Common Carp mef2 Genes: Evolution and Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Husbandry, Embryo Manipulation and Tissue Collection
2.2. RNA Extraction, cDNA Synthesis and Gene Cloning
2.3. Phylogenetic Reconstruction, Recombination Detection and Synteny Analysis
2.4. Whole-Mount In Situ Hybridization
3. Results
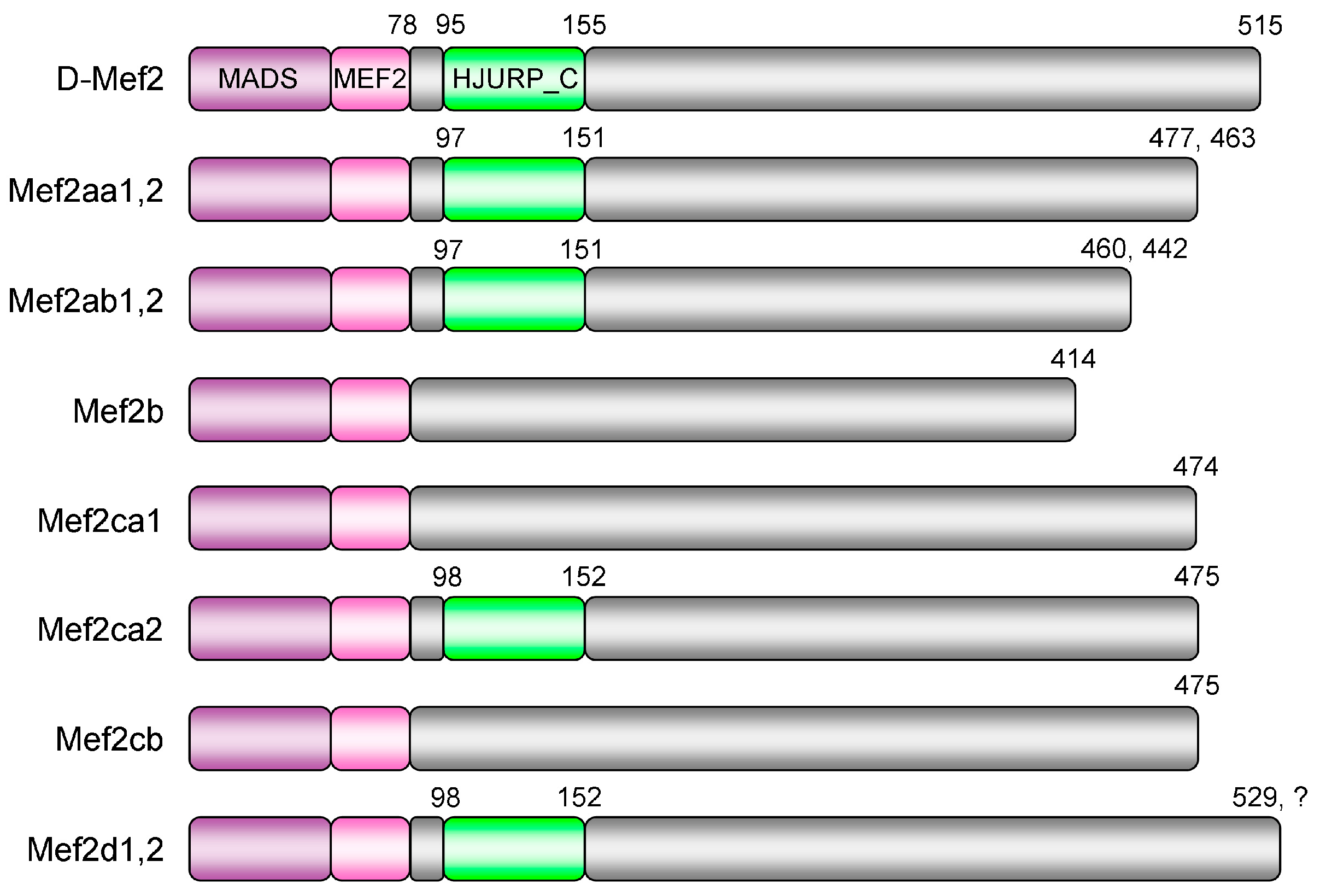
3.1. Multiple mef2 Genes in C. carpio
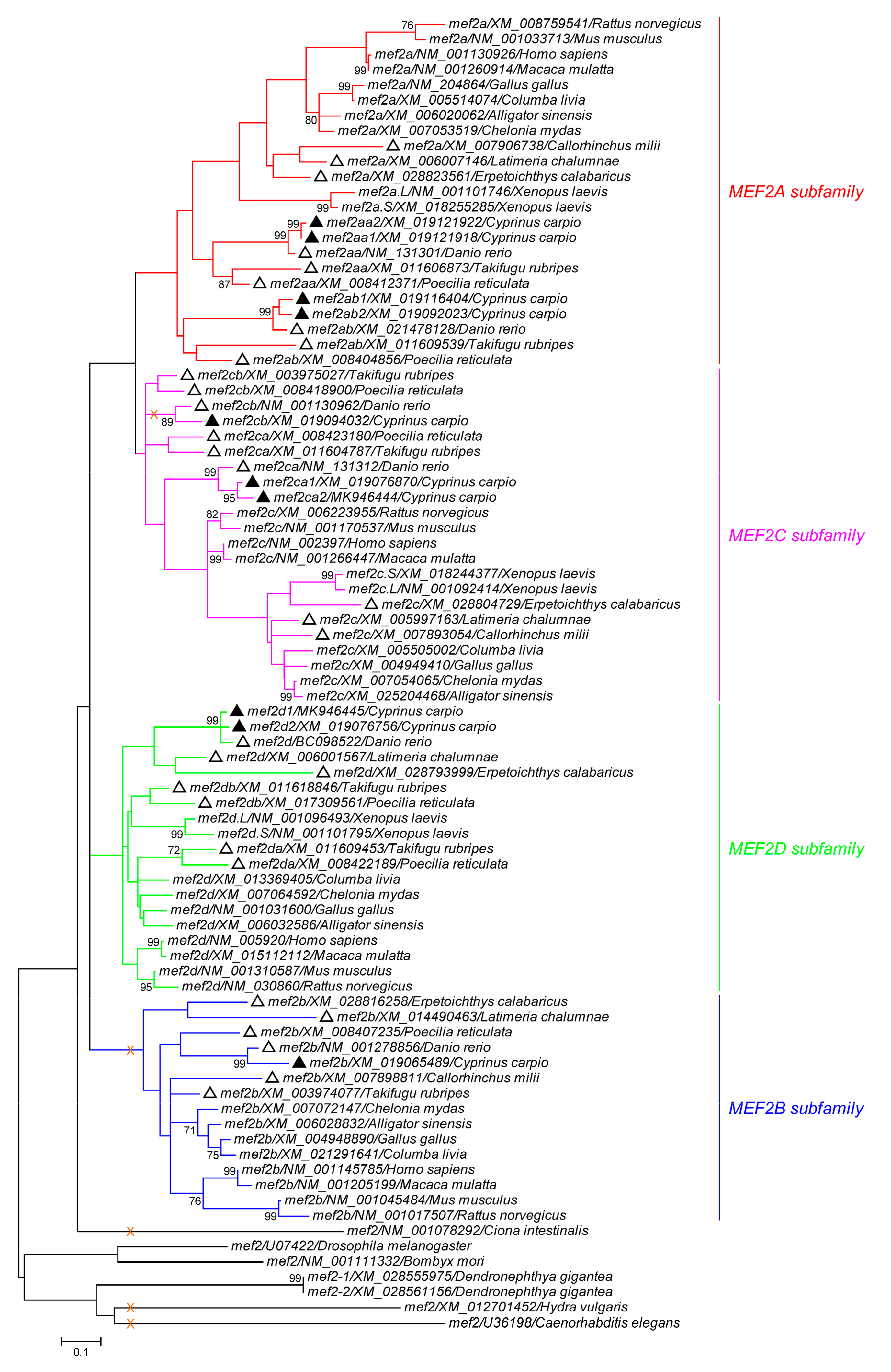
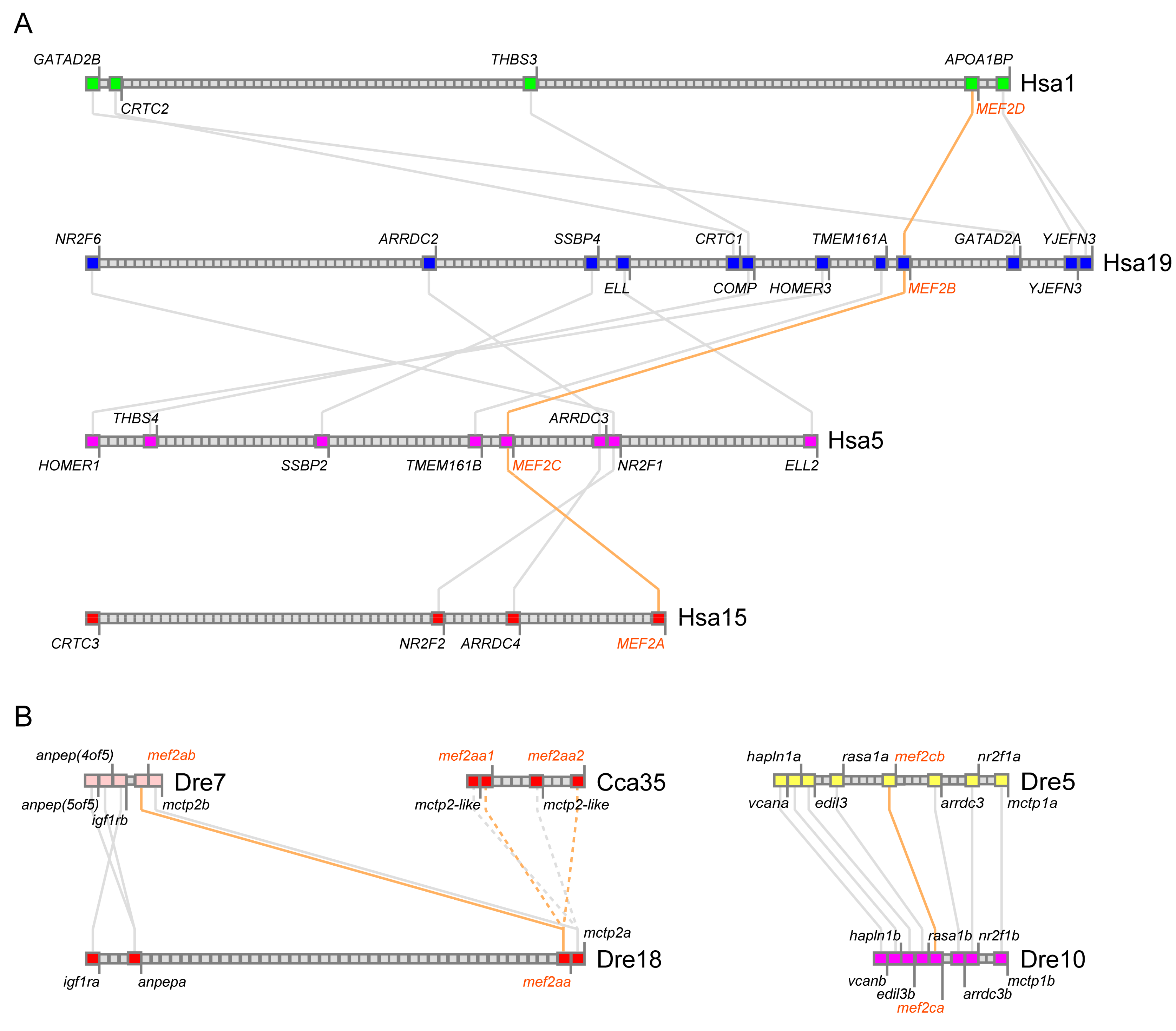
3.2. Phylogeny and Conserved Synteny of mef2 Genes
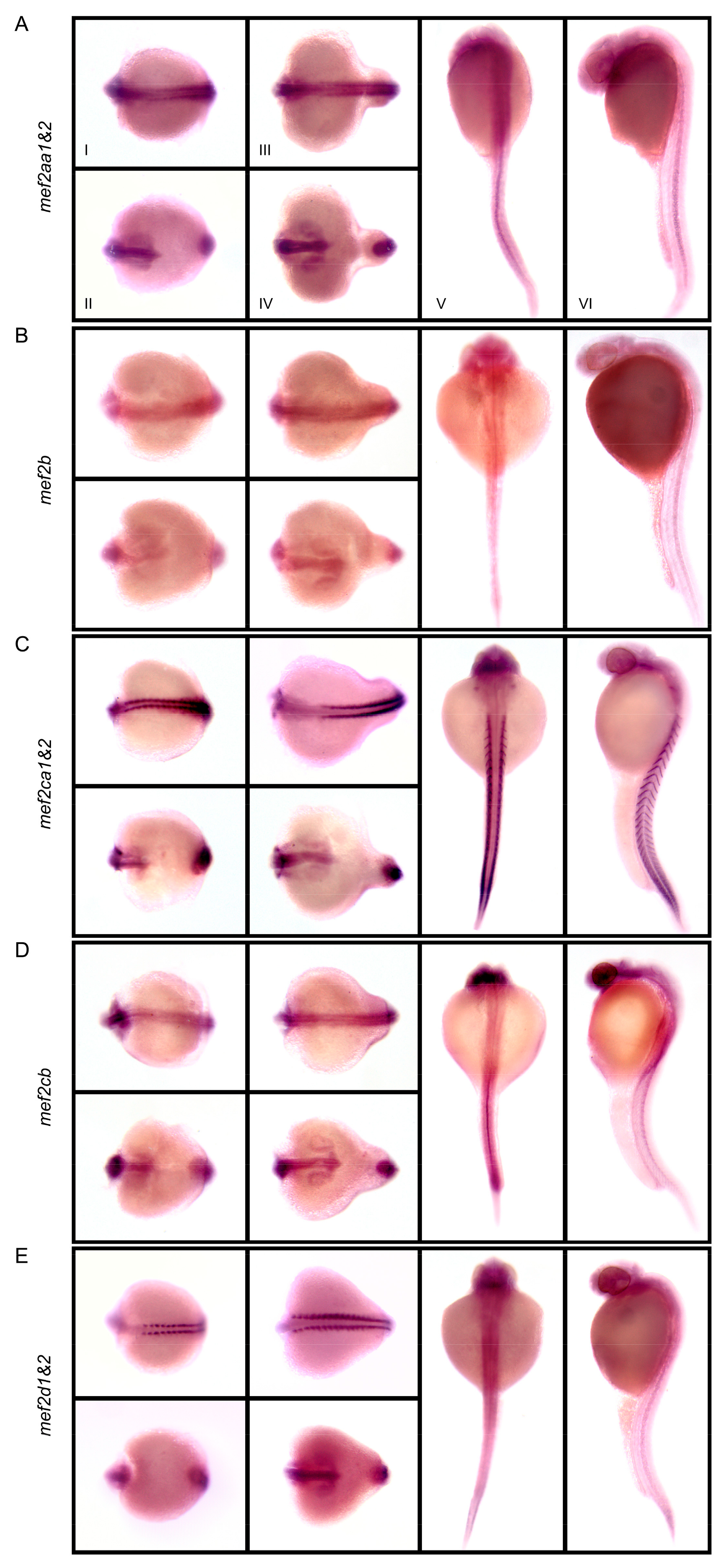
3.3. Embryonic Expression Patterns of C. carpio mef2 Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shore, P.; Sharrocks, A.D. The MADS-box family of transcription factors. Eur. J. Biochem. 1995, 229, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Black, B.L.; Olson, E.N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 1998, 14, 167–196. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Olson, E.N. MEF2: A central regulator of diverse developmental programs. Development 2007, 134, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, E.; Hancock, W.W.; Brancolini, C. MEF2 and the tumorigenic process, hic sunt leones. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.B. The MEF2 family and the brain: From molecules to memory. Cell Tissue Res. 2013, 352, 179–190. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, T.A.; Zhang, C.L.; Olson, E.N. MEF2: A calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 2002, 27, 40–47. [Google Scholar] [CrossRef]
- Naya, F.J.; Olson, E. MEF2: A transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell Biol. 1999, 11, 683–688. [Google Scholar] [CrossRef]
- Wu, W.; de Folter, S.; Shen, X.; Zhang, W.; Tao, S. Vertebrate paralogous MEF2 genes: Origin, conservation, and evolution. PLoS ONE 2011, 6, e17334. [Google Scholar] [CrossRef]
- Taylor, M.V.; Hughes, S.M. Mef2 and the skeletal muscle differentiation program. Semin. Cell Dev. Biol. 2017, 72, 33–44. [Google Scholar] [CrossRef]
- Ticho, B.S.; Stainier, D.Y.; Fishman, M.C.; Breitbart, R.E. Three zebrafish MEF2 genes delineate somitic and cardiac muscle development in wild-type and mutant embryos. Mech. Dev. 1996, 59, 205–218. [Google Scholar] [CrossRef]
- Hinits, Y.; Pan, L.; Walker, C.; Dowd, J.; Moens, C.B.; Hughes, S.M. Zebrafish Mef2ca and Mef2cb are essential for both first and second heart field cardiomyocyte differentiation. Dev. Biol. 2012, 369, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, M.; Badodi, S.; Polacchini, A.; Baruffaldi, F.; Battini, R.; Hughes, S.M.; Hinits, Y.; Molinari, S. Distinct functions of alternatively spliced isoforms encoded by zebrafish mef2ca and mef2cb. Biochim. Biophys. Acta 2014, 1839, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Yogev, O.; Williams, V.C.; Hinits, Y.; Hughes, S.M. eIF4EBP3L acts as a gatekeeper of TORC1 in activity-dependent muscle growth by specifically regulating Mef2ca translational initiation. PLoS Biol. 2013, 11, e1001679. [Google Scholar] [CrossRef] [PubMed]
- Lazic, S.; Scott, I.C. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev. Biol. 2011, 354, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hinits, Y.; Hughes, S.M. Mef2s are required for thick filament formation in nascent muscle fibres. Development 2007, 134, 2511–2519. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.M.; Gerster, T. A zebrafish homolog of the serum response factor gene is highly expressed in differentiating embryonic myocytes. Mech. Dev. 1999, 81, 217–221. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, L.; Dong, Y.; Jiang, Q.; Gui, Y.; Zhong, T.P.; Song, H. Myocyte-specific enhancer factor 2A is essential for zebrafish posterior somite development. Mech. Dev. 2006, 123, 783–791. [Google Scholar] [CrossRef]
- Lv, F.; Zhu, C.; Yan, X.; Wang, X.; Liu, D. Generation of a mef2aa:EGFP transgenic zebrafish line that expresses EGFP in muscle cells. Fish Physiol. Biochem. 2017, 43, 287–294. [Google Scholar] [CrossRef]
- Thisse, B.; Heyer, V.; Lux, A.; Alunni, V.; Degrave, A.; Seiliez, I.; Kirchner, J.; Parkhill, J.P.; Thisse, C. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 2004, 77, 505–519. [Google Scholar]
- Chen, L.; Cheng, B.; Li, L.; Zhan, S.; Wang, L.; Zhong, T.; Chen, Y.; Zhang, H. The molecular characterization and temporal-spatial expression of myocyte enhancer factor 2 genes in the goat and their association with myofiber traits. Gene 2015, 555, 223–230. [Google Scholar] [CrossRef]
- Li, H.; Zhang, F.M.; Guo, H.Y.; Zhu, Y.Y.; Yuan, J.D.; Yang, G.W.; An, L.G. Molecular characterization of hepcidin gene in common carp (Cyprinus carpio L.) and its expression pattern responding to bacterial challenge. Fish. Shellfish Immunol. 2013, 35, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, G.W.; Ma, F.; Li, T.; Yang, H.T.; Rombout, J.H.W.M.; An, L.G. Molecular characterization of a fish-specific toll-like receptor 22 (TLR22) gene from common carp (Cyprinus carpio L.): Evolutionary relationship and induced expression upon immune stimulants. Fish Shellfish Immunol. 2017, 63, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, X.; Wang, X.; Li, J.; Liu, G.; Kuang, Y.; Xu, J.; Zheng, X.; Ren, L.; Wang, G.; et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.J.; Liu, D.Z.; Liu, R.R.; Zhu, Y.Y.; Li, T.; Zhang, F.M.; An, L.G.; Yang, G.W.; Li, H. Non-mammalian Toll-like receptor 18 (Tlr18) recognizes bacterial pathogens in common carp (Cyprinus carpio L.): Indications for a role of participation in the NF-kappa B signaling pathway. Fish Shellfish Immunol. 2018, 72, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, H.; Peng, S.Q.; Zhang, F.M.; An, L.G.; Yang, G.W. Molecular characterization and expression pattern of X box-binding protein-1 (XBP1) in common carp (Cyprinus carpio L.): Indications for a role of XBP1 in antibacterial and antiviral immunity. Fish Shellfish Immunol. 2017, 67, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.W.; Guo, H.Y.; Li, H.; Shan, S.J.; Zhang, X.Q.; Rombout, J.H.W.M.; An, L.G. Molecular characterization of LEAP-2 cDNA in common carp (Cyprinus carpio L.) and the differential expression upon a Vibrio anguillarum stimulus; indications for a significant immune role in skin. Fish Shellfish Immunol. 2014, 37, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.J.; Liu, D.Z.; Wang, L.; Zhu, Y.Y.; Zhang, F.M.; Li, T.; An, L.G.; Yang, G.W. Identification and expression analysis of irak1 gene in common carp (Cyprinus carpio L.): Indications for a role of antibacterial and antiviral immunity. J. Fish Biol. 2015, 87, 241–255. [Google Scholar] [CrossRef]
- Zhang, F.M.; Liu, D.Z.; Wang, L.; Li, T.; Chang, Q.; An, L.G.; Yang, G.W. Characterization of IgM-binding protein: A pIgR-like molecule expressed by intestinal epithelial cells in the common carp (Cyprinus carpio L.). Vet. Immunol. Immunopathol. 2015, 167, 30–35. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Qi, C.C.; Shan, S.J.; Zhang, F.M.; Li, H.; An, L.G.; Yang, G.W. Characterization of common carp (Cyprinus carpio L.) interferon regulatory factor 5 (IRF5) and its expression in response to viral and bacterial challenges. BMC Vet. Res. 2016, 12. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Xing, W.X.; Shan, S.J.; Zhang, S.Q.; Li, Y.Q.; Li, T.; An, L.; Yang, G.W. Characterization and immune response expression of the Rig-I-like receptor mda5 in common carp Cyprinus carpio. J. Fish Biol. 2016, 88, 2188–2202. [Google Scholar] [CrossRef]
- Shan, S.J.; Qi, C.C.; Zhu, Y.Y.; Li, H.; An, L.G.; Yang, G.W. Expression profile of carp IFN correlate with the up-regulation of interferon regulatory factor-1 (IRF-1) in vivo and in vitro: The pivotal molecules in antiviral defense. Fish Shellfish Immunol. 2016, 52, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ding, L.; Li, Y.; Zhang, X.; Liang, Y.; Sun, X.; Teng, C.B. Identification and profiling of microRNAs from skeletal muscle of the common carp. PLoS ONE 2012, 7, e30925. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- He, C.Q.; Liu, Y.X.; Wang, H.M.; Hou, P.L.; He, H.B.; Ding, N.Z. New genetic mechanism, origin and population dynamic of bovine ephemeral fever virus. Vet. Microbiol. 2016, 182, 50–56. [Google Scholar] [CrossRef] [PubMed]
- He, M.; An, T.Z.; Teng, C.B. Evolution of mammalian and avian bornaviruses. Mol. Phylogenet. Evol. 2014, 79, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- He, M.; He, C.Q.; Ding, N.Z. Natural recombination between tobacco and tomato mosaic viruses. Virus Res. 2012, 163, 374–379. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Guan, S.Y.; He, C.Q. Evolution of Rice stripe virus. Mol. Phylogenet. Evol. 2017, 109, 343–350. [Google Scholar] [CrossRef]
- Catchen, J.M.; Conery, J.S.; Postlethwait, J.H. Automated identification of conserved synteny after whole genome duplication. Genome Res. 2009, 19, 1497–1505. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]
- Sun, G.J.; Pan, J.; Liu, K.C.; Wang, S.F.; Wang, X.; Wang, X.M. Molecular cloning and expression analysis of P-selectin glycoprotein ligand-1 from zebrafish (Danio rerio). Fish Physiol. Biochem. 2012, 38, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.Z.; Qi, Q.R.; Gu, X.W.; Zuo, R.J.; Liu, J.; Yang, Z.M. De novo synthesis of sphingolipids is essential for decidualization in mice. Theriogenology 2018, 106, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Dehal, P.; Boore, J.L. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005, 3, e314. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, J.; Cabau, C.; Nguyen, T.; Jouanno, E.; Severac, D.; Braasch, I.; Journot, L.; Pontarotti, P.; Klopp, C.; Postlethwait, J.H.; et al. Gene evolution and gene expression after whole genome duplication in fish: The PhyloFish database. BMC Genom. 2016, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Amores, A.; Force, A.; Yan, Y.L.; Joly, L.; Amemiya, C.; Fritz, A.; Ho, R.K.; Langeland, J.; Prince, V.; Wang, Y.L.; et al. Zebrafish hox clusters and vertebrate genome evolution. Science 1998, 282, 1711–1714. [Google Scholar] [CrossRef] [PubMed]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.L.; Postlethwait, J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [PubMed]
- Teufel, A.I.; Johnson, M.M.; Laurent, J.M.; Kachroo, A.H.; Marcotte, E.M.; Wilke, C.O. The many nuanced evolutionary consequences of duplicated genes. Mol. Biol. Evol. 2019, 36, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Arora, J.; Isambert, H. Identification of ohnolog genes originating from whole genome duplication in early vertebrates, based on synteny comparison across multiple genomes. PLoS Comput. Biol. 2015, 11, e1004394. [Google Scholar] [CrossRef] [PubMed]
- Stemple, D.L. Structure and function of the notochord: An essential organ for chordate development. Development 2005, 132, 2503–2512. [Google Scholar] [CrossRef]
- Kobiyama, A.; Nihei, Y.; Hirayama, Y.; Kikuchi, K.; Suetake, H.; Johnston, I.A.; Watabe, S. Molecular cloning and developmental expression patterns of the MyoD and MEF2 families of muscle transcription factors in the carp. J. Exp. Biol. 1998, 201, 2801–2813. [Google Scholar]
| Species | Gene No. | Gene Name | |
|---|---|---|---|
| Invertebrate | |||
| Saccharomyces cerevisiae | 2 | RLM1, SMP1 | |
| Dendronephthya gigantea | 2 | MEF2-1, MEF2-21 | |
| Vertebrate | |||
| cartilaginous fish | Callorhinchus milii | 3 | mef2a, -b, -c |
| bony fish | Ictaluruspunctatus | 5 | mef2aa, -ab, -b, -c, -d |
| Danio rerio | 6 | mef2aa, -ab, -b, -ca, -cb, -d | |
| Poecilia reticulata | 7 | mef2aa, -ab, -b, -ca, -cb, -da, -db | |
| Haplochromis burtoni | 11 | mef2aa1, -aa2, -ab1, -ab2, -b1,-b2, -ca, -cb, -da1, -da2, -db1 | |
| Salmo salar | 12 | mef2a1, -a2, -b1, -b2, -ca1, -ca2,-cb1, -cb2, -da1, -da2, -db1, -db21 | |
| amphibian | Xenopus laevis | 6 | mef2a.L, -a.S, -c.L, -c.S, -d.L, -d.S |
| mammal | Ornithorhynchus anatinus | 3 | MEF2A, -B, -C |
| Gene 1 | ID | Chr 2 | Location |
|---|---|---|---|
| mef2aa1 | 109108809 | 35 | NC_031731 (6415225..6510272) |
| mef2aa2 | 109108812 | 35 | NC_031731 (6804879..6863032) |
| mef2ab1 | 109103080 | 29 | NC_031725 (2966793..2992778) |
| mef2ab2 | 109076298 | Un | LN594175 (30006..48944) |
| mef2b pseudo | 109047862 | 44 | NC_031740 (10912568..10929359) |
| mef2b | 109047821 | 44 | NC_031740 (11026408..11033466) |
| mef2ca1 | 109059697 | Un | LN591062 (partial) |
| mef2ca2 | 109059699 | Un | LN591062 (partial) |
| mef2cb pseudo | 109078847 | Un | LN594801 (406945..466454) |
| mef2cb | 109078846 | Un | LN594801 (519901..748916) |
| mef2d13 | ? | ? | LN591056 + NC_031744 + NC_031735 (partial) |
| mef2d2 | 109059568 | Un | LN591056 (partial) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Zhou, D.; Ding, N.-Z.; Teng, C.-B.; Yan, X.-C.; Liang, Y. Common Carp mef2 Genes: Evolution and Expression. Genes 2019, 10, 588. https://doi.org/10.3390/genes10080588
He M, Zhou D, Ding N-Z, Teng C-B, Yan X-C, Liang Y. Common Carp mef2 Genes: Evolution and Expression. Genes. 2019; 10(8):588. https://doi.org/10.3390/genes10080588
Chicago/Turabian StyleHe, Mei, Di Zhou, Nai-Zheng Ding, Chun-Bo Teng, Xue-Chun Yan, and Yang Liang. 2019. "Common Carp mef2 Genes: Evolution and Expression" Genes 10, no. 8: 588. https://doi.org/10.3390/genes10080588
APA StyleHe, M., Zhou, D., Ding, N.-Z., Teng, C.-B., Yan, X.-C., & Liang, Y. (2019). Common Carp mef2 Genes: Evolution and Expression. Genes, 10(8), 588. https://doi.org/10.3390/genes10080588




