Synergistic Activity of Mobile Genetic Element Defences in Streptococcus pneumoniae
Abstract
1. Introduction
2. Materials and Methods
2.1. Quantification of tvr Locus Variation
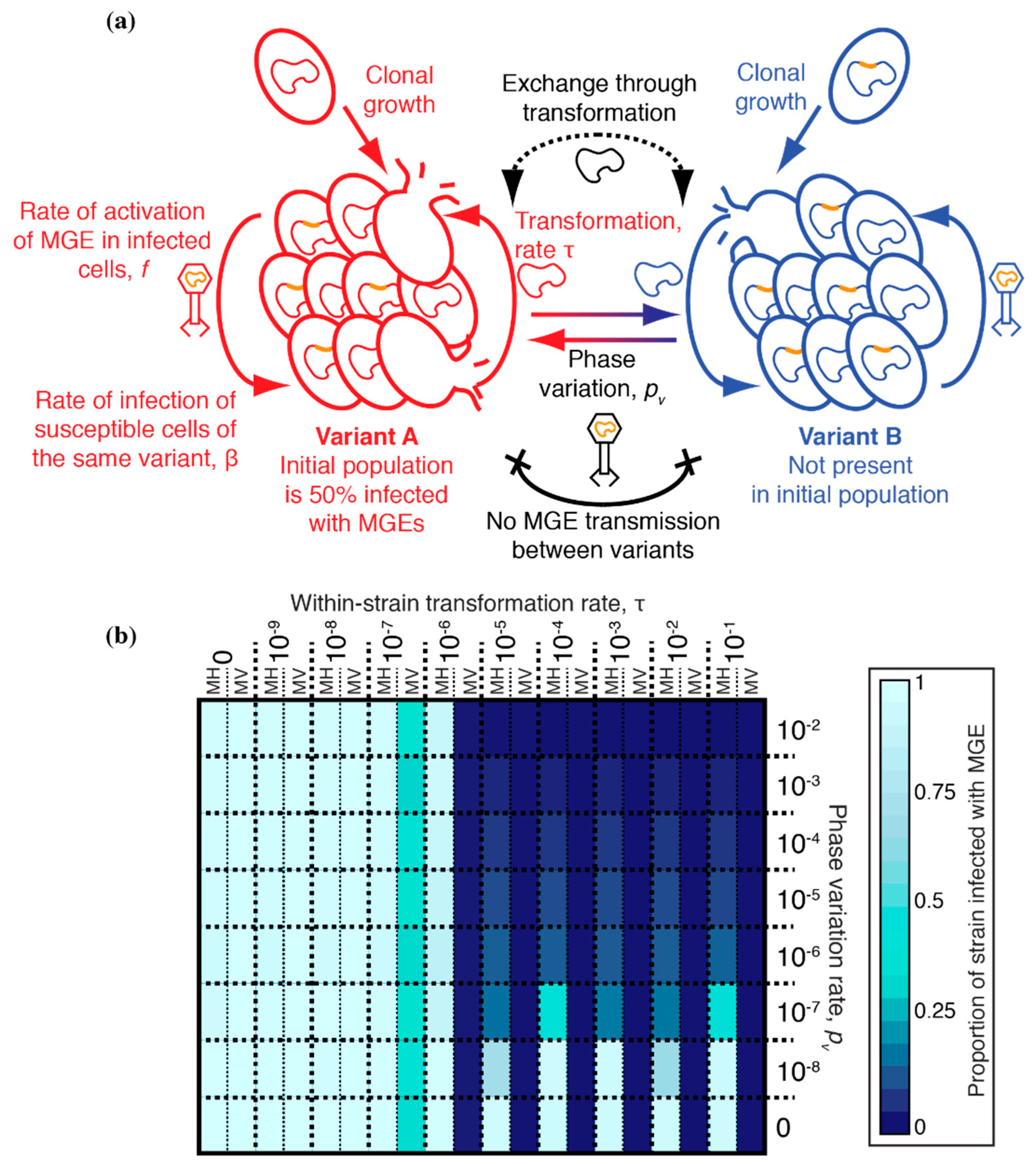
2.2. Structure of the Compartmental Model
2.3. Analysis of Simulations
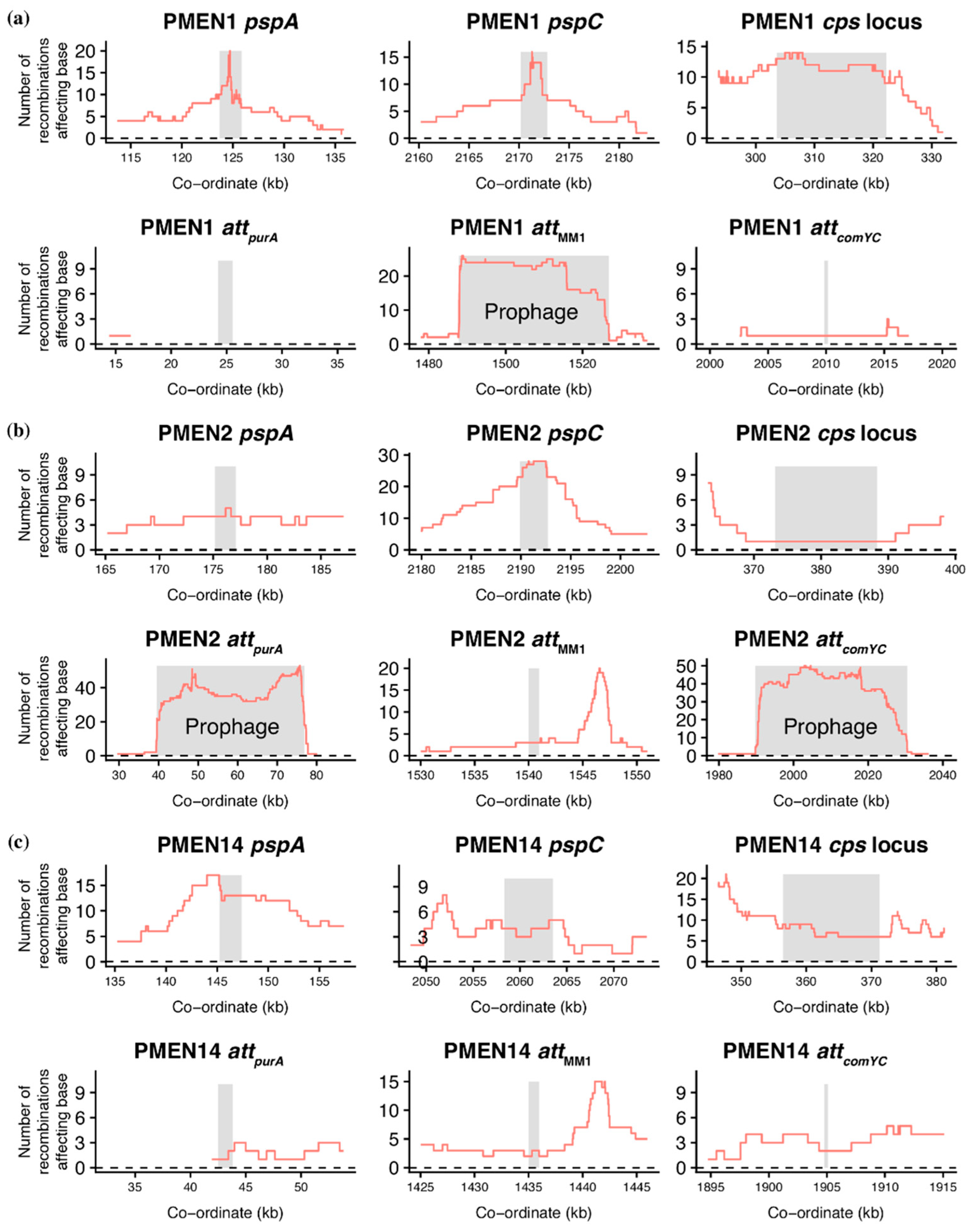
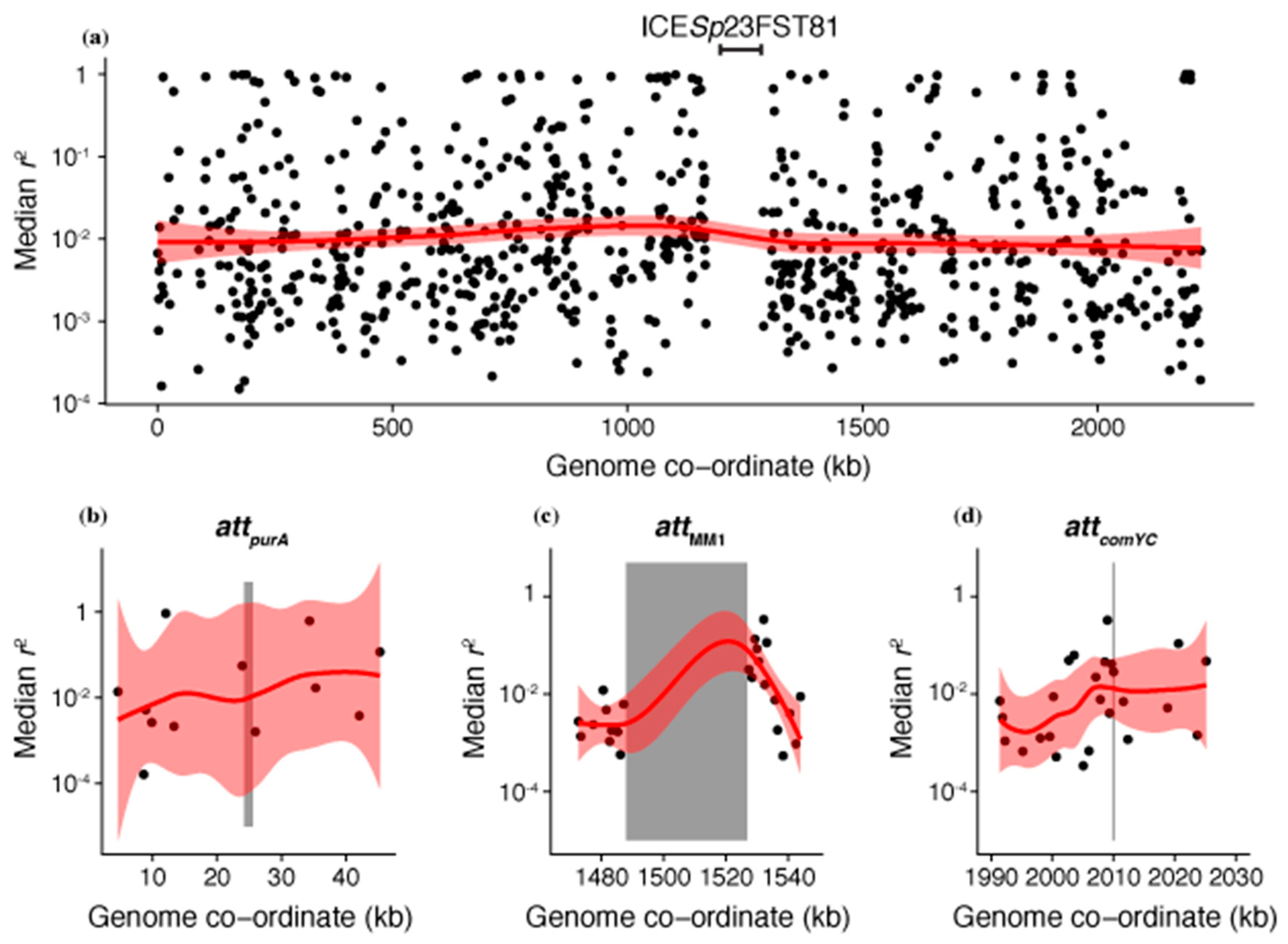
2.4. Analysis of Genomic Data
3. Results
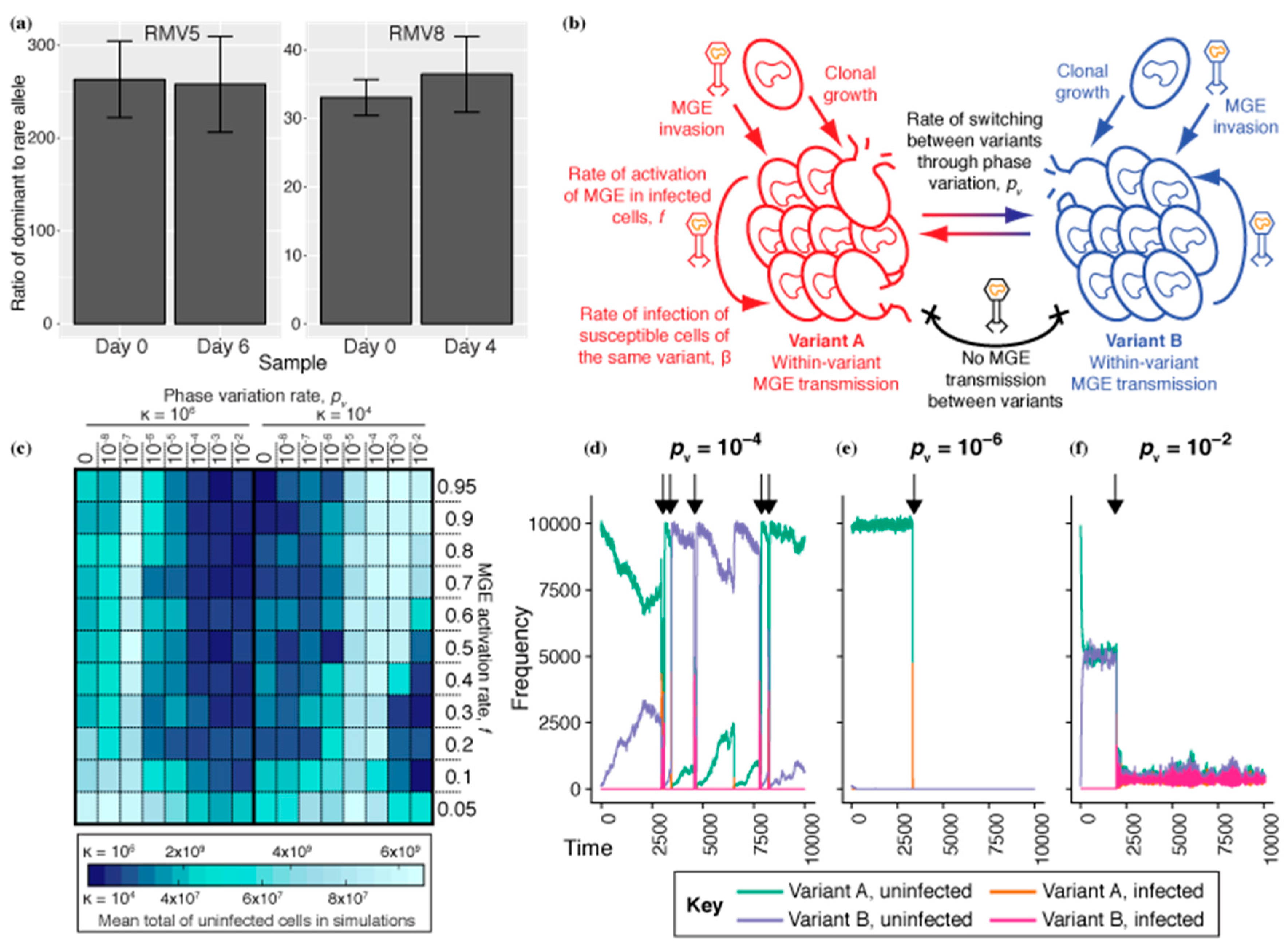
3.1. Constraining the Optimal Rate of Phase Variation for RMSs
3.2. Phase Variable RMS Are Undermined by Vertical Transmission of MGEs
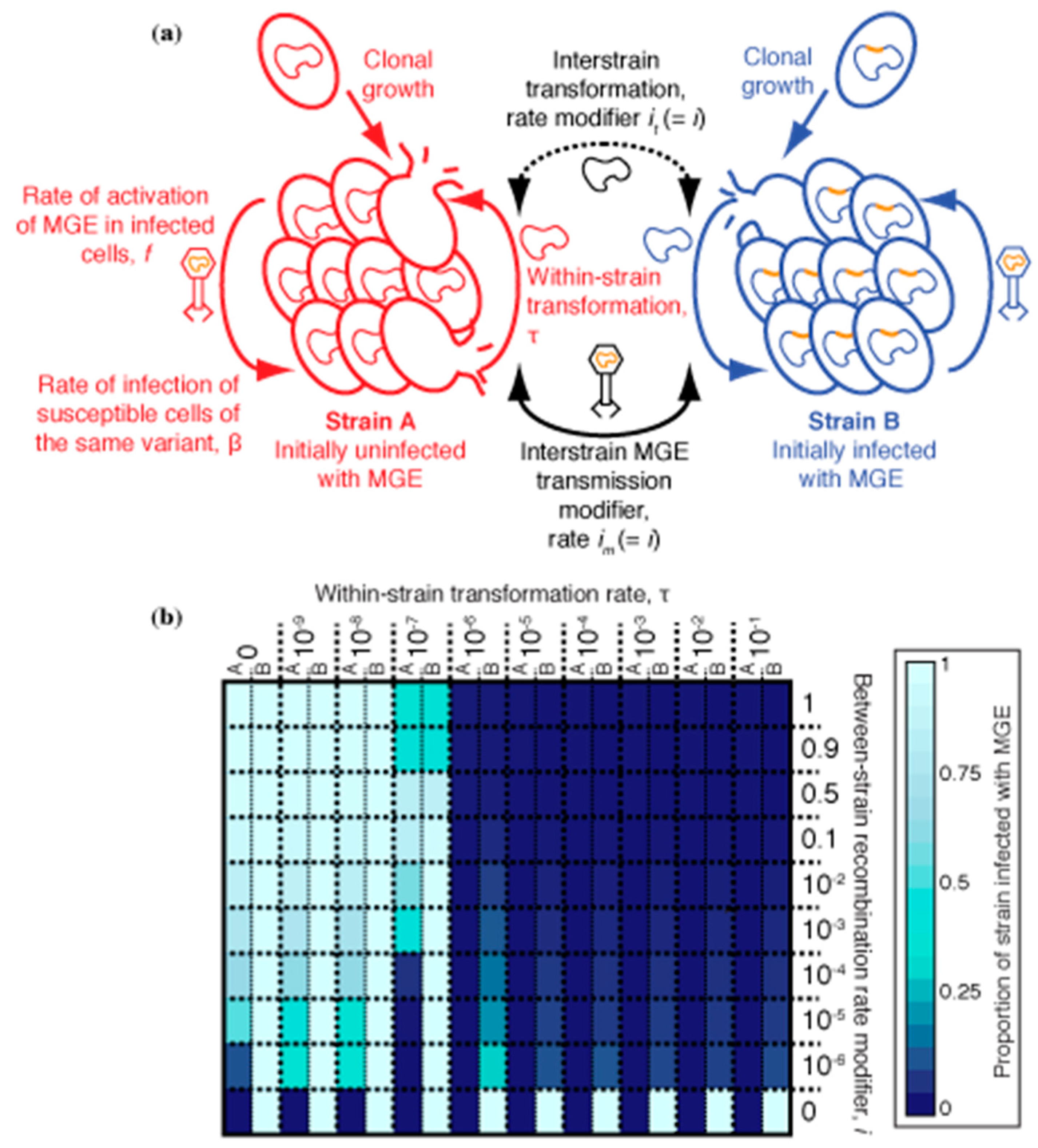
3.3. Between-Strain Transformation and the Inhibition of MGE Transmission
3.4. Partitioning Within-Strain and Between-Strain Recombination
3.5. Synergistic Combinations of S. pneumoniae ‘Immune System’ Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lees, J.A.; Harris, S.R.; Tonkin-Hill, G.; Gladstone, R.A.; Lo, S.; Weiser, J.N.; Corander, J.; Bentley, S.D.; Croucher, N.J. Fast and flexible bacterial genomic epidemiology with PopPUNK. Genome Res. 2019, 29, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, R.A.; Lo, S.W.; Lees, J.A.; Croucher, N.J.; van Tonder, A.J.; Corander, J.; Page, A.J.; Marttinen, P.; Bentley, L.J.; Ochoa, T.J.; et al. International genomic definition of pneumococcal lineages, to contextualise disease, antibiotic resistance and vaccine impact. EBioMedicine 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Løchen, A.; Bentley, S.D. Pneumococcal Vaccines: Host Interactions, Population Dynamics, and Design Principles. Annu. Rev. Microbiol. 2018, 72, 521–549. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Hinds, J.; Turner, C.; Jankhot, A.; Gould, K.; Bentley, S.D.; Nosten, F.; Goldblatt, D. Improved detection of nasopharyngeal cocolonization by multiple pneumococcal serotypes by use of latex agglutination or molecular serotyping by microarray. J. Clin. Microbiol. 2011, 49, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Coupland, P.G.; Stevenson, A.E.; Callendrello, A.; Bentley, S.D.; Hanage, W.P. Diversification of bacterial genome content through distinct mechanisms over different timescales. Nat. Commun. 2014, 5, 5471. [Google Scholar] [CrossRef]
- Novick, R.P.; Christie, G.E.; Penadés, J.R. The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 2010, 8, 541. [Google Scholar] [CrossRef] [PubMed]
- Cury, J.; Touchon, M.; Rocha, E.P.C. Integrative and conjugative elements and their hosts: composition, distribution and organization. Nucleic Acids Res. 2017, 45, 8943–8956. [Google Scholar] [CrossRef]
- Hiller, N.L.; Janto, B.; Hogg, J.S.; Boissy, R.; Yu, S.; Powell, E.; Keefe, R.; Ehrlich, N.E.; Shen, K.; Hayes, J.; et al. Comparative Genomic Analyses of Seventeen Streptococcus pneumoniae Strains: Insights into the Pneumococcal Supragenome. J. Bacteriol. 2007, 189, 8186–8195. [Google Scholar] [CrossRef]
- Johnston, C.; Campo, N.; Bergé, M.J.; Polard, P.; Claverys, J.P. Streptococcus pneumoniae, le transformiste. Trends Microbiol. 2014, 22, 113–119. [Google Scholar] [CrossRef]
- Touchon, M.; Bernheim, A.; Rocha, E.P.C. Genetic and life-history traits associated with the distribution of prophages in bacteria. ISME J. 2016, 10, 2744–2754. [Google Scholar] [CrossRef]
- Tettelin, H.; Nelson, K.E.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Peterson, S.; Heidelberg, J.; DeBoy, R.T.; Haft, D.H.; Dodson, R.J.; et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 2001, 293, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Garcia, E.; Mitchell, T.J. Development of a prophage typing system and analysis of prophage carriage in Streptococcus pneumoniae. Appl. Environ. Microbiol. 2009, 75, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Severina, E.; Tomasz, A. A high incidence of prophage carriage among natural isolates of Streptococcus pneumoniae. J. Bacteriol. 1999, 181, 3618–3625. [Google Scholar] [PubMed]
- Bikard, D.; Hatoum-Aslan, A.; Mucida, D.; Marraffini, L.A. CRISPR interference can prevent natural transformation and virulence acquisition during in vivo bacterial infection. Cell Host Microbe 2012, 12, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Terns, M.P.; Terns, R.M. CRISPR-based adaptive immune systems. Curr. Opin. Microbiol. 2011, 14, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Vincze, T.; Posfai, J.; Macelis, D. REBASE-a database for DNA restriction and modification: Enzymes, genes and genomes. Nucleic Acids Res. 2015, 43, D298–D299. [Google Scholar] [CrossRef]
- Leprohon, P.; Gingras, H.; Ouennane, S.; Moineau, S.; Ouellette, M. A genomic approach to understand interactions between Streptococcus pneumoniae and its bacteriophages. BMC Genomics 2015, 16, 972. [Google Scholar] [CrossRef]
- Eutsey, R.A.; Powell, E.; Dordel, J.; Salter, S.J.; Clark, T.A.; Korlach, J.; Ehrlich, G.D.; Hiller, N.L. Genetic Stabilization of the Drug-Resistant PMEN1 Pneumococcus Lineage by Its Distinctive DpnIII Restriction-Modification System. MBio 2015, 6, e00173-15. [Google Scholar] [CrossRef]
- Manso, A.S.; Chai, M.H.; Atack, J.M.; Furi, L.; De Ste Croix, M.; Haigh, R.; Trappetti, C.; Ogunniyi, A.D.; Shewell, L.K.; Boitano, M.; et al. A random six-phase switch regulates pneumococcal virulence via global epigenetic changes. Nat. Commun. 2014, 5, 5055. [Google Scholar] [CrossRef]
- Li, J.; Li, J.W.; Feng, Z.; Wang, J.; An, H.; Liu, Y.; Wang, Y.; Wang, K.; Zhang, X.; Miao, Z.; et al. Epigenetic Switch Driven by DNA Inversions Dictates Phase Variation in Streptococcus pneumoniae. PLoS Pathog. 2016, 12, e1005762. [Google Scholar] [CrossRef]
- Oliver, M.B.; Basu Roy, A.; Kumar, R.; Lefkowitz, E.J.; Swords, W.E. Streptococcus pneumoniae TIGR4 Phase-Locked Opacity Variants Differ in Virulence Phenotypes. mSphere 2017, 2, e00386-17. [Google Scholar] [CrossRef] [PubMed]
- De Ste Croix, M.; Vacca, I.; Kwun, M.J.; Ralph, J.D.; Bentley, S.D.; Haigh, R.; Croucher, N.J.; Oggioni, M.R. Phase-variable methylation and epigenetic regulation by type I restriction-modification systems. FEMS Microbiol. Rev. 2017, 41, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, J.; Zhang, J.R.; Zhang, X. Qdnamod: A statistical model-based tool to reveal intercellular heterogeneity of DNA modification from SMRT sequencing data. Nucleic Acids Res. 2014, 42, 13488–13499. [Google Scholar] [CrossRef] [PubMed]
- De Ste Croix, M.; Chen, Y.; Vacca, I.; Manso, A.S.; Johnston, C.; Polard, P.; Kwun, M.J.; Bentley, S.D.; Croucher, N.J.; Bayliss, C.D.; et al. Recombination of the phase variable spnIII locus is independent of all known pneumococcal site-specific recombinases. J. Bacteriol. 2019, 201, e00233-19. [Google Scholar] [CrossRef] [PubMed]
- Kwun, M.J.; Oggioni, M.R.; De Ste Croix, M.; Bentley, S.D.; Croucher, N.J. Excision-reintegration at a pneumococcal phase-variable restriction-modification locus drives within- and between-strain epigenetic differentiation and inhibits gene acquisition. Nucleic Acids Res. 2018, 46, 11438–11453. [Google Scholar] [CrossRef]
- Li, J.W.; Li, J.; Wang, J.; Li, C.; Zhang, J.R. Molecular mechanisms of hsdS inversions in the cod locus of Streptococcus pneumoniae. J. Bacteriol. 2019, 201, e00581-18. [Google Scholar] [CrossRef]
- Kono, M.; Zafar, M.A.; Zuniga, M.; Roche, A.M.; Hamaguchi, S.; Weiser, J.N. Single Cell Bottlenecks in the Pathogenesis of Streptococcus pneumoniae. PLoS Pathog. 2016, 12, e1005887. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.A.; Croucher, N.J.; Goldblatt, D.; Nosten, F.; Parkhill, J.; Turner, C.; Turner, P.; Bentley, S.D. Genome-wide identification of lineage and locus specific variation associated with pneumococcal carriage duration. Elife 2017, 6, e26255. [Google Scholar] [CrossRef]
- Furi, L.; Crawford, L.A.; Rangel-Pineros, G.; Manso, A.S.; De Ste Croix, M.; Haigh, R.D.; Kwun, M.J.; Engelsen Fjelland, K.; Gilfillan, G.D.; Bentley, S.D.; et al. Methylation warfare: interaction of pneumococcal bacteriophages with their host. J. Bacteriol. 2019, JB.00370-19. [Google Scholar] [CrossRef]
- Johnston, C.; Martin, B.; Granadel, C.; Polard, P.; Claverys, J.P. Programmed Protection of Foreign DNA from Restriction Allows Pathogenicity Island Exchange during Pneumococcal Transformation. PLoS Pathog. 2013, 9, e1003178. [Google Scholar] [CrossRef]
- Apagyi, K.J.; Fraser, C.; Croucher, N.J. Transformation asymmetry and the evolution of the bacterial accessory genome. Mol. Biol. Evol. 2018, 35, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Mostowy, R.; Wymant, C.; Turner, P.; Bentley, S.D.; Fraser, C. Horizontal DNA Transfer Mechanisms of Bacteria as Weapons of Intragenomic Conflict. PLOS Biol. 2016, 14, e1002394. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, O.E.; Rozen, D.E.; May, R.M.; Levin, B.R. Oscillations in continuous culture populations of Streptococcus pneumoniae: Population dynamics and the evolution of clonal suicide. Proc. R. Soc. B Biol. Sci. 2009, 276, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Engelmoer, D.J.P.; Donaldson, I.; Rozen, D.E. Conservative Sex and the Benefits of Transformation in Streptococcus pneumoniae. PLoS Pathog. 2013, 9, e1003758. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Harris, S.R.; Fraser, C.; Quail, M.A.; Burton, J.; van der Linden, M.; McGee, L.; von Gottberg, A.; Song, J.H.; Ko, K.S.; et al. Rapid pneumococcal evolution in response to clinical interventions. Science 2011, 331, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Hanage, W.P.; Harris, S.R.; McGee, L.; van der Linden, M.; de Lencastre, H.; Sá-Leão, R.; Song, J.-H.; Ko, K.S.; Beall, B.; et al. Variable recombination dynamics during the emergence, transmission and “disarming” of a multidrug-resistant pneumococcal clone. BMC Biol. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Chewapreecha, C.; Hanage, W.P.; Harris, S.R.; McGee, L.; van der Linden, M.; Song, J.-H.H.; Ko, K.S.; de Lencastre, H.; Turner, C.; et al. Evidence for soft selective sweeps in the evolution of pneumococcal multidrug resistance and vaccine escape. Genome Biol. Evol. 2014, 6, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Page, A.J.; Connor, T.R.; Delaney, A.J.; Keane, J.A.; Bentley, S.D.; Parkhill, J.; Harris, S.R. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015, 43, e15. [Google Scholar] [CrossRef] [PubMed]
- Corander, J.; Fraser, C.; Gutmann, M.U.; Arnold, B.; Hanage, W.P.; Bentley, S.D.; Lipsitch, M.; Croucher, N.J. Frequency-dependent selection in vaccine-associated pneumococcal population dynamics. Nat. Ecol. Evol. 2017, 1, 1950–1960. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Pfeifer, B.; Wittelsbuerger, U.; Ramos-Onsins, S.E.; Lercher, M.J. PopGenome: An Efficient Swiss Army Knife for Population Genomic Analyses in R. Mol. Biol. Evol. 2014, 31, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.M.; Levin, B.R. The population biology of bacterial viruses: Why be temperate. Theor. Popul. Biol. 1984, 26, 93–117. [Google Scholar] [CrossRef]
- Bull, J.J.; Molineux, I.J.; Rice, W.R. Selection of Benevolence in a Host-Parasite System. Evolution (N. Y.) 1991, 45, 875–882. [Google Scholar]
- Kot, W.; Sabri, M.; Gingras, H.; Ouellette, M.; Tremblay, D.M.; Moineau, S. Complete Genome Sequence of Streptococcus pneumoniae Virulent Phage MS1. Genome Announc. 2017, 5, e00333-17. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Croucher, N.J.; Hiller, N.L.; Hu, F.Z.; Ehrlich, G.D.; Bentley, S.D.; García, E.; Mitchell, T.J. Comparative genomic analysis of ten Streptococcus pneumoniae temperate bacteriophages. J. Bacteriol. 2009, 191, 4854–4862. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, A.B.; Harrold, C.L.; Rezaei Javan, R.; Van Tonder, A.J.; McDonnell, A.J.; Edwards, B.A. Pneumococcal prophages are diverse, but not without structure or history. Sci. Rep. 2017, 7, 42976. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Harris, S.R.; Barquist, L.; Parkhill, J.; Bentley, S.D. A high-resolution view of genome-wide pneumococcal transformation. PLoS Pathog. 2012, 8, e1002745. [Google Scholar] [CrossRef]
- Mell, J.C.; Lee, J.Y.; Firme, M.; Sinha, S.; Redfield, R.J. Extensive Cotransformation of Natural Variation into Chromosomes of Naturally Competent Haemophilus influenzae. Genes|Genomes|Genetics 2014, 4, 717–731. [Google Scholar] [CrossRef]
- Chewapreecha, C.; Harris, S.R.; Croucher, N.J.; Turner, C.; Marttinen, P.; Cheng, L.; Pessia, A.; Aanensen, D.M.; Mather, A.E.; Page, A.J.; et al. Dense genomic sampling identifies highways of pneumococcal recombination. Nat. Genet. 2014, 46, 305–309. [Google Scholar] [CrossRef]
- Croucher, N.J.; Finkelstein, J.A.; Pelton, S.I.; Mitchell, P.K.; Lee, G.M.; Parkhill, J.; Bentley, S.D.; Hanage, W.P.; Lipsitch, M. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat. Genet. 2013, 45, 656–663. [Google Scholar] [CrossRef]
- Yahara, K.; Didelot, X.; Jolley, K.A.; Kobayashi, I.; Maiden, M.C.J.; Sheppard, S.K.; Falush, D. The landscape of realized homologous recombination in pathogenic bacteria. Mol. Biol. Evol. 2016, 33, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Harris, S.R.; Grad, Y.H.; Hanage, W.P. Bacterial genomes in epidemiology- present and future. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 0120202. [Google Scholar] [CrossRef] [PubMed]
- Cowley, L.A.; Petersen, F.C.; Junges, R.; Jimson, D.; Jimenez, M.; Morrison, D.A.; Hanage, W.P. Evolution via recombination: Cell-to-cell contact facilitates larger recombination events in Streptococcus pneumoniae. PLoS Genet. 2018, 14, e1007410. [Google Scholar] [CrossRef] [PubMed]
- Power, P.M.; Bentley, S.D.; Parkhill, J.; Moxon, E.R.; Hood, D.W. Investigations into genome diversity of Haemophilus influenzae using whole genome sequencing of clinical isolates and laboratory transformants. BMC Microbiol. 2012, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Didelot, X.; Wilson, D.J. ClonalFrameML: Efficient Inference of Recombination in Whole Bacterial Genomes. PLoS Comput. Biol. 2015, 11, e1004041. [Google Scholar] [CrossRef] [PubMed]
- Touchon, M.; Bobay, L.M.; Rocha, E.P.C. The chromosomal accommodation and domestication of mobile genetic elements. Curr. Opin. Microbiol. 2014, 22, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Walker, D.; Romero, P.; Lennard, N.; Paterson, G.K.; Bason, N.C.; Mitchell, A.M.; Quail, M.A.; Andrew, P.W.; Parkhill, J.; et al. Role of conjugative elements in the evolution of the multidrug-resistant pandemic clone Streptococcus pneumoniaeSpain23F ST81. J Bacteriol 2009, 191, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Kapoor, M. Effect of intrastrain variation in the amount of capsular polysaccharide on genetic transformation of Streptococcus pneumoniae: Implications for virulence studies of encapsulated strains. Infect. Immun. 1999, 67, 3690–3692. [Google Scholar]
- Romero, P.; Llull, D.; García, E.; Mitchell, T.J.; López, R.; Moscoso, M. Isolation and characterization of a new plasmid pSpnP1 from a multidrug-resistant clone of Streptococcus pneumoniae. Plasmid 2007, 58, 51–60. [Google Scholar] [CrossRef]
- Oliveira, P.H.; Touchon, M.; Rocha, E.P.C. The interplay of restriction-modification systems with mobile genetic elements and their prokaryotic hosts. Nucleic Acids Res. 2014, 42, 10618–10631. [Google Scholar] [CrossRef]
- Fukuyo, M.; Sasaki, A.; Kobayashi, I. Success of a suicidal defense strategy against infection in a structured habitat. Sci. Rep. 2012, 2, 238. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.S.; Hartman, H.; Levin, S.A. Coevolutionary arms races between bacteria and bacteriophage. Proc. Natl. Acad. Sci. USA 2005, 102, 9535–9540. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Campo, J.J.; Le, T.Q.; Liang, X.; Bentley, S.D.; Hanage, W.P.; Lipsitch, M. Diverse evolutionary patterns of pneumococcal antigens identified by pangenome-wide immunological screening. Proc. Natl. Acad. Sci. USA 2017, 114, E357–E366. [Google Scholar] [CrossRef] [PubMed]
- Wielgoss, S.; Bergmiller, T.; Bischofberger, A.M.; Hall, A.R. Adaptation to parasites and costs of parasite resistance in mutator and nonmutator bacteria. Mol. Biol. Evol. 2016, 33, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Tock, M.R.; Dryden, D.T.F. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 2005, 8, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.J.; Gutmann, M.U.; Grad, Y.H.; Sheppard, S.K.; Corander, J.; Lipsitch, M.; Hanage, W.P. Weak epistasis may drive adaptation in recombining bacteria. Genetics 2018, 208, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Masala, G.L.; Lipsitch, M.; Bottomley, C.; Flasche, S. Exploring the role of competition induced by non-vaccine serotypes for herd protection following pneumococcal vaccination. J. R. Soc. Interface 2017, 14, 20170620. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, R.; Croucher, N.J.; Hanage, W.P.; Harris, S.R.; Bentley, S.; Fraser, C. Heterogeneity in the Frequency and Characteristics of Homologous Recombination in Pneumococcal Evolution. PLoS Genet. 2014, 10, e1004300. [Google Scholar] [CrossRef]
- Redfield, R.J. Genes for breakfast: The have-your-cake and-eat-it-too of bacterial transformation. J. Hered. 1993, 84, 400–404. [Google Scholar] [CrossRef]
- Wisniewski-Dyé, F.; Vial, L. Phase and antigenic variation mediated by genome modifications. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2008, 94, 493–515. [Google Scholar] [CrossRef]
- Slager, J.; Aprianto, R.; Veening, J.W. Deep genome annotation of the opportunistic human pathogen Streptococcus pneumoniae D39. Nucleic Acids Res. 2018, 46, 9971–9989. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.J.; Le, T.Q.; Pablo, J.V.; Hung, C.; Teng, A.A.; Tettelin, H.; Tate, A.; Hanage, W.P.; Alderson, M.R.; Liang, X.; et al. Panproteome-wide analysis of antibody responses to whole cell pneumococcal vaccination. Elife 2018, 7, e37015. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J. Model of horizontal DNA transfer within a simple bacterial community. Available online: https://github.com/nickjcroucher/mgeTransformation (accessed on 20 August 2019).
| Parameter Name | Parameter Description | Parameter Value |
|---|---|---|
| γ | Cell growth rate | 0.2 t−1 |
| κ | Environment carrying capacity | 106 or 104 |
| ω | Washout rate | 0.6 t−1 |
| τ | Transformation rate | 0 unless specified |
| φ | Transformation asymmetry | 10−3 |
| β | Rate of MGE horizontal transmission | 1.25 × 10−4 t−1 (ML); 10−6 t−1 (MH); 10−3 t−1 (MV), unless specified |
| b | Mean MGE burst size | 10 (ML); 10 (MH); 5 (MV) |
| f | Frequency of MGE activation | Always specified (ML); 5 × 10−2 (MH); 5 × 10−3 (MV) |
| cM | Cost of carrying MGE | 0.5 (ML); 7.5 × 10−2 (MH); 2.5 × 10−3 (MV) |
| a | Host cell death on MGE activation | Yes (ML); Yes (MH); No (MV) |
| mi | MGE invasion rate | 10−7 t−1 (ML); 5 × 10−7 t−1 (MV, MH) |
| pv | Phase variation rate | Always specified |
| i | Interstrain sequence exchange rate | Always specified |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwun, M.J.; Oggioni, M.R.; Bentley, S.D.; Fraser, C.; Croucher, N.J. Synergistic Activity of Mobile Genetic Element Defences in Streptococcus pneumoniae. Genes 2019, 10, 707. https://doi.org/10.3390/genes10090707
Kwun MJ, Oggioni MR, Bentley SD, Fraser C, Croucher NJ. Synergistic Activity of Mobile Genetic Element Defences in Streptococcus pneumoniae. Genes. 2019; 10(9):707. https://doi.org/10.3390/genes10090707
Chicago/Turabian StyleKwun, Min Jung, Marco R. Oggioni, Stephen D. Bentley, Christophe Fraser, and Nicholas J. Croucher. 2019. "Synergistic Activity of Mobile Genetic Element Defences in Streptococcus pneumoniae" Genes 10, no. 9: 707. https://doi.org/10.3390/genes10090707
APA StyleKwun, M. J., Oggioni, M. R., Bentley, S. D., Fraser, C., & Croucher, N. J. (2019). Synergistic Activity of Mobile Genetic Element Defences in Streptococcus pneumoniae. Genes, 10(9), 707. https://doi.org/10.3390/genes10090707





