ošt’ádalová, I.; Babický, A. Periodization of the early postnatal development in the rat with particular attention to the weaning period. Physiol. Res. 2012, 61, S1–S7. [Google Scholar]
- Al-Sherwany, D.A.O. Feeding effects of fenugreek seeds on intake, milk yield, chemical composition of milk and some biochemical parameters in Hamdani ewes. Al-Anbar J. Vet. Sci. 2015, 8, 49–54. [Google Scholar]
- Mahgoub, A.A.; Sallam, M.T. Effect of Extract Crushed Fenugreek Seeds as Feed Additive on some Blood Parameters, Milk Yield and Its Composition of Lactating Egyptian Buffaloes. J. Anim. Poult. Prod. 2016, 7, 269–273. [Google Scholar] [CrossRef]
- Alamer, M.A.; Basiouni, G.F. Feeding effect of Fenugreek seed (Trigonella foenum-graecum L.) on lactation performance, some plasma constituent and growth hormone level in goats. Pak. J. Biol. Sci. 2005, 8, 1553–1556. [Google Scholar]
- Liu, H.; Hua, Y.; Luo, H.; Shen, Z.; Tao, X.; Zhu, X. An Herbal Galactagogue Mixture Increases Milk Production and Aquaporin Protein Expression in the Mammary Glands of Lactating Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.A.; Ita, J.R.-D.; De La Garza, R.G.; Castilla-Cortázar, I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J. Transl. Med. 2016, 14, 1–23. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, J.-K.; Chen, C. Influence of insulin on growth hormone secretion, level and growth hormone signalling. Sheng Li Xue Bao Acta Physiol. Sin. 2017, 69, 541–556. [Google Scholar]
- Birzniece, V.; Sata, A.; Ho, K.K.Y. Growth hormone receptor modulators. Rev. Endocr. Metab. Disord. 2008, 10, 145–156. [Google Scholar] [CrossRef]
- Chi, M.M.-Y.; Schlein, A.L.; Moley, K.H. High Insulin-Like Growth Factor 1 (IGF-1) and Insulin Concentrations Trigger Apoptosis in the Mouse Blastocyst via Down-Regulation of the IGF-1 Receptor**This work was supported in part by NIH through Grants RO1-HD-38061–01A1 (to K.H.M.), P60-DK-30579 (to K.H.M.), and the Washington University Clinical Nutrition Research Unit Center Grant P30-DK-56341 (to K.H.M.); and by the Burroughs Wellcome Fund through a Career Award in the Biomedical Sciences (to K.H.M.). Endocrinology 2000, 141, 4784–4792. [Google Scholar] [CrossRef][Green Version]
- Payankaulam, S.; Raicu, A.-M.; Arnosti, D.N. Transcriptional Regulation of INSR, the Insulin Receptor Gene. Genes 2019, 10, 984. [Google Scholar] [CrossRef]
- Rhodes, C.J.; White, M.F.; Leahy, J.L.; Kahn, S.E. Direct Autocrine Action of Insulin on β-Cells: Does It Make Physiological Sense? Diabetes 2013, 62, 2157–2163. [Google Scholar] [CrossRef]
- Akers, R.M. Major Advances Associated with Hormone and Growth Factor Regulation of Mammary Growth and Lactation in Dairy Cows. J. Dairy Sci. 2006, 89, 1222–1234. [Google Scholar] [CrossRef]
- Steffensen, K.R.; Gustafsson, J.-Å. Putative Metabolic Effects of the Liver X Receptor (LXR). Diabetes 2004, 53, S36–S42. [Google Scholar] [CrossRef]
- Cruz-Garcia, L.; Sanchez-Gurmaches, J.; Gutiérrez, J.; Navarro, I.; Alvarez, I.N. Role of LXR in trout adipocytes: Target genes, hormonal regulation, adipocyte differentiation and relation to lipolysis. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2012, 163, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J. Functional Role of PPARs in Ruminants: Potential Targets for Fine-Tuning Metabolism during Growth and Lactation. PPAR Res. 2013, 2013, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, J.; Zhu, J.; Sun, Y.; Yao, D.; Shi, H.; Wang, W. Regulation of the fatty acid synthase promoter by liver X receptor α through direct and indirect mechanisms in goat mammary epithelial cells. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 184, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wehrenberg, W.B.; Gaillard, R.C. Neuroendocrine Mechanisms Regulating Growth Hormone and Prolactin Secretion during Lactation. Endocrinology 1989, 124, 464–467. [Google Scholar] [CrossRef]
- Christoffel, J.; Rimoldi, G.; Wuttke, W. Effects of 8-prenylnaringenin on the hypothalamo-pituitary-uterine axis in rats after 3-month treatment. J. Endocrinol. 2006, 188, 397–405. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Park, J.; Yu, H.-N.; Kim, J.-S.; Youn, H.J.; Jung, S.H. Up-regulation of PI3K/Akt signaling by 17β-estradiol through activation of estrogen receptor-α, but not estrogen receptor-β, and stimulates cell growth in breast cancer cells. Biochem. Biophys. Res. Commun. 2005, 336, 1221–1226. [Google Scholar] [CrossRef]
- Degirmencioglu, T.; Unal, H.; Ozbilgin, S.; Kuraloglu, H. Effect of ground fenugreek seeds (Trigonella foenum-graecum) on feed consumption and milk performance in Anatolian water buffaloes. Arch. Anim. Breed. 2016, 59, 345–349. [Google Scholar] [CrossRef]
- El-Nor, S.A.; Khattab, H.; Al-Alamy, H.; Salem, F.; Abdo, M. Effect of Some Medicinal Plants Seeds in the Rations on the Productive Performance of Lactating Buffaloes. Int. J. Dairy Sci. 2007, 2, 348–355. [Google Scholar] [CrossRef]
- Hirscbberg, A.L. Hormonal regulation of appetite and food intake. Ann. Med. 1998, 30, 7–20. [Google Scholar] [CrossRef]
- Hanwell, A.; Malcolm, P. Physiological effects of lactation on the mother. Symp. Zool. Soc. Lond. 1977, 41, 297–312. [Google Scholar]
- Petit, P.; Sauvaire, Y.; Ponsin, G.; Manteghetti, M.; Fave, A.; Ribes, G. Effects of a fenugreek seed extract on feeding behaviour in the rat: Metabolic-endocrine correlates. Pharmacol. Biochem. Behav. 1993, 45, 369–374. [Google Scholar] [CrossRef]
- Petit, P.R.; Sauvaire, Y.D.; Hillaire-Buys, D.M.; Leconte, O.M.; Baissac, Y.G.; Ponsin, G.R.; Ribes, G.R. Steroid saponins from fenugreek seeds: Extraction, purification, and pharmacological investigation on feeding behavior and plasma cholesterol. Steroids 1995, 60, 674–680. [Google Scholar] [CrossRef]
- Matsushita, N.; Kato, Y.; Shimatsu, A.; Katakami, H.; Yanaihara, N.; Imura, H. Effects of VIP, TRH, GABA and dopamine on prolactin release from superfused rat anterior pituitary cells. Life Sci. 1983, 32, 1263–1269. [Google Scholar] [CrossRef]
- Xu, J.; Kirigiti, M.A.; Grove, K.L.; Smith, M.S. Regulation of Food Intake and Gonadotropin-Releasing Hormone/Luteinizing Hormone during Lactation: Role of Insulin and Leptin. Endocrinology 2009, 150, 4231–4240. [Google Scholar] [CrossRef]
- Morag, M.; Popliker, F.; Yagil, R. Effect of litter size on milk yield in the rat. Lab. Anim. 1975, 9, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Inui, A.; Tsai, M.; Amitani, H.; Asakawa, A. Stimulation of leptin secretion by insulin. Indian J. Endocrinol. Metab. 2012, 16, 543–548. [Google Scholar] [CrossRef]
- Pliquett, R.; Fuhrer, D.; Falk, S.; Zysset, S.; Von Cramon, D.; Stumvoll, M. The Effects of Insulin on the Central Nervous System—Focus on Appetite Regulation. Horm. Metab. Res. 2006, 38, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Denis, R.G.P.; Williams, G.; Vernon, R.G. Regulation of serum leptin and its role in the hyperphagia of lactation in the rat. J. Endocrinol. 2003, 176, 193–203. [Google Scholar] [CrossRef]
| Groups | N | Dam Water Intake (g/day) | Dam Food Intake (g/day) | Pup Total Weight Gain (g) | Milk Flow (g/day) |
|---|---|---|---|---|---|
| CTL | 17 | 53.93 ± 1.12 | 49.71 ± 0.79 | 36.19 ± 0.74 | 55.90 ± 2.36 |
| FEN | 16 | 55.22 ± 1.11 | 54.66 ± 1.00 | 38.75 ± 0.85 | 64.45 ± 2.35 |
| p-value | 0.422 | <0.001 | 0.029 | 0.016 | |
| Log2 (Fold Change) CTL at L12 as Reference | Fold Change CTL as Reference | 2-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Genes | Period | ||||||
| CTL | FEN | Inter | Period | Diet | |||
| Milk lipid synthesis | |||||||
| L12 (n = 6) | 0.00 ± 0.27 a,1 | 0.97 ± 0.11 b,1 | 1.95 | ||||
| Lpl | L18 (n = 6) | 0.52 ± 0.13 1 | 0.90 ± 0.43 1 | 1.31 | 0.034 | <0.001 | 0.045 |
| Inv1 (n = 11) | −2.63 ± 0.16 2 | −2.83 ± 0.16 2 | 0.87 | ||||
| L12 | 0.00 ± 0.27 a,1 | 1.10 ± 0.13 b,1 | 2.14 | ||||
| Fasn | L18 | 0.21 ± 0.17 1 | 0.25 ± 0.37 1 | 1.03 | 0.192 | <0.001 | 0.049 |
| Inv1 | −5.10 ± 0.23 2 | −4.84 ± 0.29 2 | 1.19 | ||||
| L12 | 0.00 ± 0.32 a,1 | 1.08 ± 0.14 b,1 | 2.12 | ||||
| Acaca | L18 | −0.18 ± 0.14 1 | 0.16 ± 0.41 2 | 1.26 | 0.078 | <0.001 | 0.012 |
| Inv1 | −4.24 ± 0.14 2 | −4.19 ± 0.18 3 | 1.04 | ||||
| L12 | 0.00 ± 0.16 1 | 0.31 ± 0.08 1 | 1.24 | ||||
| Acacb | L18 | 0.52 ± 0.20 1 | 0.43 ± 0.31 1 | 0.94 | 0.480 | <0.001 | 0.965 |
| Inv1 | −2.12 ± 0.18 2 | −2.37 ± 0.26 2 | 0.84 | ||||
| L12 | 0.00 ± 0.27 a,1 | 1.84 ± 0.23 b,1 | 3.59 | ||||
| Fabp3 | L18 | 0.51 ± 0.16 1 | 0.67 ± 0.38 2 | 1.12 | 0.009 | <0.001 | <0.001 |
| Inv1 | −4.37 ± 0.16 2 | −3.95 ± 0.26 3 | 1.34 | ||||
| L12 | 0.00 ± 0.24 1 | −0.03 ± 0.17 1 | 0.98 | ||||
| Fabp4 | L18 | −1.73 ± 0.29 2 | −1.75 ± 0.48 2 | 0.99 | 0.959 | <0.001 | 0.759 |
| Inv1 | −0.30 ± 0.16 1 | −0.44 ± 0.16 1 | 0.91 | ||||
| L12 | 0.00 ± 0.71 | 1.19 ± 0.16 1,2 | 2.28 | ||||
| Scd1 | L18 | 1.10 ± 0.18 | 2.10 ± 0.41 1 | 2.00 | 0.399 | 0.013 | 0.122 |
| Inv1 | 0.04 ± 0.52 | −0.03 ± 0.56 2 | 0.95 | ||||
| L12 | 0.00 ± 0.15 1 | 0.21 ± 0.03 1 | 1.16 | ||||
| Dgat1 | L18 | 1.04 ± 0.17 2 | 0.85 ± 0.26 2 | 0.88 | 0.273 | <0.001 | 0.564 |
| Inv1 | −0.50 ± 0.06 3 | −0.33 ± 0.08 3 | 1.12 | ||||
| L12 | 0.00 ± 0.36 1,2 | −0.74 ± 0.20 1 | 0.60 | ||||
| Dgat2 | L18 | −0.33 ± 0.10 1 | −0.25 ± 0.42 1-2 | 1.05 | 0.068 | 0.005 | 0.322 |
| Inv1 | 0.65 ± 0.17 2 | 0.12 ± 0.23 2 | 0.69 | ||||
| L12 | 0.00 ± 0.43 1 | 0.50 ± 0.08 1 | 1.41 | ||||
| Plin2 | L18 | 1.51 ± 0.16 2 | 1.43 ± 0.26 2 | 0.95 | 0.267 | <0.001 | 0.069 |
| Inv1 | −0.37 ± 0.13 1 | 0.16 ± 0.16 1 | 1.44 | ||||
| Milk lactose synthesis | |||||||
| L12 | 0.00 ± 0.37 1 | 0.49 ± 0.21 1 | 1.40 | ||||
| Glut1 | L18 | −0.52 ± 0.14 1 | −0.06 ± 0.29 1 | 1.38 | 0.310 | <0.001 | 0.031 |
| Inv1 | −2.08 ± 0.07 2 | −2.04 ± 0.08 2 | 1.03 | ||||
| L12 | 0.00 ± 0.10 1 | 0.33 ± 0.06 1 | 1.26 | ||||
| Pgm1 | L18 | −0.61 ± 0.11 2 | −0.60 ± 0.18 2 | 1.01 | 0.032 | <0.001 | 0.591 |
| Inv1 | −0.05 ± 0.05 1 | −0.26 ± 0.08 3 | 0.86 | ||||
| L12 | 0.00 ± 0.35 a,1 | 1.03 ± 0.13 b,1 | 2.05 | ||||
| Ugp2 | L18 | −0.67 ± 0.24 1 | −0.48 ± 0.44 2 | 1.14 | 0.042 | <0.001 | 0.039 |
| Inv1 | −2.49 ± 0.11 2 | −2.56 ± 0.06 3 | 0.95 | ||||
| L12 | 0.00 ± 0.26 a,1 | 1.23 ± 0.13 b,1 | 2.35 | ||||
| B4galt1 | L18 | 0.74 ± 0.33 2 | 0.88 ± 0.27 1 | 1.11 | 0.009 | <0.001 | 0.001 |
| Inv1 | −3.22 ± 0.06 3 | −3.06 ± 0.09 2 | 1.11 | ||||
| L12 | 0.00 ± 0.58 a,1 | 1.28 ± 0.12 b,1 | 2.42 | ||||
| Lalba | L18 | 1.65 ± 0.15 2 | 1.64 ± 0.30 1 | 0.99 | 0.166 | <0.001 | 0.019 |
| Inv1 | −1.44 ± 0.28 3 | −0.86 ± 0.25 2 | 1.49 | ||||
| Milk protein synthesis | |||||||
| L12 | 0.00 ± 0.41 1 | 0.83 ± 0.11 1 | 1.78 | ||||
| Slc7a5 | L18 | 1.12 ± 0.25 2 | 0.74 ± 0.24 1 | 0.77 | 0.086 | <0.001 | 0.164 |
| Inv1 | −1.73 ± 0.16 3 | −1.31 ± 0.26 2 | 1.34 | ||||
| L12 | 0.00 ± 0.60 1 | 1.18 ± 0.06 b,1 | 2.27 | ||||
| Csn2 | L18 | 1.48 ± 0.12 2 | 1.36 ± 0.30 1 | 0.92 | 0.104 | <0.001 | 0.013 |
| Inv1 | −2.13 ± 0.22 3 | −1.37 ± 0.24 2 | 1.69 | ||||
| L12 | 0.00 ± 0.90 1 | 1.47 ± 0.11 1 | 2.77 | ||||
| Wap | L18 | 1.58 ± 0.05 1 | 1.48 ± 0.28 1 | 0.93 | 0.329 | <0.001 | 0.156 |
| Inv1 | −2.29 ± 0.54 2 | −1.91 ± 0.42 2 | 1.30 | ||||
| Lactocyte water inflow | |||||||
| L12 | 0.00 ± 0.45 1 | 0.58 ± 0.11 | 1.50 | ||||
| Aqp1 | L18 | 1.24 ± 0.10 2 | 0.57 ± 0.26 | 0.63 | 0.006 | <0.001 | 0.458 |
| Inv1 | −0.39 ± 0.10 1 | 0.05 ± 0.14 | 1.36 | ||||
| L12 | 0.00 ± 0.38 1 | 0.63 ± 0.48 1 | 1.55 | ||||
| Aqp3 | L18 | 1.64 ± 0.35 2 | 1.82 ± 0.28 1 | 1.13 | 0.751 | <0.001 | 0.240 |
| Inv1 | −0.91 ± 0.30 1 | −0.75 ± 0.17 2 | 1.12 | ||||
| Log2 (Fold Change) CTL at L12 as Reference | Fold Change) CTL as Reference | 2-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Genes | Period | ||||||
| CTL | FEN | Inter | Period | Diet | |||
| Lipid β-oxidation | |||||||
| L12 (n = 6) | 0.00 ± 0.75 a,1 | 1.94 ± 0.64 b,1 | 3.84 | ||||
| Cpt1a | L18 (n = 6) | −0.26 ± 0.53 1 | 0.29 ± 0.46 2 | 1.46 | 0.021 | <0.001 | 0.007 |
| Inv1 (n = 11) | 3.28 ± 0.05 2 | 3.22 ± 0.08 3 | 0.96 | ||||
| L12 | 0.00 ± 0.52 a,1 | 1.47 ± 0.40 b,1 | 2.77 | ||||
| Acads | L18 | 0.19 ± 0.08 1 | 0.39 ± 0.13 2 | 1.15 | 0.007 | <0.001 | 0.002 |
| Inv1 | 1.28 ± 0.06 2 | 1.41 ± 0.07 1 | 1.09 | ||||
| Krebs cycle initiation | |||||||
| L12 | 0.00 ± 0.53 1 | 0.53 ± 0.23 1 | 1.45 | ||||
| Pdha1 | L18 | 0.32 ± 0.08 1 | 0.59 ± 0.22 1 | 1.21 | 0.465 | <0.001 | 0.071 |
| Inv1 | −1.46 ± 0.05 2 | −1.39 ± 0.06 2 | 1.05 | ||||
| L12 | 0.00 ± 0.40 1 | 0.70 ± 0.25 1 | 1.62 | ||||
| Cs | L18 | 0.10 ± 0.07 1 | 0.32 ± 0.20 1 | 1.16 | 0.246 | <0.001 | 0.013 |
| Inv1 | −1.20 ± 0.04 2 | −1.03 ± 0.07 2 | 1.12 | ||||
| Oxidative phosphorylation | |||||||
| L12 | 0.00 ± 0.53 1 | 0.74 ± 0.25 1 | 1.68 | ||||
| Mtco1 | L18 | 0.52 ± 0.17 1 | 0.35 ± 0.18 1 | 0.89 | 0.148 | <0.001 | 0.099 |
| Inv1 | −0.90 ± 0.07 2 | −0.61 ± 0.12 2 | 1.22 | ||||
| L12 | 0.00 ± 0.50 | 0.55 ± 0.23 1 | 1.47 | ||||
| Atp5f1a | L18 | −0.24 ± 0.15 | 0.07 ± 0.18 1 | 1.24 | 0.251 | <0.001 | 0.073 |
| Inv1 | −0.61 ± 0.03 | −0.64 ± 0.07 2 | 0.98 | ||||
| Antioxidant enzymes | |||||||
| L12 | 0.00 ± 0.12 1 | −0.24 ± 0.08 | 0.85 | ||||
| Sod1 | L18 | −0.55 ± 0.14 2 | −0.48 ± 0.18 | 1.05 | 0.285 | 0.012 | 0.142 |
| Inv1 | −0.22 ± 0.08 1,2 | −0.48 ± 0.08 | 0.83 | ||||
| L12 | 0.00 ± 0.18 | −0.18 ± 0.10 | 0.88 | ||||
| Cat | L18 | −0.85 ± 0.13 | −0.85 ± 0.21 | 1.00 | 0.458 | 0.021 | 0.726 |
| Inv1 | −0.51 ± 0.28 | −0.11 ± 0.23 | 1.32 | ||||
| L12 | 0.00 ± 0.18 | 0.20 ± 0.13 1 | 1.14 | ||||
| Gpx1 | L18 | 0.14 ± 0.13 | 0.38 ± 0.23 1 | 1.18 | 0.310 | <0.001 | 0.680 |
| Inv1 | −0.47 ± 0.17 | −0.71 ± 0.17 2 | 0.85 | ||||
| Log2 (Fold Change) CTL at L12 as Reference | Fold Change CTL as Reference | 2-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Genes | Period | ||||||
| CTL | FEN | Inter | Period | Diet | |||
| Lipid metabolism regulatory factors | |||||||
| L12 (n = 6) | 0.00 ± 0.37 1 | 0.40 ± 0.52 1 | 1.32 | ||||
| Srebf1 | L18 (n = 6) | −2.11 ± 0.29 2 | −1.76 ± 0.62 2 | 1.27 | 0.605 | <0.001 | 0.543 |
| Inv1 (n = 11) | −3.03 ± 0.18 2 | −3.24 ± 0.21 3 | 0.87 | ||||
| L12 | 0.00 ± 0.46 1 | 0.77 ± 0.24 1 | 1.71 | ||||
| Pparg | L18 | −0.07 ± 0.28 1 | −0.12 ± 0.39 2 | 0.97 | 0.097 | <0.001 | 0.432 |
| Inv1 | −0.93 ±0.10 2 | −1.19 ± 0.10 3 | 0.84 | ||||
| L12 | 0.00 ± 0.49 1 | 0.55 ± 0.38 1 | 1.46 | ||||
| Spot14 | L18 | −0.03 ± 0.40 1 | 0.23 ± 0.39 1 | 1.19 | 0.698 | <0.001 | 0.324 |
| Inv1 | −1.28 ± 24 2 | −1.28 ± 0.18 2 | 1.00 | ||||
| L12 | 0.00 ± 0.61 a,1 | 1.24 ± 0.37 b | 2.38 | ||||
| Lxra | L18 | 0.52 ± 0.17 1-2 | 0.44 ± 0.27 | 0.95 | 0.024 | 0.053 | 0.086 |
| Inv1 | 1.08 ± 0.07 2 | 1.00 ± 0.13 | 0.95 | ||||
| Protein synthesis regulatory factors | |||||||
| L12 | 0.00 ± 0.48 1 | 0.59 ± 0.24 1 | 1.51 | ||||
| Akt1 | L18 | −0.02 ± 0.08 1 | −0.10 ± 0.18 2 | 0.94 | 0.209 | <0.001 | 0.128 |
| Inv1 | −0.81 ± 0.05 2 | −0.65 ± 0.05 2 | 1.05 | ||||
| L12 | 0.00 ± 0.56 a | 0.88 ± 0.23 b,1 | 1.84 | ||||
| Mtor | L18 | 0.34 ± 0.13 | 0.09 ± 0.27 2 | 0.84 | 0.040 | 0.189 | 0.223 |
| Inv1 | 0.06 ± 0.05 | 0.06 ± 0.04 2 | 1.00 | ||||
| L12 | 0.00 ± 0.51 | 0.78 ± 0.34 1 | 1.71 | ||||
| Stat5 | L18 | 0.17 ± 0.13 | 0.09 ± 0.26 1,2 | 0.94 | 0.164 | 0.004 | 0.212 |
| Inv1 | −0.36 ± 0.08 | −0.35 ± 0.13 2 | 1.01 | ||||
| Log2 (Fold Change) CTL at L12 as Reference | Fold Change CTL as Reference | 2-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Genes | Period | ||||||
| CTL | FEN | Inter | Period | Diet | |||
| Lactogenic hormone receptors | |||||||
| L12 (n = 6) | 0.00 ± 0.49 1 | 0.40 ± 0.26 1 | 1.32 | ||||
| Prlr | L18 (n = 6) | −0.32 ± 0.30 1 | −0.11 ± 0.49 1 | 1.15 | 0.499 | <0.001 | 0.642 |
| Inv1 (n = 11) | −1.84 ± 0.16 2 | −2.09 ± 0.22 2 | 0.84 | ||||
| L12 | 0.00 ± 0.64 1 | 0.84 ± 0.32 1 | 1.79 | ||||
| Insr | L18 | 2.29 ± 0.19 2 | 2.35 ± 0.18 2 | 1.04 | 0.283 | <0.001 | 0.082 |
| Inv1 | −0.85 ± 0.09 3 | −0.67 ± 0.11 3 | 1.13 | ||||
| L12 | 0.00 ± 0.62 1 | 0.79 ± 0.69 1 | 1.72 | ||||
| Oxtr | L18 | −0.52 ± 0.37 1 | −0.58 ± 0.87 1 | 0.96 | 0.649 | 0.025 | 0.557 |
| Inv1 | 0.75 ± 0.22 1 | 0.73 ± 0.26 1 | 0.99 | ||||
| L12 | 0.00 ± 0.56 | 0.89 ± 0.43 | 1.86 | ||||
| Ghr | L18 | −0.14 ±0.23 | 0.34 ± 0.43 | 1.40 | 0.286 | 0.405 | 0.097 |
| Inv1 | 0.07 ±0.18 | 0.01 ± 0.18 | 0.96 | ||||
| L12 | 0.00 ± 0.59 a,1 | 1.37 ± 0.52 b,1,2 | 2.60 | ||||
| Igf1r | L18 | 0.89 ± 0.11 1 | 0.96 ± 0.38 1 | 1.05 | 0.057 | <0.001 | 0.033 |
| Inv1 | 1.87 ± 0.19 2 | 1.97 ± 0.10 2 | 1.07 | ||||
| L12 | 0.00 ± 0.54 1 | 0.34 ± 0.18 1 | 1.27 | ||||
| Esr1 | L18 | 1.57 ± 0.14 2 | 1.09 ± 0.32 1 | 0.72 | 0.204 | <0.001 | 0.547 |
| Inv1 | −2.02 ± 0.24 3 | −1.44 ±0.17 2 | 1.49 | ||||
| Log2 (Fold Change) CTL at L12 as Reference | Fold Change CTL as Reference | 2-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Genes | Period | ||||||
| CTL | FEN | Inter | Period | Diet | |||
| Pituitary lactogenic hormones | |||||||
| L12 (n = 5) | 0.00 ± 0.06 1 | −0.15 ± 0.20 1 | 0.90 | ||||
| Prl | L18 (n = 5) | −0.14 ± 0.15 1 | −0.20 ± 0.11 1 | 0.95 | 0.291 | <0.001 | 0.652 |
| Inv1 (n = 10) | −1.18 ± 0.06 2 | −0.93 ± 0.14 2 | 1.19 | ||||
| L12 | 0.00 ± 0.25 | −0.34 ± 0.05 1 | 0.79 | ||||
| Gh1 | L18 | 0.02 ± 0.19 | 0.21 ± 0.19 2 | 1.14 | 0.143 | 0.110 | 0.925 |
| Inv1 | −0.24 ± 0.09 | −0.06 ± 0.10 1-2 | 1.14 | ||||
| L12 | 0.00 ± 0.48 | 0.70 ± 0.12 | 1.62 | ||||
| Oxt | L18 | 0.16 ± 0.44 a | 1.08 ± 0.10 b | 1.88 | 0.050 | 0.469 | 0.007 |
| Inv1 | 0.42 ± 0.08 | 0.38 ± 0.12 | 0.97 | ||||
| Receptors of lactogenic hormone activators | |||||||
| L12 | 0.00 ± 0.05 1 | −0.13 ± 0.06 1 | 0.91 | ||||
| Drd2 | L18 | −0.39 ± 0.04 2 | −0.45 ± 0.07 2 | 0.96 | 0.040 | <0.001 | 0.984 |
| Inv1 | −0.46 ± 0.06 a,2 | −0.27 ± 0.06 b,1,2 | 1.14 | ||||
| L12 | 0.00 ± 0.16 1 | 0.42 ± 0.21 1 | 1.34 | ||||
| Trhr | L18 | 0.78 ± 0.10 2 | 0.99 ± 0.10 2 | 1.15 | 0.551 | <0.001 | 0.035 |
| Inv1 | 0.55 ± 0.13 2 | 0.67 ± 0.07 1-2 | 1.09 | ||||
| L12 | 0.00 ± 0.25 | 0.35 ± 0.11 1 | 1.27 | ||||
| Vipr2 | L18 | 0.65 ± 0.16 | 1.07 ± 0.07 2 | 1.34 | 0.375 | <0.001 | 0.061 |
| Inv1 | 0.22 ± 0.15 | 0.25 ± 0.14 1 | 1.02 | ||||
| L12 | 0.00 ± 0.16 1 | −0.65 ± 0.44 1 | 0.64 | ||||
| Ghrhr | L18 | 0.46 ± 0.33 1 | 1.20 ± 0.21 2 | 1.68 | 0.069 | <0.001 | 0.382 |
| Inv1 | −2.31 ± 0.15 2 | −1.77 ± 0.27 3 | 1.45 | ||||
| L12 | 0.00 ± 0.08 | 0.11 ± 0.09 | 1.08 | ||||
| Ar | L18 | 0.27 ± 0.08 | 0.18 ± 0.09 | 0.94 | 0.420 | 0.152 | 0.565 |
| Inv1 | 0.11 ± 0.04 | 0.19 ± 0.07 | 1.06 | ||||
| L12 | 0.00 ± 0.14 1 | 0.26 ± 0.35 1 | 1.20 | ||||
| Esr1 | L18 | 0.56 ± 0.12 1-2 | 0.69 ± 0.09 1-2 | 1.09 | 0.508 | <0.001 | 0.823 |
| Inv1 | 1.35 ± 0.16 2 | 1.10 ± 0.27 2 | 0.84 | ||||
| Estrogenic effect biomarker | |||||||
| L12 | 0.00 ± 0.29 | −0.09 ± 0.35 1 | 0.94 | ||||
| C3 | L18 | 0.24 ± 0.18 a | 1.31 ± 0.15 b,2 | 2.10 | 0.017 | 0.006 | 0.006 |
| Inv1 | 0.61 ± 0.26 | 1.00 ± 0.13 2 | 1.31 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevrin, T.; Boquien, C.-Y.; Gandon, A.; Grit, I.; de Coppet, P.; Darmaun, D.; Alexandre-Gouabau, M.-C. Fenugreek Stimulates the Expression of Genes Involved in Milk Synthesis and Milk Flow through Modulation of Insulin/GH/IGF-1 Axis and Oxytocin Secretion. Genes 2020, 11, 1208. https://doi.org/10.3390/genes11101208
Sevrin T, Boquien C-Y, Gandon A, Grit I, de Coppet P, Darmaun D, Alexandre-Gouabau M-C. Fenugreek Stimulates the Expression of Genes Involved in Milk Synthesis and Milk Flow through Modulation of Insulin/GH/IGF-1 Axis and Oxytocin Secretion. Genes. 2020; 11(10):1208. https://doi.org/10.3390/genes11101208
Chicago/Turabian StyleSevrin, Thomas, Clair-Yves Boquien, Alexis Gandon, Isabelle Grit, Pierre de Coppet, Dominique Darmaun, and Marie-Cécile Alexandre-Gouabau. 2020. "Fenugreek Stimulates the Expression of Genes Involved in Milk Synthesis and Milk Flow through Modulation of Insulin/GH/IGF-1 Axis and Oxytocin Secretion" Genes 11, no. 10: 1208. https://doi.org/10.3390/genes11101208
APA StyleSevrin, T., Boquien, C.-Y., Gandon, A., Grit, I., de Coppet, P., Darmaun, D., & Alexandre-Gouabau, M.-C. (2020). Fenugreek Stimulates the Expression of Genes Involved in Milk Synthesis and Milk Flow through Modulation of Insulin/GH/IGF-1 Axis and Oxytocin Secretion. Genes, 11(10), 1208. https://doi.org/10.3390/genes11101208






