The Draft Genome of the Endangered Sichuan Partridge (Arborophila rufipectus) with Evolutionary Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequencing, Assembly, and Genome Data
2.2. Characterization of Repeat Content
2.3. Analyses of Olfactory Receptor (ORs) Genes
2.4. Gene Family and Positive Selection
2.5. Demography Reconstruction
3. Results
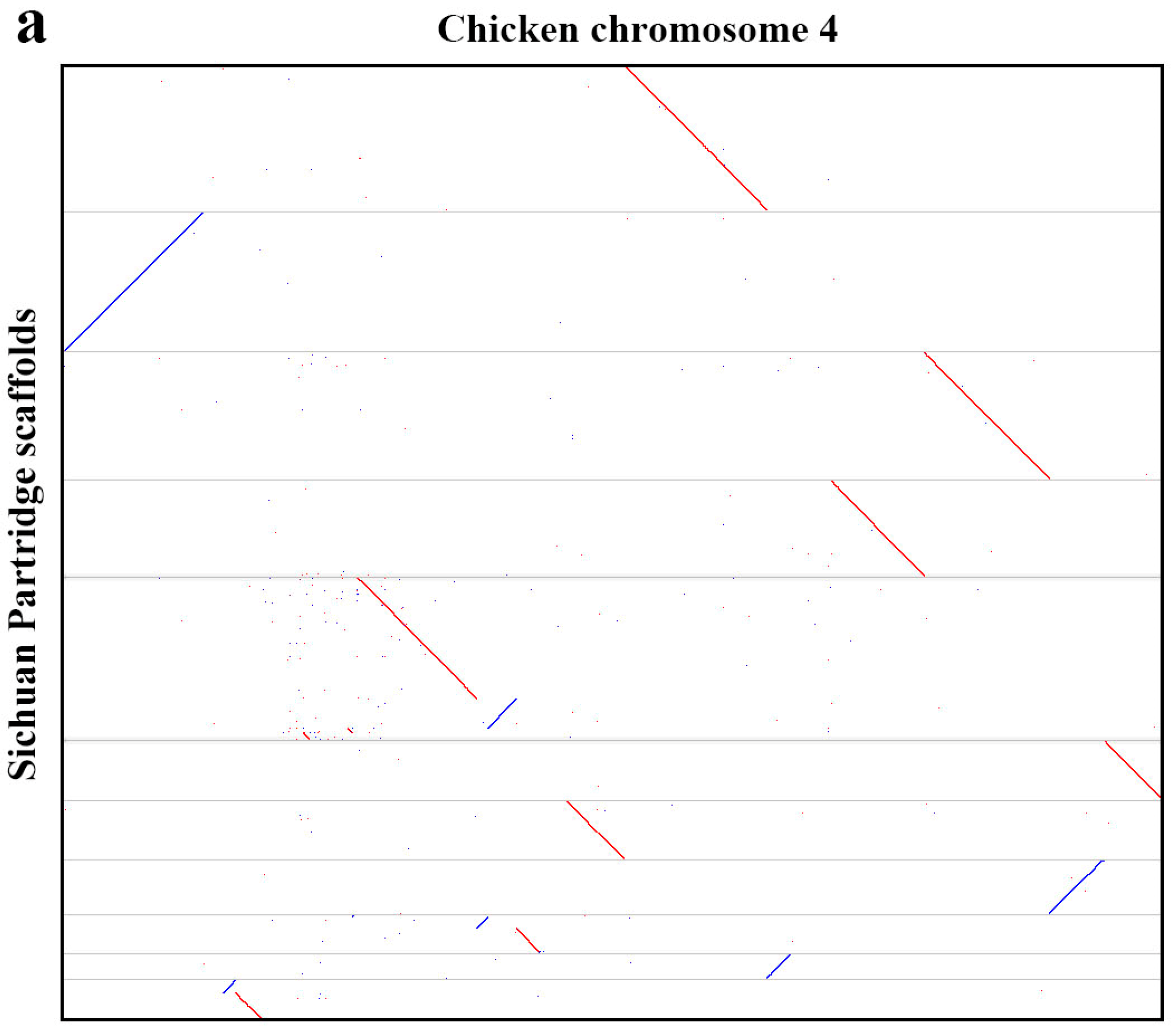
3.1. Genome Sequencing, Assembly, and Quality Assessment
3.2. Genome Characterization
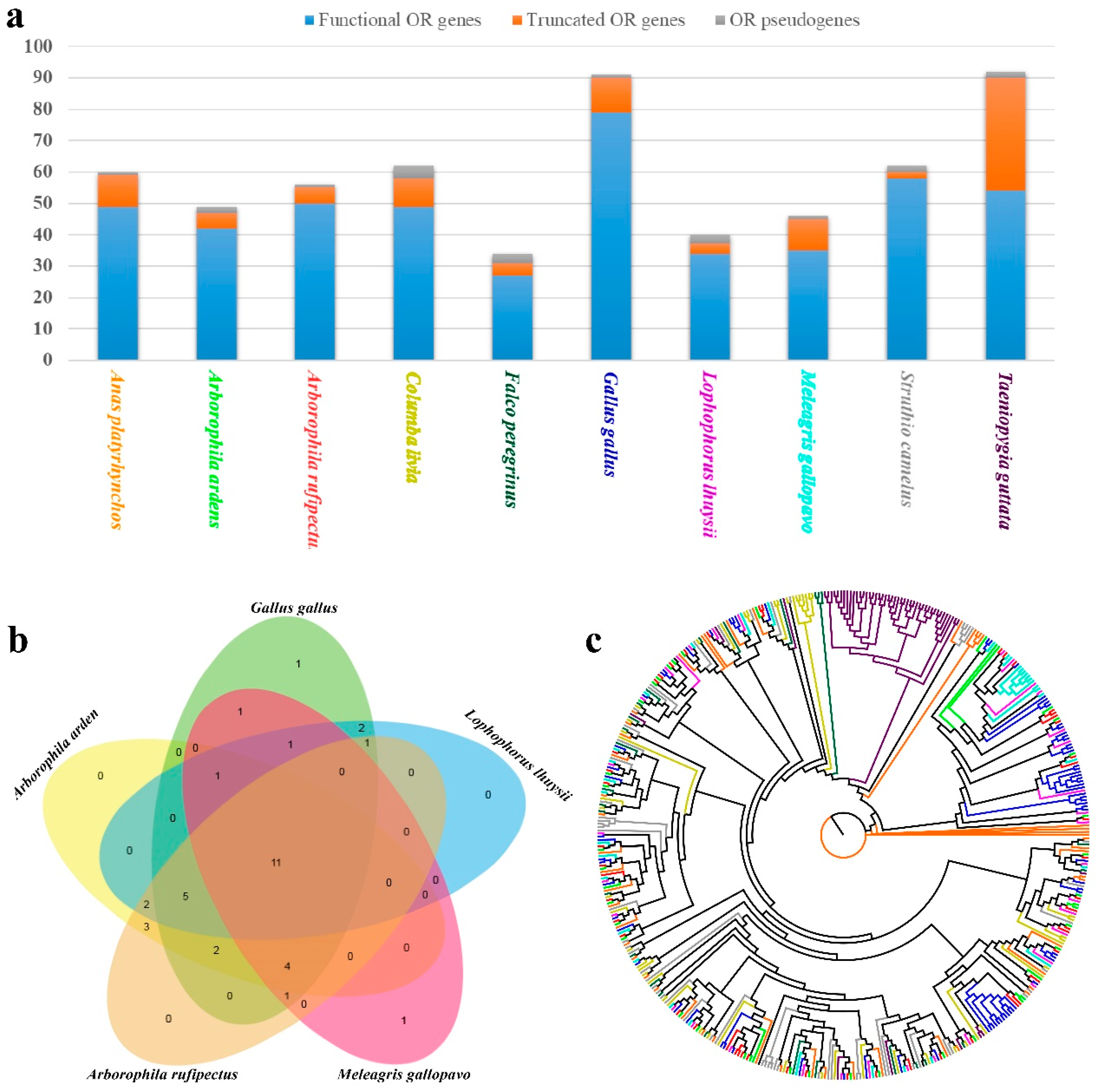
3.3. Olfactory Receptors (ORs): Composition, Classification, and Conserved Motifs
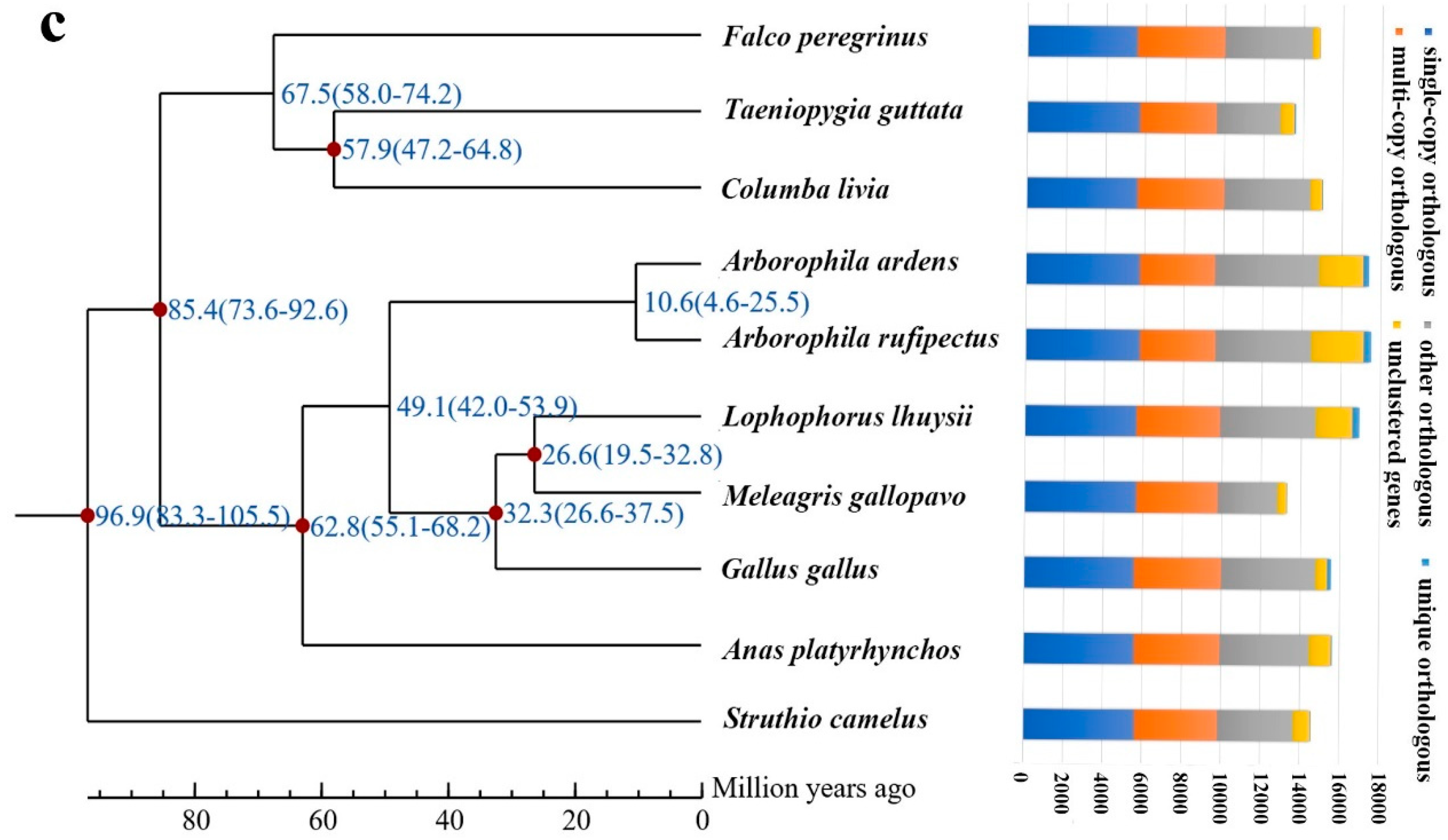
3.4. Bird Phylogeny, Divergence and Evolution of Gene Families
3.5. Positive Selection in the Sichuan Partridge
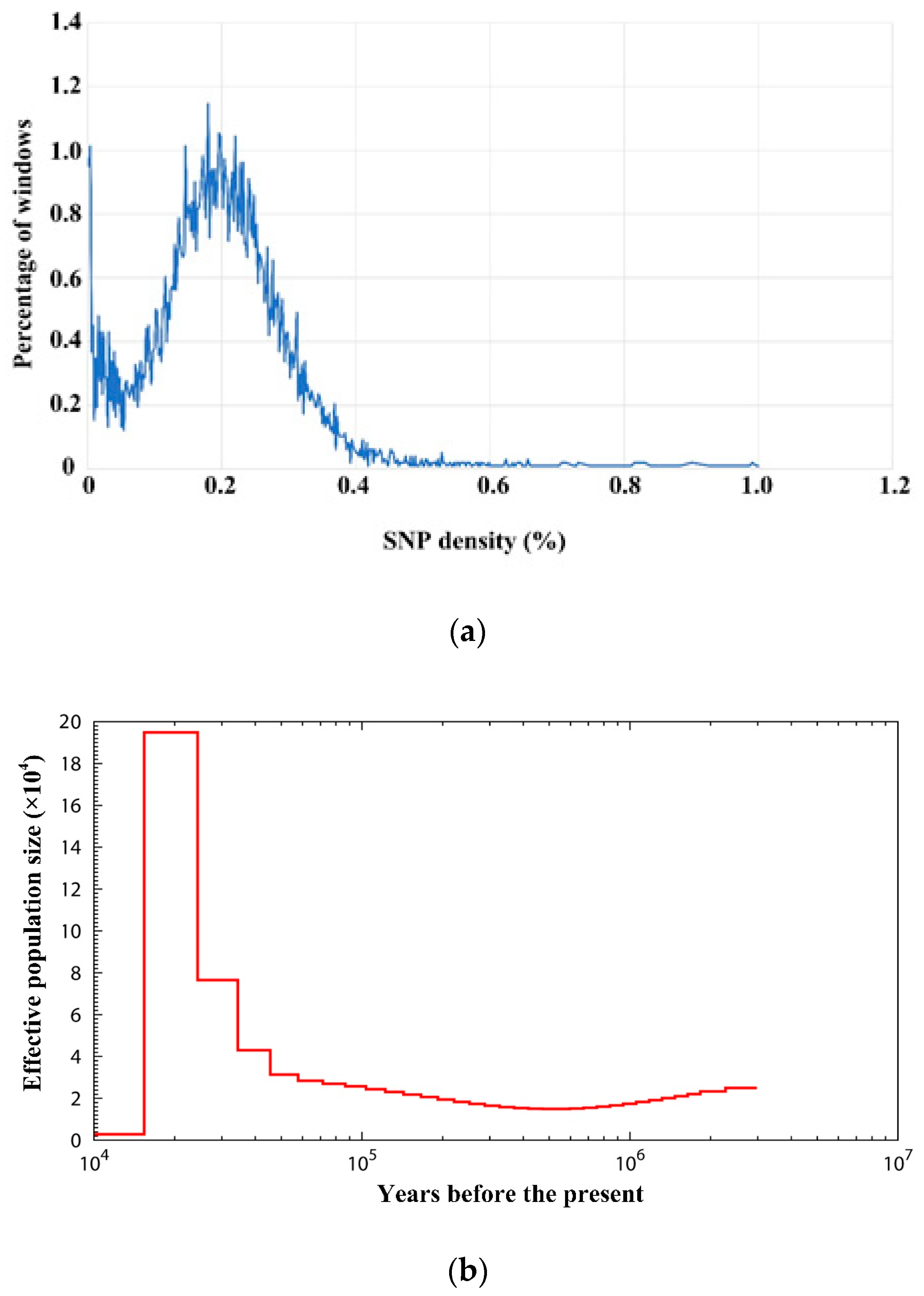
3.6. Demography Reconstruction
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, L.; Dai, B.; Zeng, B.; Zhang, X.; Chen, B.; Yue, B.; Li, J. The complete mitochondrial genome of the Sichuan Hill Partridge (Arborophila rufipectus) and a phylogenetic analysis with related species. Gene 2009, 435, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Dowell, S.D.; Martins, R.P.; Williams, S.R. Conservation status of the Sichuan Hill Partridge. Bird Conserv. Int. 1998, 8, 349–359. [Google Scholar]
- Li, C.; Hu, J.; Yu, Z. Distribution and habitat selection of the Sichuan Hill-Partridge. Chin. J. Zool. 2003, 38, 46–51. [Google Scholar]
- Liao, W.B.; Li, C.; Hu, J.C.; Lu, X. Habitat utilization of Sichuan Hill Partridge (Arborophila rufipectus) in the non-breeding period in Laojunshan nature reserve. Zool. Res. 2007, 28, 172–178. [Google Scholar]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Boetzer, M.; Henkel, C.V.; Jansen, H.J.; Butler, D.; Pirovano, W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 2011, 7, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.; Bradnam, K.; Korf, I. CEGMA: A pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics 2007, 23, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Zhou, C.; Zheng, S.; Jiang, X.; Liang, W.; Price, M.; Fan, Z.; Meng, Y.; Yue, B. First complete genome sequence in Arborophila and comparative genomics reveals the evolutionary adaptation of Hainan Partridge (Arborophila ardens). Avian Res. 2018, 9, 45. [Google Scholar] [CrossRef]
- Cui, K.; Li, W.; James, J.G.; Peng, C.; Jin, J.; Yan, C.; Fan, Z.; Du, L.; Price, M.; Wu, Y.; et al. The first draft genome of Lophophorus: A step forward for Phasianidae genomic diversity and conservation. Genomics 2018, 29. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, C.; Liu, Q.; Zhang, X.; Yue, B. Krait: An ultrafast tool for genome-wide survey of microsatellites and primer design. Bioinformatics 2017, 34, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, G.; Yu, H.; Geng, Y.; Wu, W.; Tu, H.; Price, M.; Fan, Z.; Meng, Y.; Yue, B. Genome-wide analysis reveals the genomic features of the turkey vulture (Cathartes aura) as a scavenger. Mol. Genet. Genom. 2019, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Jin, J.; Peng, C.; Wen, Q.; Wang, G.; Wei, W.; Jiang, X.; Price, M.; Cui, K.; Meng, Y.; et al. Comparative genomics sheds light on the predatory lifestyle of accipitrids and owls. Sci. Rep. 2019, 9, 2249. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Glusman, G.; Bahar, A.; Sharon, D.; Pilpel, Y.; White, J.; Lancet, D. The olfactory receptor gene superfamily: Data mining, classification, and nomenclature. Mamm. Genome 2000, 11, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology, Stanford, CA, USA, 14–17 August 1994. [Google Scholar]
- Li, L.; Stoeckert, C.J.; Roos, D.S. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003, 13, 2178. [Google Scholar] [CrossRef] [PubMed]
- Nick, G.; Ari, L. WebPRANK: A phylogeny-aware multiple sequence aligner with interactive alignment browser. BMC Bioinform. 2010, 11, 1–7. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qian, X.; Lang, Y.; Luo, Y.; Xu, J.; Pan, S.; Hui, Y.; Gou, C.; Cai, Y.; Hao, M.; et al. Genome sequence of ground tit Pseudopodoces humilis and its adaptation to high altitude. Genome Biol. 2013, 14, R29. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, Y.; Wang, Z.; Li, X.; Chen, W.; Tao, Z.; Shen, J.; Tian, Y.; Wang, D.; Li, G.; et al. The goose genome sequence leads to insights into the evolution of waterfowl and susceptibility to fatty liver. Genome Biol. 2015, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mao, X.; Cai, T.; Luo, J.; Wei, L. KOBAS server: A web-based platform for automated annotation and pathway identification. Nucleic Acids Res. 2006, 34, W720–W724. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Inference of human population history from individual whole-genome sequences. Nature 2011, 475, 493. [Google Scholar] [CrossRef]
- Seabury, C.M.; Dowd, S.E.; Seabury, P.M.; Raudsepp, T.; Brightsmith, D.J.; Liboriussen, P.; Halley, Y.; Fisher, C.A.; Owens, E.; Viswanathan, G.; et al. A multi-platform draft de novo genome assembly and comparative analysis for the scarlet macaw (Ara macao). PLoS ONE 2013, 8, e62415. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Long, J.A.; Zimin, A.V.; Aslam, L.; Beal, K.; Blomberg, L.A.; Bouffard, P.; Burt, D.W.; Crasta, O.; Crooijmans, R.P.; et al. Multi-platform next-generation sequencing of the domestic turkey (Meleagris gallopavo): Genome assembly and analysis. PLoS Biol. 2010, 8, e1000475. [Google Scholar] [CrossRef]
- Warren, W.C.; Clayton, D.F.; Ellegren, H.; Arnold, A.P.; Hillier, L.W.; Künstner, A.; Searle, S.; White, S.; Vilella, A.J.; Fairley, S.; et al. The genome of a songbird. Nature 2010, 464, 757. [Google Scholar] [CrossRef]
- Halley, Y.A.; Dowd, S.E.; Decker, J.E.; Seabury, P.M.; Bhattarai, E.; Johnson, C.D.; Rollins, D.; Tizard, I.R.; Brightsmith, D.J.; Peterson, M.J.; et al. A draft de novo genome assembly for the northern bobwhite (Colinus virginianus) reveals evidence for a rapid decline in effective population size beginning in the Late Pleistocene. PLoS ONE 2014, 9, e90240. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Lee, K.; Choi, H.; Choi, M.K.; Le, M.T.; Song, N.; Kim, J.H.; Seo, H.G.; Oh, J.W.; Lee, K.; et al. The complete swine olfactory subgenome: Expansion of the olfactory gene repertoire in the pig genome. BMC Genom. 2012, 13, 584. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Nguyen, D.T.; Choi, M.; Cha, S.Y.; Kim, J.H.; Dadi, H.; Seo, H.G.; Seo, K.; Chun, T.; Park, C. Analysis of cattle olfactory subgenome: The first detail study on the characteristics of the complete olfactory receptor repertoire of a ruminant. BMC Genom. 2013, 14, 596. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Pan, S.; Wang, J.; Dixon, A.; He, J.; Muller, M.G.; Ni, P.; Hu, L.; Liu, Y.; Hou, H.; et al. Peregrine and saker falcon genome sequences provide insights into evolution of a predatory lifestyle. Nat. Genet. 2013, 45, 563. [Google Scholar] [CrossRef] [PubMed]
- Le Duc, D.; Renaud, G.; Krishnan, A.; Almén, M.S.; Huynen, L.; Prohaska, S.J.; Ongyerth, M.; Bitarello, B.D.; Schiöth, H.B.; Hofreiter, M.; et al. Kiwi genome provides insights into evolution of a nocturnal lifestyle. Genome Biol. 2015, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Bu, P.; Jian, Z.; Koshy, J.; Shen, Y.; Yue, B.; Fan, Z. The olfactory subgenome and specific odor recognition in forest musk deer. Anim. Genet. 2019, 50, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.Y.; Liang, X.F.; He, S. Genome-wide identification and characterization of olfactory receptor genes in Chinese perch, Siniperca chuatsi. Genes 2019, 10, 178. [Google Scholar] [CrossRef]
- Nadachowska-Brzyska, K.; Li, C.; Smeds, L.; Zhang, G.; Ellegren, H. Temporal dynamics of avian populations during Pleistocene revealed by whole-genome sequences. Curr. Biol. 2015, 18, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Likelihood and Bayes estimation of ancestral population sizes in hominoids using data from multiple loci. Genetics 2002, 162, 1811–1823. [Google Scholar]
- Jensen, P. Behaviour of pigs. In The Ethology of Domestic Animals; Jensen, P., Ed.; CABI Publishing: Wallingford, UK, 2002. [Google Scholar]
- Bouissou, M.F.; Boissy, A.; Le Neindre, P.; Veissier, I. The social behaviour of cattle. In Social Behaviour in Farm Animals; Leeling, L.J., Gonyou, H.W., Eds.; CABI Publishing: Wallingford, UK, 2001. [Google Scholar]
- Crowe, T.M. Phylogenetics, biogeography and classification of, and character evolution in, gamebirds (Aves: Galliformes): Effects of character exclusion, data partitioning and missing data. Cladistics 2006, 22, 495–532. [Google Scholar] [CrossRef]
- Chen, D.; Braun, E.L.; Forthman, M.; Kimball, R.T.; Zhang, Z. A simple strategy for recovering ultraconserved elements, exons, and introns from low coverage shotgun sequencing of museum specimens: Placement of the partridge genus Tropicoperdix within the galliformes. Mol. Phylogenet. Evol. 2018, 129, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Hosner, P.A.; Faircloth, B.C.; Glenn, T.C.; Braun, E.L.; Kimball, R.T. Avoiding missing data biases in phylogenomic inference: An empirical study in the landfowl (Aves: Galliformes). Mol. Biol. Evol. 2016, 33, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Hosner, P.A.; Tobias, J.A.; Braun, E.L.; Kimball, R.T. How do seemingly non-vagile clades accomplish trans-marine dispersal? Trait and dispersal evolution in the landfowl (Aves: Galliformes). Proc. R. Soc. B Biol. Sci. 2017, 284, 1854. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Dai, B.; Wen, L.; Chen, B.; Dowell, S.; Zhang, Z. Unusual incubation behavior and embryonic tolerance of hypothermia in the Sichuan partridge (Arborophila rufipectus). J. Ornithol. 2017, 158, 707–715. [Google Scholar] [CrossRef]






| Insert Size (bp) | Read Length (bp) | Raw Data | Clean Data | ||||
|---|---|---|---|---|---|---|---|
| Read Number | Total Bases (Gb) | Sequencing Depth (x) | Read Number | Total Bases (Gb) | Sequencing Depth (x) | ||
| 250 | 150 | 485,619,976 | 72.84 | 66.80 | 476,794,840 | 71.52 | 65.62 |
| 500 | 150 | 267,992,818 | 40.20 | 36.88 | 263,165,590 | 39.47 | 36.22 |
| 800 | 150 | 423,275,628 | 63.49 | 58.25 | 414,846,834 | 62.23 | 57.09 |
| 2000 | 150 | 207,573,892 | 31.14 | 28.57 | 167,760,376 | 25.16 | 23.09 |
| 5000 | 150 | 433,978,936 | 65.10 | 59.73 | 408,121,682 | 61.22 | 56.17 |
| 10,000 | 150 | 265,968,600 | 39.90 | 36.60 | 231,043,688 | 34.66 | 31.80 |
| 15,000 | 150 | 96,333,412 | 14.45 | 13.26 | 96,333,412 | 14.45 | 13.26 |
| 20,000 | 150 | 138,791,494 | 20.82 | 19.10 | 98,033,406 | 14.71 | 13.49 |
| Type | A. rufipectus | A. ardens | G. gallus | M. gallopavo | L. lhuysii | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (bp) | Percentage (%) | Length (bp) | Percentage (%) | Length (bp) | Percentage (%) | Length (bp) | Percentage (%) | Length (bp) | Percentage (%) | |
| SINEs | 531,088 | 0.05 | 532,392 | 0.05 | 611,338 | 0.05 | 565,262 | 0.05 | 557,053 | 0.06 |
| LINEs | 69,173,064 | 6.35 | 67,925,543 | 6.50 | 81,486,693 | 6.62 | 82,214,258 | 7.29 | 70,584,167 | 6.97 |
| LTR elements | 19,342,763 | 1.77 | 13,540,223 | 1.30 | 26,993,586 | 2.19 | 23,893,383 | 2.12 | 14,107,359 | 1.39 |
| DNA elements | 11,459,489 | 1.05 | 11,554,622 | 1.11 | 13,381,436 | 1.09 | 11,677,720 | 1.03 | 11,403,896 | 1.13 |
| Unclassified | 716,293 | 0.07 | 767,625 | 0.07 | 1,772,312 | 0.14 | 1,182,696 | 0.10 | 632,586 | 0.06 |
| Total | 101,222,697 | 9.29 | 94,320,405 | 9.02 | 124,245,365 | 10.10 | 119,533,319 | 10.59 | 97,285,061 | 9.61 |
| Types | A. rufipectus | A. ardens | G. gallus | M. gallopavo | L. lhuysii | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Counts | Length (bp) | Percentage (%) | Counts | Length (bp) | Percentage (%) | Counts | Length (bp) | Percentage (%) | Counts | Length (bp) | Percentage (%) | Counts | Length (bp) | Percentage (%) | |
| Mono- | 263,050 | 4,738,050 | 71.58 | 317,580 | 5,538,023 | 74.56 | 216,419 | 3,462,413 | 52.44 | 140,879 | 1,959,747 | 13.91 | 209,830 | 3,535,260 | 71.75 |
| Di- | 23,602 | 427,292 | 6.42 | 24,677 | 457,440 | 5.79 | 31,485 | 590,712 | 7.63 | 26,237 | 584,466 | 22.28 | 20,669 | 376,944 | 7.07 |
| Tri- | 22,277 | 402,633 | 6.06 | 23,886 | 445,965 | 5.61 | 27,148 | 487,461 | 6.58 | 20,303 | 446,127 | 21.97 | 18,649 | 335,742 | 6.38 |
| Tetra- | 42,231 | 896,888 | 11.49 | 42,527 | 968,896 | 9.98 | 47,327 | 1,114,116 | 11.47 | 37,434 | 814,944 | 21.77 | 29,203 | 611,568 | 9.99 |
| Penta- | 14,233 | 460,685 | 3.87 | 15,035 | 572,380 | 3.53 | 55,616 | 2,187,015 | 13.48 | 13,247 | 418,520 | 31.59 | 11,498 | 500,615 | 3.93 |
| Hexa- | 2120 | 84,402 | 0.58 | 2251 | 92,466 | 0.53 | 34,679 | 1,610,112 | 8.4 | 3390 | 113,010 | 33.34 | 2581 | 105,420 | 0.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Tu, H.; Yu, H.; Zheng, S.; Dai, B.; Price, M.; Wu, Y.; Yang, N.; Yue, B.; Meng, Y. The Draft Genome of the Endangered Sichuan Partridge (Arborophila rufipectus) with Evolutionary Implications. Genes 2019, 10, 677. https://doi.org/10.3390/genes10090677
Zhou C, Tu H, Yu H, Zheng S, Dai B, Price M, Wu Y, Yang N, Yue B, Meng Y. The Draft Genome of the Endangered Sichuan Partridge (Arborophila rufipectus) with Evolutionary Implications. Genes. 2019; 10(9):677. https://doi.org/10.3390/genes10090677
Chicago/Turabian StyleZhou, Chuang, Hongmei Tu, Haoran Yu, Shuai Zheng, Bo Dai, Megan Price, Yongjie Wu, Nan Yang, Bisong Yue, and Yang Meng. 2019. "The Draft Genome of the Endangered Sichuan Partridge (Arborophila rufipectus) with Evolutionary Implications" Genes 10, no. 9: 677. https://doi.org/10.3390/genes10090677
APA StyleZhou, C., Tu, H., Yu, H., Zheng, S., Dai, B., Price, M., Wu, Y., Yang, N., Yue, B., & Meng, Y. (2019). The Draft Genome of the Endangered Sichuan Partridge (Arborophila rufipectus) with Evolutionary Implications. Genes, 10(9), 677. https://doi.org/10.3390/genes10090677







