Detection of Quantitative Trait Loci (QTL) Associated with the Fruit Morphology of Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Development and Experimental Design
2.2. Phenotypic Analysis
2.3. Linkage Map Construction and QTL Analysis
3. Results
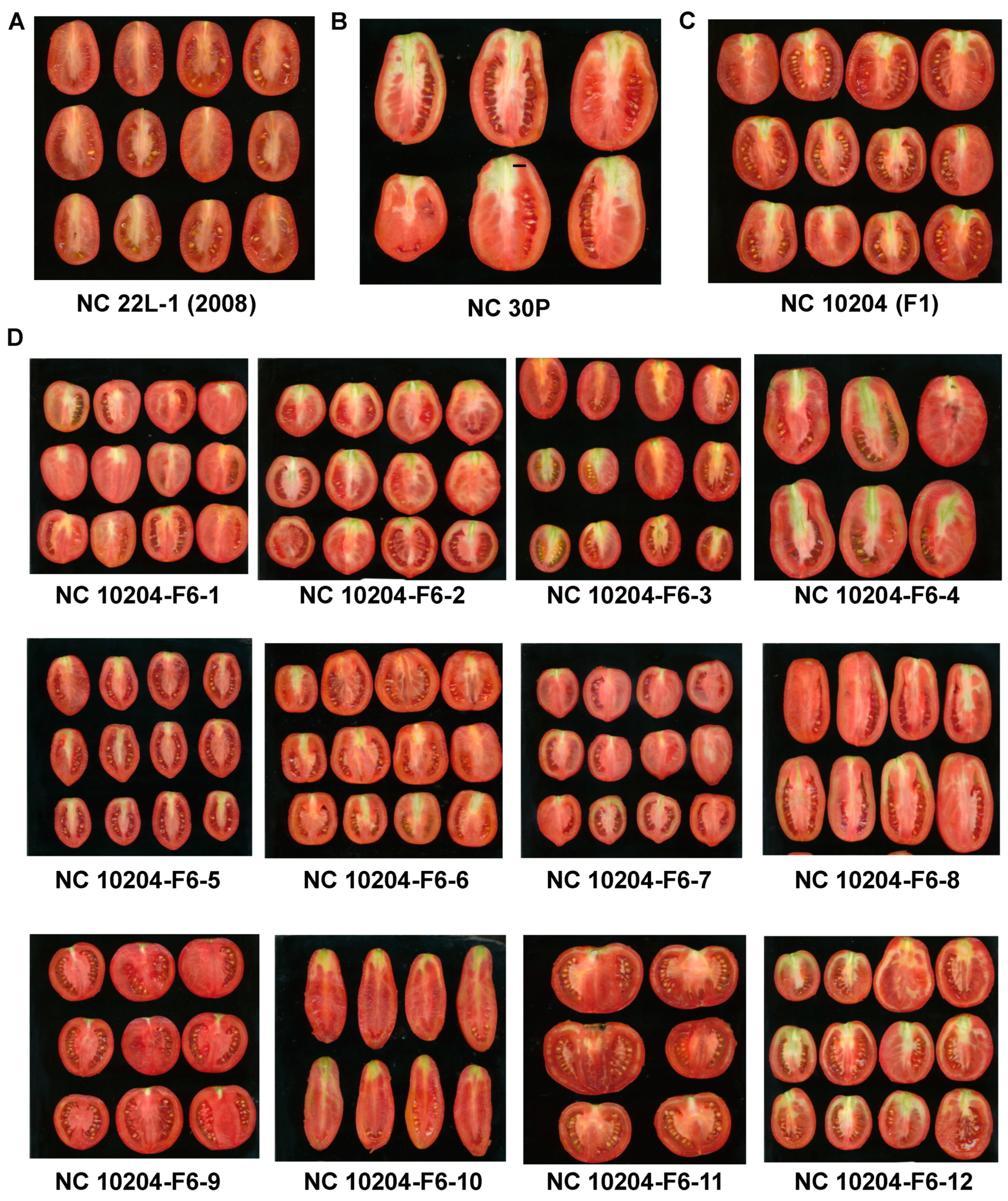
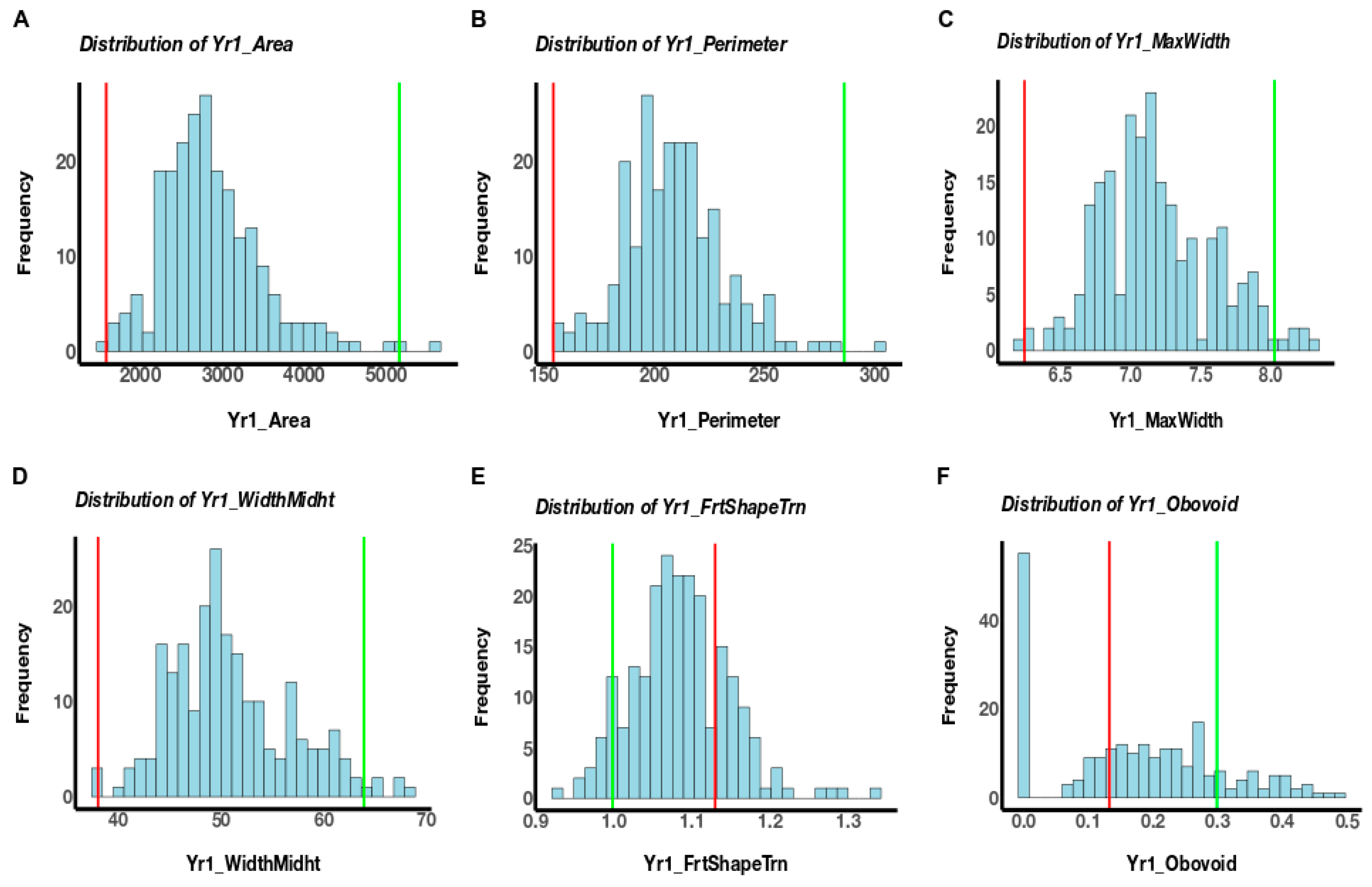
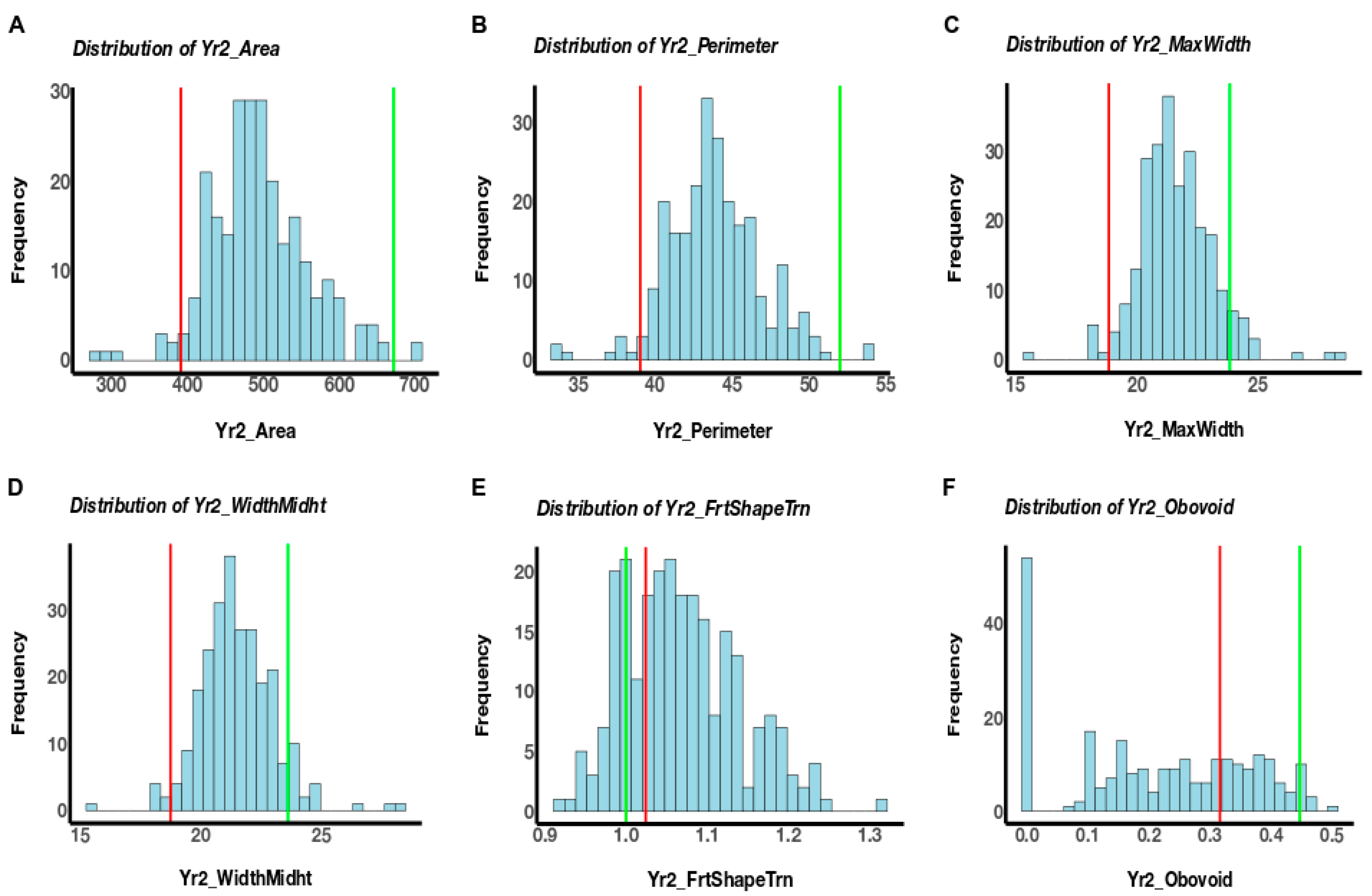
3.1. Phenotypic Variation
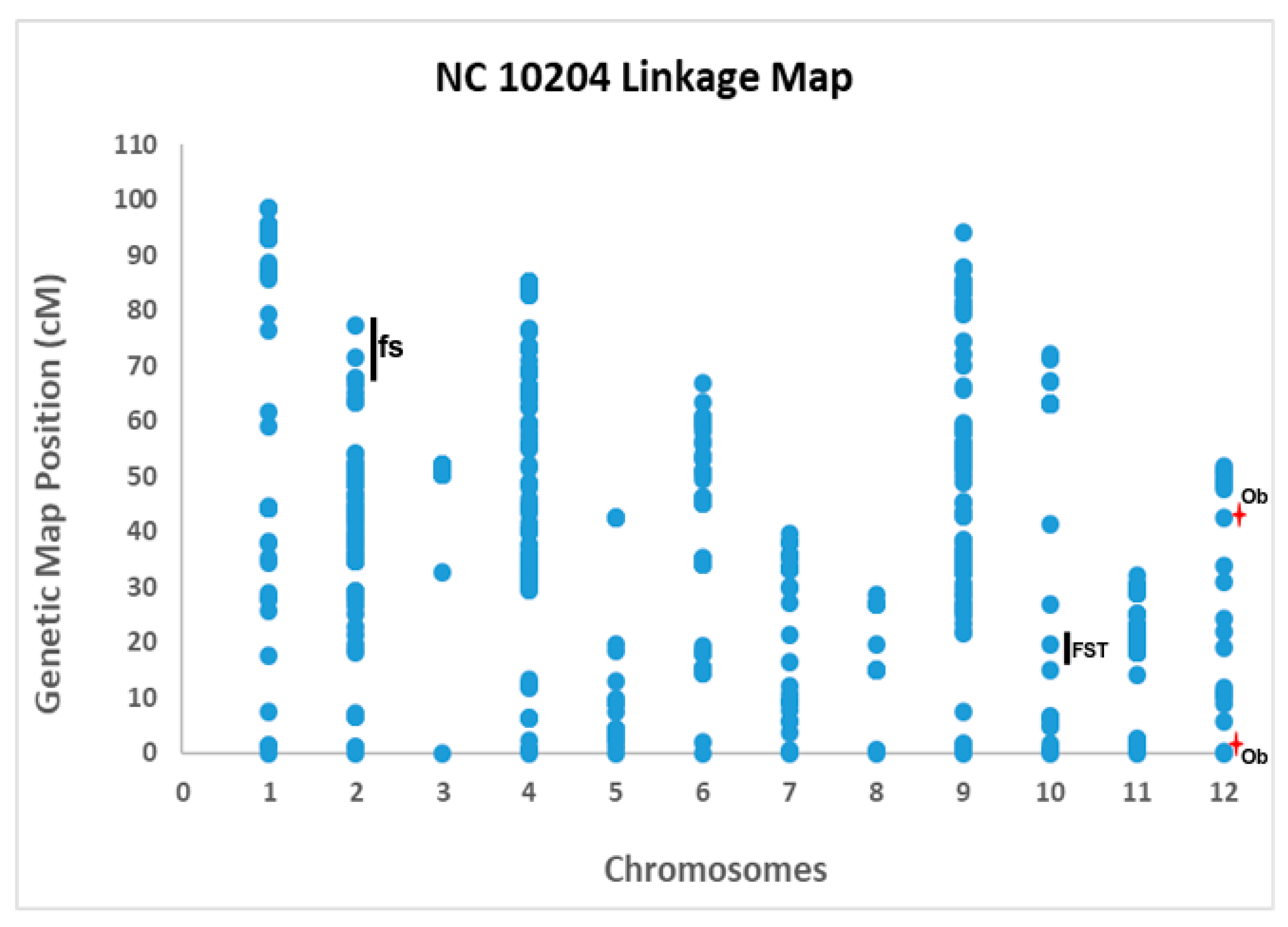
3.2. Linkage Mapping and QTL Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AMRC. Tomatoes; Agricultural Marketing Resource Center: Washington, DC, USA, 2018. [Google Scholar]
- Merk, H.L.; Ashrafi, H.; Foolad, M.R. Selective genotyping to identify late blight resistance genes in an accession of the tomato wild species Solanum pimpinellifolium. Euphytica 2012, 187, 63–75. [Google Scholar] [CrossRef]
- Rodriguez, G.R.; Munos, S.; Anderson, C.; Sim, S.C.; Michel, A.; Causse, M.; Gardener, B.B.M.; Francis, D.; van der Knaap, E. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 2011, 156, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Visa, S.; Cao, C.X.; Gardener, B.M.; van der Knaap, E. Modeling of tomato fruits into nine shape categories using elliptic fourier shape modeling and Bayesian classification of contour morphometric data. Euphytica 2014, 200, 429–439. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Zhang, N.; Sauvage, C.; Munos, S.; Blanca, J.; Canizares, J.; Diez, M.J.; Schneider, R.; Mazourek, M.; McClead, J.; et al. A cytochrome P450 regulates a domestication trait in cultivated tomato. Proc. Natl. Acad. Sci. USA 2013, 110, 17125–17130. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Barrero, L.S.; Tanksley, S.D. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 2008, 40, 800–804. [Google Scholar] [CrossRef]
- Frary, A.; Nesbitt, T.C.; Grandillo, S.; van der Knaap, E.; Cong, B.; Liu, J.P.; Meller, J.; Elber, R.; Alpert, K.B.; Tanksley, S.D. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef]
- Liu, J.P.; Van Eck, J.; Cong, B.; Tanksley, S.D. A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc. Natl. Acad. Sci. USA 2002, 99, 13302–13306. [Google Scholar] [CrossRef]
- Munos, S.; Ranc, N.; Botton, E.; Berard, A.; Rolland, S.; Duffe, P.; Carretero, Y.; Le Paslier, M.C.; Delalande, C.; Bouzayen, M.; et al. Increase in tomato locule number is controlled by two Single Nucleotide Polymorphisms located near WUSCHEL. Plant Physiol. 2011, 156, 2244–2254. [Google Scholar] [CrossRef]
- Xiao, H.; Jiang, N.; Schaffner, E.; Stockinger, E.J.; van der Knaap, E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 2008, 319, 1527–1530. [Google Scholar] [CrossRef]
- Van der Knaap, E.; Chakrabarti, M.; Chu, Y.H.; Clevenger, J.P.; Illa-Berenguer, E.; Huang, Z.J.; Keyhaninejad, N.; Mu, Q.; Sun, L.; Wang, Y.P.; et al. What lies beyond the eye: The molecular mechanisms regulating tomato fruit weight and shape. Front. Plant Sci. 2014, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, M.J.; van der Knaap, E. A comparative analysis into the genetic bases of morphology in tomato varieties exhibiting elongated fruit shape. Theor. Appl. Genet. 2008, 116, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Grandilio, S.; Ku, H.M.; Tanksley, S.D. Characterization of fs8.1, a major QTL influencing fruit shape in tomato. Mol. Breed. 1996, 2, 251–260. [Google Scholar] [CrossRef]
- Ku, H.M.; Grandillo, S.; Tanksley, S.D. fs8.1, a major QTL, sets the pattern of tomato carpel shape well before anthesis. Theor. Appl. Genet. 2000, 101, 873–878. [Google Scholar] [CrossRef]
- Clevenger, J. Metabolic and Genomic Analysis of Elongated Fruit Shape in Tomato (Solanum lycopersicum). Master’s Thesis, Ohio State University, Wooster, OH, USA, 2012. [Google Scholar]
- Rodríguez, G.R.; Kim, H.J.; van der Knaap, E. Mapping of two suppressors of OVATE (sov) loci in tomato. Heredity 2013, 111, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, L.; Duffe, P.; Buret, M.; Servin, B.; Hospital, F.; Causse, M. Marker-assisted introgression of five QTLs controlling fruit quality traits into three tomato lines revealed interactions between QTLs and genetic backgrounds. Theor. Appl. Genet. 2004, 109, 658–668. [Google Scholar] [CrossRef]
- Wurschum, T. Mapping QTL for agronomic traits in breeding populations. Theor. Appl. Genet. 2012, 125, 201–210. [Google Scholar] [CrossRef]
- Sim, S.-C.; Durstewitz, G.; Plieske, J.; Wieseke, R.; Ganal, M.W.; Van Deynze, A.; Hamilton, J.P.; Buell, C.R.; Causse, M.; Wijeratne, S. Development of a large SNP genotyping array and generation of high-density genetic maps in tomato. PLoS ONE 2012, 7, e40563. [Google Scholar] [CrossRef]
- Brewer, M.T.; Lang, L.X.; Fujimura, K.; Dujmovic, N.; Gray, S.; van der Knaap, E. Development of a controlled vocabulary and software application to analyze fruit shape variation in tomato and other plant species. Plant Physiol. 2006, 141, 15–25. [Google Scholar] [CrossRef]
- Rodríguez, G.; Moyseenko, J.; Robbins, M.; Morejón, N.; Francis, D.; van der Knaap, E. Tomato Analyzer: A useful software application to collect accurate and detailed morphological and colorimetric data from two-dimensional objects. J. Vis. Exp. 2010, 37, e1856. [Google Scholar] [CrossRef]
- Gardner, R.G.; Panthee, D.R. ‘Plum Regal’ Fresh-market plum tomato hybrid and its parents, NC 25P and NC 30P. HortScience 2010, 45, 824–825. [Google Scholar] [CrossRef]
- Ivors, K. Commercial Production of Staked Tomatoes in the Southeast; North Carolina State University: Raleigh, NC, USA, 2010. [Google Scholar]
- The SAS System, Version 9.4 for Windows, 9th ed.; SAS Institute: Cary, NC, USA, 2012.
- Kabelka, E.; Franchino, B.; Francis, D.M. Two loci from Lycopersicon hirsutum LA407 confer resistance to strains of Clavibacter michiganensis subsp michiganensis. Phytopathology 2002, 92, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Van Ooijen, J.W. Joinmap 4.0, Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Plant Research International: Wageningen, The Netherlands, 2006. [Google Scholar]
- Stam, P. Construction of integrated genetic linkage maps by means of a new computer package: Join Map. Plant J. 1993, 3, 739–744. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Windows QTL Cartographer, V2.5; Department of Statistics, North Carolina State University: Raleigh, NC, USA, 2012.
- Panthee, D.R.; Piotrowski, A.; Ibrahem, R. Mapping Quantitative Trait Loci (QTL) for Resistance to Late Blight in Tomato. Int. J. Mol. Sci. 2017, 18, 1589. [Google Scholar] [CrossRef] [PubMed]
- Panthee, D.R.; Pantalone, V.R.; Sams, C.E.; Saxton, A.M.; West, D.R.; Orf, J.H.; Killam, A.S. Quantitative trait loci controlling sulfur containing amino acids, methionine and cysteine, in soybean seeds. Theor. Appl. Genet. 2006, 112, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.; Desplat, N.; Huang, B.E.; Desgroux, A.; Bruguier, L.; Bouchet, J.P.; Le, Q.H.; Chauchard, B.; Verschave, P.; Causse, M. Potential of a tomato MAGIC population to decipher the genetic control of quantitative traits and detect causal variants in the resequencing era. Plant Biotechnol. J. 2015, 13, 565–577. [Google Scholar] [CrossRef]
- Ben Chaim, A.; Borovsky, Y.; Rao, G.; Gur, A.; Zamir, D.; Paran, I. Comparative QTL mapping of fruit size and shape in tomato and pepper. Isr. J. Plant Sci. 2006, 54, 191–203. [Google Scholar] [CrossRef]
- Brewer, M.T.; Moyseenko, J.B.; Monforte, A.J.; van der Knaap, E. Morphological variation in tomato: A comprehensive study of quantitative trait loci controlling fruit shape and development. J. Exp. Bot. 2007, 58, 1339–1349. [Google Scholar] [CrossRef]
- Lin, T.; Zhu, G.T.; Zhang, J.H.; Xu, X.Y.; Yu, Q.H.; Zheng, Z.; Zhang, Z.H.; Lun, Y.Y.; Li, S.; Wang, X.X.; et al. Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 2014, 46, 1220–1226. [Google Scholar] [CrossRef]
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Bombarely, A.; Fisher-York, T.; Pujar, A.; Foerster, H.; et al. The Sol Genomics Network (SGN)-from genotype to phenotype to breeding. Nucleic Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Van Houten, J.; Gonzalez, G.; Xiao, H.; van der Knaap, E. Genome-wide identification, phylogeny and expression analysis of SUN, OFP and YABBY gene family in tomato. Mol. Genet. Genom. 2013, 288, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Chusreeaeom, K.; Ariizumi, T.; Asamizu, E.; Okabe, Y.; Shirasawa, K.; Ezura, H. A novel tomato mutant, Solanum lycopersicum elongated fruit1 (Slelf1), exhibits an elongated fruit shape caused by increased cell layers in the proximal region of the ovary. Mol. Genet. Genom. 2014, 289, 399–409. [Google Scholar] [CrossRef] [PubMed]
| Category of Trait | Trait | F2:3(Year 2014) | F2:3(Year 2014) | Correlation between Generations c | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Dev | Min. | Max. | Geno Effect a | Shapiro-Wilk Normality Test of Residuals b | H2 | Mean | Std. Dev | Min. | Max. | Geno Effect | Shapiro-Wilk Normality Test of Residuals | H2 | |||
| Fruit Size | A | 2836 | 549.2 | 1640 | 5095 | <0.0001 | 0.4236 | 0.70 | 247,274 | 49,705 | 130,233 | 416,683 | <0.0001 | <2.2 × 1016 nn | 0.61 | 0.25 * |
| P | 207.8 | 20.38 | 155 | 281.9 | <0.0001 | 0.3971 | 0.69 | 1929 | 206.2 | 1403 | 2566 | <0.0001 | <2.2 × 1016 nn | 0.64 | 0.25 * | |
| WMH | 50.62 | 5.39 | 40.8 | 66.45 | <0.0001 | 0.06062 nn | 0.73 | 460.9 | 50.34 | 300.3 | 599.83 | <0.0001 | <2.2 × 1016 nn | 0.64 | 0.24 * | |
| MW | 51.46 | 5.4 | 42.3 | 67.26 | <0.0001 | 0.04489 | 0.73 | 466.2 | 50.35 | 304.1 | 601.75 | <0.0001 | <2.2 × 1016 nn | 0.73 | 0.24 * | |
| Fruit Shape | FST | 1.18 | 0.13 | 0.87 | 1.77 | <0.0001 | 0.00201 nn | 0.56 | 1.15 | 0.14 | 0.87 | 1.51 | <0.0001 | 0.001386 nn | 0.67 | 0.52 *** |
| Ob | 0.05 | 0.05 | 0 | 0.24 | <0.0001 | 4.77 × 106 nn | 0.64 | 0.07 | 0.06 | 0 | 0.23 | <0.0001 | 5.18 × 1011 nn | 0.77 | 0.48 *** | |
| Variable | P | A | WMH | MW | FST | Ob |
|---|---|---|---|---|---|---|
| P | 1 | |||||
| A | 0.99 ***a | 1 | ||||
| WMH | 0.8 *** | 0.86 *** | 1 | |||
| MW | 0.81 *** | 0.86 *** | 0.99 *** | 1 | ||
| FST | −0.19 * | −0.18 * | ns b | ns | 1 | |
| Ob | 0.22 * | ns | −0.18 * | ns | −0.77 *** | 1 |
| Variable | P | A | WMH | MW | FST | Ob |
|---|---|---|---|---|---|---|
| P | 1 | |||||
| A | 0.98 ***a | 1 | ||||
| WMH | 0.70 *** | 0.79 *** | 1 | |||
| MW | 0.73 *** | 0.81 *** | 0.99 ***b | 1 | ||
| FST | −0.2 * | ns | ns | ns | 1 | |
| Ob | 0.33 *** | 0.23 * | ns | ns | −0.83 *** | 1 |
| Source | Perimeter | Area | Width Mid Height | Maximum Width | Fruit Shape Triangle | Obovoid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F Value | Pr > F | F Value | Pr > F | F Value | Pr > F | F Value | Pr > F | F Value | Pr > F | F Value | Pr > F | |
| Genotype | 1.5 | 0.0213 * | 1.55 | 0.0148 * | 1.45 | 0.0316 * | 1.35 | 0.0648 | 2.59 | <0.0001 *** | 2.52 | <0.0001 *** |
| Year | 607.3 | 0.0002 *** | 2579.94 | <0.0001 *** | 2055.84 | <0.0001 *** | 2508.6 | <0.0001 *** | 2.48 | 0.1256 | 2.44 | 0.151 |
| Genotype * Year | 3.31 | <0.0001 *** | 4.94 | <0.0001 *** | 5.31 | <0.0001 *** | 3.59 | <0.0001 *** | 2.01 | <0.0001 *** | 2.39 | <0.0001 *** |
| Replication (Year) | 17.99 | <0.0001 *** | 0.44 | 0.6433 | 3.27 | 0.0401 * | 23.25 | <0.0001 *** | 0.37 | 0.6897 | 1.94 | 0.1457 |
| Chromosome | Markers | Length (cM) |
|---|---|---|
| 1 | 58 | 98.6 |
| 2 | 101 | 77.4 |
| 3 | 60 | 52.1 |
| 4 | 212 | 85.3 |
| 5 | 30 | 42.6 |
| 6 | 60 | 66.8 |
| 7 | 36 | 39.6 |
| 8 | 15 | 28.8 |
| 9 | 138 | 94.1 |
| 10 | 31 | 72.1 |
| 11 | 112 | 30.1 |
| 12 | 33 | 51.9 |
| Total | 886 | 739.4 |
| Category of Traits | Traits | Chr. | Closest Markers | Genetic Map Position (cM) | Physical Map Position (bp) | Threshold LOD | LOD Score | Additive Value | R2 (%) | Nature of QTL |
|---|---|---|---|---|---|---|---|---|---|---|
| Fruit Size | Yr1_P | 2 | solcap_snp_sl_21867 | 77.44 | 47,948,927 | 3.7 | 6.9 | −7.76 | 6.9 | a consistent; confirmatory |
| Yr2_P | 2 | solcap_snp_sl_50066 | 67.7 | 44,783,686 | 3.6 | 4.7 | −2.17 | 17.2 | consistent; confirmatory | |
| Yr1_A | 2 | solcap_snp_sl_21867 | 75.44 | 47,948,927 | 3.8 | 7.8 | −228.27 | 8.3 | consistent; confirmatory | |
| Yr2_A | 2 | solcap_snp_sl_42324 | 66.73 | 44,069,445 | 3.7 | 5.4 | −50.58 | 20.0 | consistent; confirmatory | |
| Yr1_WMH | 2 | solcap_snp_sl_21867 | 77.44 | 47,948,927 | 3.9 | 7.7 | −2.23 | 8.8 | consistent; confirmatory | |
| Yr2_WMH | 2 | solcap_snp_sl_21867 | 77.44 | 47,948,927 | 3.7 | 3.7 | −0.72 | 15.8 | consistent; confirmatory | |
| Yr1_MW | 2 | solcap_snp_sl_21867 | 72.44 | 47,948,927 | 3.6 | 6.9 | −0.16 | 8.3 | consistent; confirmatory | |
| Yr2_MW | 2 | solcap_snp_sl_21867 | 76.44 | 47,948,927 | 3.6 | 3.6 | −0.73 | 15.6 | consistent; confirmatory | |
| Fruit Shape | Yr1_FST | 10 | solcap_snp_sl_9598, solcap_snp_sl_16517 | 22.77 | 4,260,136;57,327,585 | 3.7 | 4.0 | 0.03 | 10.2 | consistent; novel |
| Yr2_FST | 10 | solcap_snp_sl_34373, solcap_snp_sl_9598 | 16.07 | 3,991,802;4,260,136 | 3.6 | 5.4 | 0.05 | 24.2 | consistent; novel | |
| Yr1_Ob | 12 | solcap_snp_sl_1573, solcap_snp_sl_58869 | 1.26 | 4,038,812;5,029,856 | 3.7 | 4.9 | −0.10 | 34.0 | b not consistent; putative QTL; might be novel | |
| Yr2_Ob | 12 | solcap_snp_sl_24755, solcap_snp_sl_31628 | 43.48 | 7,801,435;64,210,355 | 3.7 | 4.2 | 0.06 | 7.1 | not consistent; putative QTL; might be novel |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, P.; McNellie, J.; Panthee, D.R. Detection of Quantitative Trait Loci (QTL) Associated with the Fruit Morphology of Tomato. Genes 2020, 11, 1117. https://doi.org/10.3390/genes11101117
Adhikari P, McNellie J, Panthee DR. Detection of Quantitative Trait Loci (QTL) Associated with the Fruit Morphology of Tomato. Genes. 2020; 11(10):1117. https://doi.org/10.3390/genes11101117
Chicago/Turabian StyleAdhikari, Pragya, James McNellie, and Dilip R. Panthee. 2020. "Detection of Quantitative Trait Loci (QTL) Associated with the Fruit Morphology of Tomato" Genes 11, no. 10: 1117. https://doi.org/10.3390/genes11101117
APA StyleAdhikari, P., McNellie, J., & Panthee, D. R. (2020). Detection of Quantitative Trait Loci (QTL) Associated with the Fruit Morphology of Tomato. Genes, 11(10), 1117. https://doi.org/10.3390/genes11101117






