Abstract
Over the last years, a growing body of evidence suggests that gut microbial communities play a fundamental role in many aspects of human health and diseases. The gut microbiota is a very dynamic entity influenced by environment and nutritional behaviors. Considering the influence of such a microbial community on human health and its multiple mechanisms of action as the production of bioactive compounds, pathogens protection, energy homeostasis, nutrients metabolism and regulation of immunity, establishing the influences of different nutritional approach is of pivotal importance. The very low carbohydrate ketogenic diet is a very popular dietary approach used for different aims: from weight loss to neurological diseases. The aim of this review is to dissect the complex interactions between ketogenic diet and gut microbiota and how this large network may influence human health.
1. Introduction
1.1. The Human Gut Microbiota and the Microbiome
The human gut microbiota, that means the types of organisms that are present in an environmental habitat, consisting of trillions of microbial cells and thousands of bacterial species [1]. It encompasses ~10−13 microorganisms belonging to the three domains of life Bacteria, Archaea and Eukarya and it is involved in several and different functions [2,3]. Microbiome is the collection of the genes and their functions and, due to the new genetic and bioinformatics technologies, the study of the gut microbiome has been radically transformed. The use of the newest platform next generation sequencing (NGS) enables the sequencing of a thousand to million DNA molecules of bacteria in one sequence run (metagenomics) [4] and through this microbial sequencing has been finally possible the understanding of how different microorganisms are present in different tracts of human body [5]. These new omics-technologies allow scientists to discover the role of bacterial genes in human health [6].
Several studies suggest that a mammalian host establishes their core microbiota at birth [7]; the colonization of the gastrointestinal tract by microorganisms, begins within a few hours of birth and concludes around three to four years of age. The nature of the colonic microbiota is driven by several factors such as breast feeding, geographical location, genetics, age and gender [8].
The impact of food (macronutrients) on gut microbiota composition is growing up in interest, especially with respect of specifically dietary fibers. It has been shown that dietary patterns composed by non-refined foods and a high intake of “microbiota accessible carbohydrate” (MACs), are capable to support the growth of specialist microbes producing short chain fatty acids (SCFAs): the prominent energy source for human colonocytes and the signaling key molecules between the gut microbiota and the host [9]. Controversially, the typical pattern of Western diet, high fat-high sugar and low fibers, reduces the production of SCFAs shifting the gastrointestinal microbiota metabolism to the production of detrimental metabolites, favoring the expansion of bacteria associated with chronic inflammation [10].
The composition of the microbiome is influenced by many factors [11] and the stability of the microbiome, reached between two to five years of age, is overlooked by Bacteroidetes, the largest phylum of gram-negative bacteria associated with both beneficial and detrimental effects on health [12,13]. However, the Firmicutes to Bacteroidetes ratio is regarded to be significant for the gut health, the ratio is clearly linked with increasing body mass index (BMI) [14] and the levels of these two dominant bacterial species are known to shift dramatically with aging, especially Bifidobacterium and Lactobacillus genera [15].
1.2. Bioactive Products
The microorganisms living in our gut influence the host through the production of bioactive metabolites, which are able to regulate many biological pathways involved in immunity and energy production. The bacterial population of the large intestine digests carbohydrates, proteins and lipids left undigested by the small intestine. Indigested substances, named “microbiota accessible carbohydrates” (MACs), are represented by the walls of plant cell, cellulose, hemicelluloses and pectin and resistant starch; these polymers undergo microbial degradation and subsequent fermentation [3]. It is really fascinating that the genome of gut bacteria, different from the human genome, encoded several highly specified enzymes able to digest and ferment complex biomacromolecules by hydrolyzing the glycosidic bonds [16,17].
More important, microorganisms have the ability to produce a great amount of B12 and K vitamins, essential for human health, especially for the daily vitamin K intake that is most frequently insufficient [18,19].
The prominent end-products of fermentation in the colon are short chain fatty acids (SCFAs) such as butyrate (C4H7O2-) produced especially by Firmicutes, propionate (C3H502-) by Bacteroidetes and acetate (C2H402) by anaerobes; they represent the greatest source of energy for intestinal absorptive cells. [20,21].
SCFAs contribute to the regulation of the systemic immune function, to the direct appropriate immune response to pathogen and they influence the resolution of inflammation [22].
Moreover, specific bacteria have their own ability to produce many neuroendocrine hormones and neuroactive compounds involved in key aspect of neurotransmission, thus, microbial endocrinology interconnects the science of microbiology with neurobiology. As a matter of fact, γ amino butyric acid (GABA), the major inhibitory neurotransmitter of mammalian central nervous system [23], has been demonstrated to be produced by strains of Lactobacilli and Bifidobacteria, more specifically by Lactobacillus brevis, Bifidobacterium dentium, Bifidobacterium adolescentis and Bifidobacterium infantis [24,25]. Lactobacillus rhamnosus has been demonstrated for its therapeutical potential in modulating the expression of central GABA receptors, mediating depression and anxiety-like behaviors [26].
Furthermore, another important mediator of the gut-brain axis is serotonin (5-hydroxytryptamine 5-HT) that is produced by the enterochromaffin cells of the gastrointestinal tract. It is a metabolite of the amino acid tryptophan and plays a pivotal role in the regulation of several functions such as the mood.
The 95% of serotonin is stored in enterochromaffin cells and enteric neurons, while only the 5% is found in the central nervous system. Kim and colleagues found that germ-free mice have a two-fold decrease of the serotonin blood’s level as compared with commonly mice [27].
However, the gut peripheral serotonin is unable to overstep the blood brain barrier; this serotonin acts on lumen, mucosa, circulating platelets and it is grandly implicated in the gut peristalsis and intestinal anti-inflammation [28,29]. Jun Namking and colleagues suggested that the regulation of the peripheral serotonin might be an adequate tool for the treatment of obesity by the increasing of insulin sensitivity [30].
1.3. Interindividual Variability of Microbiota
The variability among people and the adaptability of gut microbiota to substantial changes have permitted the manipulation of various external factors, restoring both the biological functions and richness of microbiota [31]. The fact that human microbial community is strictly influenced by diet, and, a good ecological community is connected with a better health, offers a range of opportunity for improving human’s health by changing the microbiota composition through different patterns of diet [32,33,34].
The availability of a huge variety and combination of nutrients promotes the selective enrichment of microorganisms, but both the quality and quantity of the macronutrients have an effect on the structure and function of the microbiome [35].
It has been demonstrated the high fat–high sugar Western diet negatively impacts gut health [36] and a high fat diet is closely related to inflammation [37], however, several studies [38,39,40] suggested the necessity to consider the structure and the function of different fatty acids. De Wit and collaborators [41] showed that specific type of fatty acids affect the gut microbiota in different way and, more recently, it has been said that monounsaturated fatty acid’s (MUFA’s) and polyunsaturated fatty acid’s (PUFA’s) omega 3 may be the control key of low-grade systemic inflammation, gut inflammation and as well as obesity [39].
For these reasons, specialized and restricted dietary regimens adopted as a treatment of some diseases, such as low FODMAP for the irritable bowel syndrome and ketogenic diet for refractory epilepsy, should be investigated for their influence on human microbiota [40,42]. These patterns, by reducing or excluding certain type of foods, may affect positively or negatively the microbiota composition and its related influence on host physiology [43,44,45]. That is the case of very low carbohydrate ketogenic diet (VLCKD), a nutritional approach growing up in interest not only for neurological disorders but also for being a “lose-it-quick-plan” [45,46]. VLCKD, by the drastic reduction of the carbohydrate intake, showed an impairment both on the diversity and richness of gut microbiota [47].
1.4. Very Low Carbohydrate Ketogenic Diet (VLCKD)
The very low carbohydrate ketogenic diet (VLCKD) is a dietary protocol that has been used since the 1920 as a treatment for refractory epilepsy [48] and it is currently getting popularity as a potential therapy for obesity and related metabolic disorders [49]. Due to the typical pattern of VLCKD, a hot topic in research and an evolving area of study has been the effect of ketogenic diet on the gut microbiome [50,51,52,53].
Ketogenic diet permits a very low carbohydrate consumption (around 5% to 10% of total caloric intake or below 50 g per day), as a mean to enhance ketone production [54].
Originally, VLCKD had been used as a treatment for epileptic patients that failed to respond to anticonvulsant medication [55]. Currently it has become popular for its benefits extended to neurodegenerative diseases, metabolic diseases and obesity [45]. Recently, VLCKD has been demonstrated to be a powerful tool for some neurodegenerative disease such as autism spectrum disorder (ASD), Alzheimer’s disease [46], glucose transporter 1 deficiency syndrome [56] and auto immune multiple sclerosis (AIMS) [57]. Given the fact that VLCKD is a highly restricted dietary pattern, nowadays, there has been the necessity of formulating new features of the VLCKD, such as the popular modified Atkins diet (MAD) and low glycemic index diet (LGIT) [58,59].
These new patterns have been demonstrated as a successful tool able to reduce seizure symptoms, as well as they reveal a similar outcome, with lower side effects, while compared to the traditional regimes of VLCKD [60,61,62]. LGIT, different from the modified Atkins regime, involves avoiding high glycemic carbohydrates to stabilize blood glucose since it has been shown that stable glucose levels are associated with seizure control [63]. Due to the MAD and LGIT people may choose in a more flexible way the meal they want to consume, they do not have to calculate the specific ketogenic ratio but they may focus on ensuring sufficient and appropriate fats, both in quantity and quality.
1.5. Physiology of Ketosis
The very low carbohydrate ketogenic diet (VLCKD) share several pathways that have been found during fasting state [64]. After several days of drastically reduction of carbohydrate intake, at least <20 g/d or 5% of total daily energy intake, the glucose in the body results insufficient for both fat oxidation (oxaloacetate in tricarboxylic acid cycle TCA) and energy required for the central nervous system forcing the organism to use fats as a primary fuel source [65].
However, fat free acids do not provide energy for the brain because they are not capable to overstep the blood brain barrier: This energy is provided by ketone bodies.
Ketone bodies, 3 hydroxybutyrate (3HB), acetate and acetoacetate (AcAc) are produced in the liver through the process of ketogenesis. Ketogenesis takes place especially in the mitochondria of liver cells where fatty acids reach the mitochondria via carnitine palmitoyltransferase and then breaks down into their metabolites, generating acetyl CoA. The enzyme thiolase (or acetyl coenzyme A acetyltransferase) converts two molecules of acetyl-CoA into acetoacetyl-CoA. Acetoacetyl-CoA is then converted to HMG-CoA due to the enzyme HMG-CoA synthase. Lastly, HMG-CoA lyase converts HMG-CoA to acetoacetate, which can be decarboxylated in acetone or, via β-hydroxybutyrate dehydrogenase, transformed in β-hydroxybutyrate.
The less abundant ketone body is acetone while 3HB plays a main role in the human body under low carbon hydrate diet [66].
The global view of how VLCKD may influence the gut’s health is shown in Figure 1.
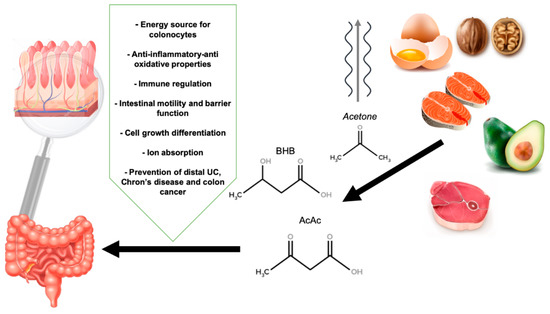
Figure 1.
The influence of a very low carbohydrate ketogenic diet and ketone bodies in gut health. BHB: β-hydroxybutyrate, AcAc: Acetoacetate.
2. Methods
We performed a systematic review from February to March 2019; we used the electronic databases Pubmed, (MEDLINE) and Google scholar. We adopted the MeSH term through the function “MeSH Database” within Pubmed. The terms combined with Boolean operators AND, OR, NOT have been “gut microbiota”, “gut microbiome”, “intestinal microbiome”, “ketogenic diet”, with “ketogenic”, “fat”. Eligibility criteria included full-text articles, written in English, available online from 2015 to 2019; specific studies in which authors investigated the effect of the ketogenic diet on gut microbiota and declared no conflict of interest. We decided to include both in vivo and in vitro studies, ranging from randomized controlled trials to case-control and, to emphasize the effects of diet in “fixed” conditions, we included as well animal studies.
3. Results
How VLCKD Affects the Gut Microbiome
As the ketogenic diet seems to gain consensus [63], little is still known about its impact on the gut microbiota.
Only few experimental studies sought the relationship between VLCKD and gut microbiome [47,50,52,53,67,68,69,70] investigating how VLCKD impacts composition and characteristics of intestinal microorganisms. The effects of VLCKD on gut microbiome have been explored in mice and humans with mixed results. Our systematic review included nine studies and the major findings have been schematically reported (Table 1).

Table 1.
Main findings of the effects of Ketogenic diet (KD) on gut microbiome.
Recently, [53] it has been explored the role of VLCKD on gut microbiota related to the anti-seizure effect on mice. In this study, they found that mice, within four days of being on a diet, had significant changes in gut bacterial taxonomy. Two species of bacteria, Akkermansia and Parabacteriodes were significantly increased in mice that were fed ketogenic diets and gnotobiotic colonization with these microorganisms revealed an anti-seizure effect in germ-free mice or treated with antibiotics.
The increase of these two bacteria species in the gut led to a decreased production of γ-glutamyl transpeptidase by the gut microbiome, the enzyme catalyzes the transfer of γ-glutamyl functional groups from molecules such as glutathione to an acceptor that may be an amino acid forming glutamate [71].
Moreover, they observed a decrease in subset of ketogenic γ-glutatamylated (GG) amino acids (i.e., γ-glutamyl-leucine) both in the gut and blood. GG amino acids are supposed to have transport properties across the blood–brain barrier, different from non-γ-glutamylated forms [72]: This property is involved in glutamate and GABA biosynthesis [73].
This fact, in turn, had the effect of increasing the ratio of GABA to glutamate in the brain of mice. The researchers suggested that VLCKD-microbiota-related limitation in GG amino acids plays a pivotal role on anti-seizure effect, confirmed by the previous studies showing GGT activity to modify the electrical activity of seizure [53].
The ketogenic diet, composed by short fatty acids SFAs, monounsaturated fatty acids MUFAs and polyunsaturated fatty acids PUFAs, has been provided for 16 weeks by Ma and colleagues [51] and it reveals that mice had a variety of neurovascular improvement strictly linked to a lower risk of developing Alzheimer’s disease. These beneficial effects may be connected with the changing on gut microbiota composition, more specifically with the growing quota of beneficial bacteria, Akkermansia Muciniphila and Lactobacillus, which have the ability of generating short chain fatty acids SCFAs. Interestingly, they found a reduction in pro-inflammatory microbes such as Desulfovibrio and Turicibacter. The VLCKD however, decreased the overall microbial α diversity due to the low carbohydrate (complex carbohydrate) content of diet, which is fundamental for the microorganism in order to breakdown them and producing energy [52].
A 2016 study [67] investigated whether or not a VLCKD could ensure benefits in the gut microbiome in murine model of autism. The authors administrated a VLCKD for several days (10–14) observing changes in gut microbiome; they concluded that the VLCKD had an “anti-microbial” effect by decreasing the overall richness of microorganisms both in cecal and fecal matter, and as well as improved the ratio of Firmicutes to Bacteroides species. A lowered firmicutes: bacteroides ratio is common in ASD and the VLCKD, by improving this ratio, was able to enhance ASD behavioral symptoms. Lastly, different from the above-mentioned studies, the VLCKD decreased the number of A. muciniphila bacteria species, resulting in similar levels to those found in the control groups.
It has been described the connection between microbiome, VLCKD and the potential role playing in multiple sclerosis (MS) [52]. A common attribute of the AIMS is the damage and affliction of “colonic bio-fermentative function”. The fermentative process which allow the production of beneficial byproducts such as SFCA, is impaired, thus, the dysbiotic colonic bacteria ferment foods into dangerous compounds affecting the organism. The VLCKD completely restored the microbial biofermentative mass and normalizing the concentration of the colonic microbiome. The authors [52] showed a biphasic effect of VLCKD: first there has been a dramatic decrease in richness and bacterial diversity, but, after 12 weeks, bacterial concentration began to recover back to baseline and, after 23–24 weeks, it showed a significant increase in bacterial concentration above baseline.
A study in children by Xie and colleagues [68], investigated the connection between microbiome and refractory epilepsy in 14 epileptic and 30 healthy infants. Patients with epilepsy demonstrated an imbalance of gut microbiota before starting the VLCKD. The authors found a higher amount of pathogenic proteobacteria (Escherichia, Salmonella and Vibrio), which significantly decreased after VLCKD treatment and an increase of Bacterioidetes both in healthy subjects and in patients. Bacteroides spp. are strictly connected with the digestion and metabolism of high-fat nutrients and the regulation of the secretion of 6–17 interleukins in dendritic cells, which is connected with the seizure effects on epileptic patients [74]. Researchers suggest that VLCKD can reduce these symptoms by developing changes on microbiota diversity.
Zhang et al. sought the differences in the microbiota of pediatric patients fed a ketogenic diet [69]. They investigated the difference between responders (seizure frequency was reduced or stopped) and non-responders (no effect on seizure). They found increased amount of Bacteroides and decreased amounts in Firmicutes and Actinobacteria, in responders. On the other hand, Clostridia, Ruminococcus and Lachnospiraceae (Firmicutes phylum) increased in non-responders. These data demonstrated that ketogenic diet alters the gut microbiome of pediatric patients, suggesting that the gut microbiome should be taken into account as a biomarker of efficacy of anti-seizure treatment. As regard patients affected by Glucose Transporter 1 Deficiency Syndrome [50], it has been showed a significant increase in Desulfovibrio spp. in six patients, after 3 months of intervention. Desulfovibrio spp is a genus belonging to a heterogeneous group of sulfate-reducing, motile, anaerobic bacteria related to the inflammatory status of the gut layer mucosa [75]. Authors suggested that in case of dysbiosis, it might be a good option the use of an extra-supplementation with pre or probiotics to maintain the “ecological balance” of gut microbiota [50].
Recently, a study in epileptic children found a reduction of Bifidobacteria, as well as E. rectale and Dialister, which are correlated with health promoting benefits such as the prevention of colorectal cancer, IBS and necrotizing entercolitis [76]. Researcher identified a relative abundance of Actinobacteria and Escherichia coli that may be due to the VLCKD restricted on carbohydrate. It should be stressed that through the analysis of the 29SEED subsystem, scientists revealed a depletion of those pathways responsible of the degradation of carbohydrates [70].
4. Discussion
4.1. Friend or Enemies?
All the papers that have been chosen for depicting the crossing mechanisms, revealed supposed connections between gut microbiome, ketogenic diets and systemic effects. Some findings are demonstrated through “omics” analyses, some are only assumed. As it can be seen, there are several and controversy findings suggesting the necessity of a deeper understanding. The picture (Figure 2) aims to highlight the supposed major effects of ketogenic diet on different tissues and gut microbiota, along with how tissues may be influenced by gut microbiota diversity.
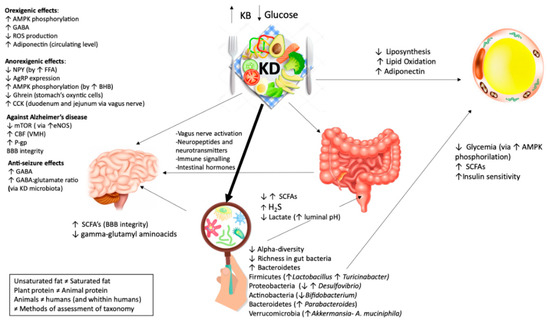
Figure 2.
Effects of ketogenic diet on different tissues and the microbiome. KD has a contradictory role on hunger but the net effect is anorexigenic. KD Exerts orexigenic effects: The increase of brain GABA (γ-aminobutyric acid) through BHB (β-hydroxybutyric acid); the increase of AMP (adenosine monophosphate -activated protein) phosphorylation via BHB; the increase of circulating level of adiponectin; the decreases of ROS (reactive oxygen species). KD Exerts anorexigenic effect: the increase of circulating post meal FFA (free fatty acids); a maintained meal’s response of CCK (cholecystokinin); a decrease of circulating ghrelin; a decrease of AMP phosphorylation; a decrease of AgRP (agouti-related protein) expression. KD has positive effects on Alzheimer’s disease through: an increase levels of CBF (cerebral blood flow) in VMH (ventromedial hypothalamus); a decrease expression of mTOR (mammalian target of rapamycin) by the increase of the level of eNOS (endothelial nitric oxide synthase) protein expression; an increased expression of P-gp (P-glycoprotein), which transport Aβ (amyloid-β) plaques; an improvement of BBB’s (blood–brain-barrier) integrity. KD has beneficial effects on epileptic seizure by the modulation of hippocampal GABA/glutamate ratio. It exerts anti-seizure effects through: An increase level of GABA, an increase content of GABA: glutamate ratio. KD plays a main role on fat loss. It exerts positive effects on adipose tissue through: a decrease of liposynthesis, an increase of lipid oxidation and an increase in adiponectin. KD has a contradictory role on microbiome. KD generally exerts its effect through: a decrease in α diversity (the diversity in a single ecosystem/sample) and a decrease in richness (number of different species in a habitat/sample). KD influences the gut health through metabolites produced by different microbes: an increase/decrease in SCFA (short chain fatty acids), an increase in H2S (hydrogen sulfide) and a decrease in lactate. KD to microbiome to the brain: KD may influence the CNS (central nervous system) not only directly but also indirectly. The KD effects on the brain are supposed to be mediated by microbiota through an increase of SCFAs and a decrease of γ-glutamyl amino acid. A. muciniphila and Lactobacillus are known as SCFAs producers. SCFAs are transported by monocarboxylase transporters expressed at BBB. Desulfovibrio has the ability to produce hydrogen sulfide and, as a consequence, impair intestinal mucosal barrier. A reduction in Desulfovibrio and an enhancement in A. muciniphila and Lactobacillus may facilitate BBB and neurovascular amelioration. KD to microbiome to the adipose tissue: KD may indirectly influence the adipose tissue by the microbiota through a decrease in glycemia via adenosine monophosphate-activated protein kinase (AMPK) phosphorylation, an increase in insulin sensitivity and an increase in SCFAs. The great amount of A. muciniphila and Lactobacillus spp. led to the reduction of body weight and glycemia. It has been demonstrated that patient with type 2 diabetes, treated with metformin, revealed higher level of A. muciniphila, may be to the ability of metformin on decreasing body weight by the activation of AMPK pathways (amp-activated protein kinase). A. muciniphila is related with the enhancement of insulin sensitivity and Lactobacillus may be playing the same effects through SFCAs production: Several studies showed that Lactobacillus is strictly connected with body weight loss.
4.2. Factors Affecting Microbiota during a VLCKD: What Should We Consider?
4.2.1. Fats
The optimal composition of a VLCKD considers both high saturated and mono-polyunsaturated fats [54], whilst the Western diet is rich in saturated-trans fats and poor in mono-polyunsaturated fats [77].
A recent systematic review concluded that diets high in saturated fatty acids led to negative effects on the gut microbiota [78]. The authors observed that diets rich in highly monounsaturated fats affected negatively the gut microbiota decreasing bacteria richness, while diets rich in polyunsaturated fatty acids (with opposite effects when comparing omega 3 vs. omega 6 fats) did not change richness and diversity. However, to notice that only a few studies have been conducted with NGS methods or shotgun sequencing, these new technologies deliver accurate data by avoiding experimental pitfalls and biases created by the “old fashioned” sequencing methods [79]. Recently, a randomized controlled trial study [80] has revealed that a diet with a high content in fat increased Bacteroides while reducing the number of butyrate producers (Faecalibacterium and Blautia bacteria) compared with a middle-lower-fat group. The differences in fecal SCFA could be explained by the high content of carbohydrates in the middle to low-fat diets, made up of resistant starches that have been broken down and fermented. It has to be stressed that the source of fat came from soybean oil, which is highly rich in omega 6 polyunsaturated fatty acids [81]; a higher omega-6: omega-3 long-chain PUFA ratio is associated with many health risks and chronic state of inflammation [82,83,84]. Another RCT study [85] showed that a supplementation with omega 3 PUFA did not disclose any taxonomic changes both in α and β diversity (at family and genus levels) including short-chain fatty acid producers.
According to these results, different studies demonstrated that each type of fatty acid may induce different effects: The saturated fats (palm oil) induce higher liver triglyceride content in mice, as opposed to polyunsaturated fats (olive oil) [41]. Moreover, genetically modified mice, characterized by the ability of producing omega 3 (PUFAs) and fed with high fat and high sugar diet, showed a higher microbial diversity and a normal gut layer function in the distal intestine, different from non-modified-mice fed with the same macronutrients [86].
The source of fats (omega 6: Omega3, PUFAa and MUFAs) and their own quality should be highly considered when performing a very low carbohydrate dietary plan and as well as when giving general nutritional advices.
4.2.2. Sweeteners
An area of controversy in the ketogenic diet is the consumption of artificial sweeteners replacing natural sugars. Several evidences demonstrated that artificial sweeteners have a negative impact on both host and gut health. Nettleton at al. found that low calorie sweeteners, such as acesulfame potassium (Ace-K) and sucralose, disrupted the structure and function of gut microbiota and gut mucosa [87]. More recently Qiao-Ping Wang investigated, through the use of NGS, the effects of non-nutritive sweeteners (NNSs) on the gut microbioma of mice at the organism level; the study reveals that artificial sweeteners has bacteriostatic effects and as well as change the composition of microbiota [88]. These findings, according to the fact that the routine consumption of NNSs may increase the risk of cardiometabolic diseases [89], suggested that these chemical substitutes may be detrimental for human health and should be avoided [90]. However, recently, the use of stevia (also called Stevia rebaudiana) has been widely adopted as a non-nutrient but natural sweeteners. The use of Stevia lowered insulin and glucose level in 19 healthy lean and 12 obese individuals and left them satisfied and full after eating, despite the lower calorie intake [91]. Accordingly, Sharma and colleagues [92] showed a reduction of cholesterol level, triglyceride, low-density lipoprotein (LDL) and an enhancement of high-density lipoprotein (HDL) on 20 hypercholesterolemic women consuming stevia extracts. In a 2008 review, authors suggest that there are not enough information concerning the effect of stevia on gut microbioma [93], whilst others reported a possible link between nonnutritive sweeteners, including stevia, and the disruption of beneficial intestinal flora [94].
Given the fact that there is no explicit data available on gut microbiome, but, The Food and Drug Administration (FDA) considered it as “generally safe” [95], stevia might slightly be used in place of artificial and chemical sweeteners, within coffee, tea or in a unsweetened yogurt. However, further investigation need to be done considering the effect of low calorie sweeteners on gut and human health.
4.2.3. Pre and Probiotics
A proper suggestion for maintaining a healthy gut microbiota during the ketogenic diet may be the use of pre and probiotics: Increasing evidences [96,97] demonstrate their positive benefits. The major source of prebiotics is represented by fructo-oligosaccharides, inulin, lactulose galacto-oligosaccharides and trans-galacto-oligosaccharides [98]. Fermentation of prebiotics by gut microbiota produces SCFAs, which positively modulate the composition of microbiota (by increasing intestinal bifidobacteria and lactic acid bacteria), providing an energy source for colonocytes [99]. Differently, probiotics are living bacteria (especially from the genera Bifidobacterium and Lactobacillus) and yeasts that, when administrated in an adequate amount, show positive effect on human health; they are usually added to yogurts or found in “specialty food” [100,101,102]. It has been reported [103,104] that foods enriched with these microorganisms are able to recovery and improve gut microbiota, reaching the state of eubiosis. Cultured-milk products (kefir, Greek yogurt), traditional buttermilk, water kefir, fermented cheese, sauerkraut, kimchi, miso, kombucha and pickles contain several and different “friendly bacteria” such as Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgarius, Lactobacillus reuteri, Saccharomyces boulardii and Bifidobacterium bifidum [105,106,107,108].
However, despite the growing interest on fermented foods, there is a lack of epidemiological studies [104] and the majority have focused only on yogurt and cultured dairy foods [109,110]. The paucity arises from the difficulty of understanding if health benefits come from the fermentation operated by microbes or other bioactive compounds. As regard the usefulness of fermented foods during a VLCKD, they represent an excellent and palatable source of dietary fiber and essential micronutrients [111], which should be moderately provided during a VLCKD.
In our opinion since foods that have undergone deep fermentation seem to improve the gut microbiome diversity and gut health index [112] adding small portions of fermented foods to the diet may be a useful prebiotic/probiotic supplementation as well as an effective aid to digestion. A caveat should be done: It is mandatory to verify that fermented foods and beverages are able to not modify in a significant manner glycaemia and insulinaemia while maintaining a sufficient ketonemia.
It has been recently shown that parmesan (an Italian hard and dry cheese), contains “friendly bacteria” acting as probiotics and able to colonize and live in the gut of those individuals who daily consume it [113]. Thus, the moderate consumption of a high-fat fermented food is well recommended for human gut and human health.
4.2.4. Proteins
Several considerations have to be done to the different impact of different protein on gut microbiome.
The source and type of protein must be considered, especially in the field of sports, in which the intake of protein within VLCKD is fundamental to maintain lean body mass [114].
Several studies investigated how and how much different kind of protein (plant versus animal) modify microbiome [115,116,117], showing that, even though high protein diet generally impair gut health (decrease abundance and change composition) [118], several and disparate effects appear on the gut microbiota [119].
Plant-derived protein, such as mung bean protein (as a part of high fat diet), increased Bacteroidetes while decreasing Firmicutes as well as pea protein increased strains of Bifidobacterium and lactobacillus [115].
These studies demonstrated that plant-derived protein get better benefits on gut microbiome along with positive effects on the host metabolism.
To note that we did consider that no studies investigated how protein have been processed, such as thermal treatment, and the effect of the processing treatment on microbiome composition.
During a period of VLCKD, we recommend the use of a source of plant protein (veg protein) since these are more beneficial in terms of health gut microbiota.
5. Conclusions, Perspective and Future Research
In the recent years, the interest regarding the benefits of ketogenic diets is growing up and expanding well beyond the seizure control. Ketogenic diet, as well as the more flexible and less restrictive regimens MAD, LGIT is commonly adopted for weight loss in both obese patients and athlete populations. Bacteria taxa, richness and diversity are strictly influenced by ketogenic diet. A few human and animal studies have shown different results demonstrating positive effects on reshaping bacterial architecture and gut biological functions, while others reporting negative effects as a lowered diversity and an increased amount of pro-inflammatory bacteria.
Nevertheless, short period studies and with specific disease conditions have been carried out [50,52,67,68], limiting generalization to the overall population. Additionally, the microbiota of many environments may be highly variable and its plasticity could be dependent on past and specific dietary patterns [120]. In agreement with these considerations, Healey and colleagues concluded that because of the high variability among people of microbiome composition, it is actually difficult to identify how microbiota may change the diversity in relation to a specific dietary pattern [121]. According to different authors [50,70], there is the necessity to find better strategies to maximize the benefit of VLCKD. It may be useful implementing VLCKD with specific pre and probiotics, which has been found to be drastically reduced during VLCKD [50]. Additionally, promising evidence comes from randomized control trials suggesting that quality dietary fats highly affects the gut microbiota composition. Diets with a high fat content and good quality of polyunsaturated fats and plant-derived protein are able to maintain normal gut function [80,86]. In parallel, the abolition of artificial sweeteners [90] should be recommended to avoid negative effects on general health caused by alteration of gut microbiota. It has been suggested that a supplementation with prebiotics, such as inulin, lactulose, fruttooligosaccharides (FOS) and galactooligosaccharides (GOS) that increases Bifidobacteria, may prevent undesired changes in the gut microbiota [122].
Nonetheless, it is essential to point out that the modified microbiota composition, changed by VLCKD, plays a pivotal role on the itself activity of VLCKD [53,67,68]; the changes have been demonstrated to be necessary in order to provide positive effects such as the anti-seizure effect and amelioration of neurovascular function [53,69,70].
Although there are still many questions limiting the practical research on microbiome, several new developments carried on advancement in this field. Integration of omics science with the newest metagenomic methods of microbiota assessment (next generation sequencing, shotgun sequencing 16S rRNA) shall be helpful to define healthy versus unhealthy microbial operational taxonomic units (OTUs). For this purpose, the Italian Microbiome Project (http://progettomicrobiomaitaliano.org) focuses his research on the advantages and disadvantages that may arise from the genes of bacterial origin, by combining bioinformatic tools with algorithms to better link microbiota data to human health outcomes. Recently, it has been developed a machine e-learning algorithm that is able to predict a specific post-prandial glycemic response by analyzing microbiome profiling [123,124].
The observations that a ketogenic diet can modulate and reshape gut microbiota represents a potential and promising future therapeutic approach. VLCKD has been demonstrated to be a powerful tool and needs to be further refined and well formulated considering its impact on gut health. In conclusion, further research with long-term clinical trials has to be performed in order to establish safer and healthier specific dietary interventions for patients.
Take Home Message:
Practical recommendations to preserve gut health during a VLCKD:
- Introduce the use of whey and plant proteins (i.e., pea protein);
- Reduce the intake of animal protein;
- Implement fermented food and beverages (yoghurt, water and milk kefir, kimchi, fermented vegetables);
- Introduce properly prebiotics and specific probiotics (if needed);
- Reduce omega 3 to omega 6 fatty acids ratio (increase omega 3 while decreasing omega 6);
- Introduce an accurate quantity and quality of unsaturated fatty acids;
- Avoid artificial sweeteners (stevia?) and processed foods;
- Test your microbiome if needed (analysis of 16S rRNA to identify biodiversity and richness).
It is mandatory to verify that fermented foods and beverages and proteins should not modify (in a significant manner) glycaemia and insulinaemia while maintaining a sufficient ketonemia.
We need to remember as well as that the modified microbiota composition induced by VLCKD, plays a pivotal role on the itself activity of diet.
Author Contributions
All authors contributed equally to the review.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, G.M. Genomic approaches to studying the human microbiota. Nature 2012, 489, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Koedooder, R.; Mackens, S.; Budding, A.; Fares, D.; Blockeel, C.; Laven, J.; Schoenmakers, S. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum. Reprod. Update 2019, 25, 298–325. [Google Scholar] [CrossRef]
- Oulas, A.; Pavloudi, C.; Polymenakou, P.; Pavlopoulos, G.A.; Papanikolaou, N.; Kotoulas, G.; Arvanitidis, C.; Iliopoulos, I. Metagenomics: Tools and insights for analyzing next-generation sequencing data derived from biodiversity studies. Bioinform. Biol. Insights 2015, 9, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Group, N.H.W.; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The nih human microbiome project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Taneja, V. Arthritis susceptibility and the gut microbiome. Febs Lett. 2014, 588, 4244–4249. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Annalisa, N.; Alessio, T.; Claudette, T.D.; Erald, V.; Antonino, d.L.; Nicola, D.D. Gut microbioma population: An indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediat. Inflamm. 2014, 2014, 901308. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Fouhy, F.; Watkins, C.; Hill, C.J.; O’Shea, C.A.; Nagle, B.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 2019, 10, 1517. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and firmicutes/bacteroidetes ratio in an adult ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Vrieze, A.; Holleman, F.; Zoetendal, E.G.; de Vos, W.M.; Hoekstra, J.B.; Nieuwdorp, M. The environment within: How gut microbiota may influence metabolism and body composition. Diabetologia 2010, 53, 606–613. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Env. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Lundin, A.; Bok, C.M.; Aronsson, L.; Bjorkholm, B.; Gustafsson, J.A.; Pott, S.; Arulampalam, V.; Hibberd, M.; Rafter, J.; Pettersson, S. Gut flora, toll-like receptors and nuclear receptors: A tripartite communication that tunes innate immunity in large intestine. Cell. Microbiol. 2008, 10, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B. The role of gaba in the pathophysiology and treatment of anxiety disorders. Psychopharmacol. Bull. 2003, 37, 133–146. [Google Scholar] [PubMed]
- Cryan, J.F.; Kaupmann, K. Don’t worry ‘b’ happy!: A role for gaba(b) receptors in anxiety and depression. Trends Pharm. Sci. 2005, 26, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of lactobacillus strain regulates emotional behavior and central gaba receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Kim, D.Y.; Camilleri, M. Serotonin: A mediator of the brain-gut connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Namkung, J.; Kim, H.; Park, S. Peripheral serotonin: A new player in systemic energy homeostasis. Mol. Cells 2015, 38, 1023–1028. [Google Scholar]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients 2018, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from europe and rural africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zinocker, M.K.; Lindseth, I.A. The western diet-microbiome-host interaction and its role in metabolic disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory links between high fat diets and diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision nutrition and the microbiome, part I: Current state of the science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Candido, F.G.; Valente, F.X.; Grzeskowiak, L.M.; Moreira, A.P.B.; Rocha, D.; Alfenas, R.C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- Reddel, S.; Putignani, L.; Del Chierico, F. The impact of low-fodmaps, gluten-free, and ketogenic diets on gut microbiota modulation in pathological conditions. Nutrients 2019, 11, 373. [Google Scholar] [CrossRef]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Muller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef] [PubMed]
- Varju, P.; Farkas, N.; Hegyi, P.; Garami, A.; Szabo, I.; Illes, A.; Solymar, M.; Vincze, A.; Balasko, M.; Par, G.; et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: A meta-analysis of clinical studies. PLoS ONE 2017, 12, e0182942. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.M. Restriction of fodmap in the management of bloating in irritable bowel syndrome. Singap. Med. J. 2016, 57, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Bascunan, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E.; Rho, J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharm. 2012, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, N.; Burke, L.M.; Vlahovich, N.; Charlesson, B.; O’Neill, H.; Ross, M.L.; Campbell, K.L.; Krause, L.; Morrison, M. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients 2019, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Cooder, H.R. Epilepsy in children: With particular reference to the ketogenic diet. Cal. West. Med. 1933, 39, 169–173. [Google Scholar]
- Perez-Guisado, J. Ketogenic diets: Additional benefits to the weight loss and unfounded secondary effects. Arch. Lat. Nutr. 2008, 58, 323–329. [Google Scholar]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 deficiency syndrome: A 3-month prospective observational study. Clin. Nutr Espen 2017, 17, 33–37. [Google Scholar] [CrossRef]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 2018, 8, 6670. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dorffel, Y.; Loening-Baucke, V.; Gille, C.; Goktas, O.; Reisshauer, A.; Neuhaus, J.; Weylandt, K.H.; Guschin, A.; Bock, M. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018, 174, 497. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Klepper, J. GLUT1 deficiency syndrome in clinical practice. Epilepsy Res. 2012, 100, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Wang, H.S. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed. J. 2013, 36, 9–15. [Google Scholar] [CrossRef]
- Miranda, M.J.; Turner, Z.; Magrath, G. Alternative diets to the classical ketogenic diet—Can we be more liberal? Epilepsy Res. 2012, 100, 278–285. [Google Scholar] [CrossRef]
- Sharma, S.; Sankhyan, N.; Gulati, S.; Agarwala, A. Use of the modified atkins diet for treatment of refractory childhood epilepsy: A randomized controlled trial. Epilepsia 2013, 54, 481–486. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Cervenka, M.C.; Henry, B.J.; Haney, C.A.; Turner, Z. A decade of the modified Atkins diet (2003–2013): Results, insights, and future directions. Epilepsy Behav. 2013, 29, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Muzykewicz, D.A.; Lyczkowski, D.A.; Memon, N.; Conant, K.D.; Pfeifer, H.H.; Thiele, E.A. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia 2009, 50, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Canato, M.; Toniolo, L.; Bargossi, A.M.; Neri, M.; Mediati, M.; Alesso, D.; Sanna, G.; Grimaldi, K.A.; Fazzari, A.L.; et al. The ketogenic diet: An underappreciated therapeutic option? La Clinica Terapeutica 2011, 162, e145–e153. [Google Scholar] [PubMed]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The influence of meal frequency and timing on health in humans: The role of fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis; Statpearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhou, Q.; Qiu, C.Z.; Dai, W.K.; Wang, H.P.; Li, Y.H.; Liao, J.X.; Lu, X.G.; Lin, S.F.; Ye, J.H.; et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J. Gastroenterol. 2017, 23, 6164–6171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterstrom, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. npj Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.B. γ glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef] [PubMed]
- Pica, A.; Chi, M.C.; Chen, Y.Y.; d’Ischia, M.; Lin, L.L.; Merlino, A. The maturation mechanism of γ-glutamyl transpeptidases: Insights from the crystal structure of a precursor mimic of the enzyme from bacillus licheniformis and from site-directed mutagenesis studies. Biochim. Biophys. Acta 2016, 1864, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Hertz, L. The glutamate-glutamine (gaba) cycle: Importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front. Endocrinol. (Lausanne) 2013, 4, 59. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in IL-10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.C.; Harder, J.M.; Soto, I.; de Vries, W.N.; John, S.W.; Howell, G.R. Chronic consumption of a western diet induces robust glial activation in aging mice and in a mouse model of alzheimer’s disease. Sci. Rep. 2016, 6, 21568. [Google Scholar] [CrossRef]
- Wolters, M.; Ahrens, J.; Romani-Perez, M.; Watkins, C.; Sanz, Y.; Benitez-Paez, A.; Stanton, C.; Gunther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the mynewgut project. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Cao, Y.; Fanning, S.; Proos, S.; Jordan, K.; Srikumar, S. A review on the applications of next generation sequencing technologies as applied to food-related microbiome studies. Front. Microbiol. 2017, 8, 1829. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef]
- Isaac, D.M.; Alzaben, A.S.; Mazurak, V.C.; Yap, J.; Wizzard, P.R.; Nation, P.N.; Zhao, Y.Y.; Curtis, J.M.; Sergi, C.; Wales, P.W.; et al. Mixed lipid, fish oil and soybean oil parenteral lipids impact cholestasis, hepatic phytosterol and lipid composition. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 861–867. [Google Scholar] [CrossRef]
- Jang, H.; Park, K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systematic review and meta-analysis. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Noori, N.; Dukkipati, R.; Kovesdy, C.P.; Sim, J.J.; Feroze, U.; Murali, S.B.; Bross, R.; Benner, D.; Kopple, J.D.; Kalantar-Zadeh, K. Dietary omega-3 fatty acid, ratio of omega-6 to omega-3 intake, inflammation, and survival in long-term hemodialysis patients. Am. J. Kidney Dis. 2011, 58, 248–256. [Google Scholar] [CrossRef]
- Lee, H.J.; Han, Y.M.; An, J.M.; Kang, E.A.; Park, Y.J.; Cha, J.Y.; Hahm, K.B. Role of omega-3 polyunsaturated fatty acids in preventing gastrointestinal cancers: Current status and future perspectives. Expert Rev. Anticancer 2018, 18, 1189–1203. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Bidu, C.; Escoula, Q.; Bellenger, S.; Spor, A.; Galan, M.; Geissler, A.; Bouchot, A.; Dardevet, D.; Morio, B.; Cani, P.D.; et al. The transplantation of ω3 PUFA-altered gut microbiota of FAT-1 mice to wild-type littermates prevents obesity and associated metabolic disorders. Diabetes 2018, 67, 1512–1523. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Reimer, R.A.; Shearer, J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol. Behav. 2016, 164, 488–493. [Google Scholar] [CrossRef]
- Wang, Q.P.; Browman, D.; Herzog, H.; Neely, G.G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE 2018, 13, e0199080. [Google Scholar] [CrossRef]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Anton, S.D.; Martin, C.K.; Han, H.; Coulon, S.; Cefalu, W.T.; Geiselman, P.; Williamson, D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010, 55, 37–43. [Google Scholar] [CrossRef]
- Sharma, N.; Mogra, R.; Upadhyay, B. Effect of stevia extract intervention on lipid profile. Stud. Ethno-Med. 2009, 3, 137–140. [Google Scholar] [CrossRef]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Wolwer-Rieck, U.; Jeppesen, P.B.; Rogers, P.J.; Rowland, I.; Mathews, R. Stevia leaf to stevia sweetener: Exploring its science, benefits, and future potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef]
- Pepino, M.Y. Metabolic effects of non-nutritive sweeteners. Physiol. Behav. 2015, 152, 450–455. [Google Scholar] [CrossRef]
- Perrier, J.D.; Mihalov, J.J.; Carlson, S.J. Fda regulatory approach to steviol glycosides. Food Chem. Toxicol. 2018, 122, 132–142. [Google Scholar] [CrossRef]
- Finamore, A.; Roselli, M.; Donini, L.; Brasili, D.E.; Rami, R.; Carnevali, P.; Mistura, L.; Pinto, A.; Giusti, A.; Mengheri, E. Supplementation with Bifidobacterium longum Bar33 and lactobacillus helveticus bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition 2019, 63–64, 184–192. [Google Scholar] [CrossRef]
- Bagheri, S.; Heydari, A.; Alinaghipour, A.; Salami, M. Effect of probiotic supplementation on seizure activity and cognitive performance in PTZ-induced chemical kindling. Epilepsy Behav. 2019, 95, 43–50. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Interactions and competition within the microbial community of the human colon: Links between diet and health. Env. Microbiol. 2007, 9, 1101–1111. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Prebiotics and probiotics in digestive health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef]
- Nath, A.; Haktanirlar, G.; Varga, A.; Molnar, M.A.; Albert, K.; Galambos, I.; Koris, A.; Vatai, G. Biological activities of lactose-derived prebiotics and symbiotic with probiotics on gastrointestinal system. Medicina (Kaunas) 2018, 54, 18. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligne, B.; Ganzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Borresen, E.C.; Henderson, A.J.; Kumar, A.; Weir, T.L.; Ryan, E.P. Fermented foods: Patented approaches and formulations for nutritional supplementation and health promotion. Recent Pat. Food Nutr. Agric. 2012, 4, 134–140. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Chen, L.; Bhochhibhoya, A. Probiotic foods and supplements interventions for metabolic syndromes: A systematic review and meta-analysis of recent clinical trials. Ann. Nutr. Metab. 2019, 74, 224–241. [Google Scholar] [CrossRef]
- Sairanen, U.; Piirainen, L.; Grasten, S.; Tompuri, T.; Matto, J.; Saarela, M.; Korpela, R. The effect of probiotic fermented milk and inulin on the functions and microecology of the intestine. J. Dairy Res. 2007, 74, 367–373. [Google Scholar] [CrossRef]
- Yoon, H.; Park, Y.S.; Lee, D.H.; Seo, J.G.; Shin, C.M.; Kim, N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Biochem. Nutr. 2015, 57, 129–134. [Google Scholar] [CrossRef]
- Yang, S.J.; Lee, J.E.; Lim, S.M.; Kim, Y.J.; Lee, N.K.; Paik, H.D. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 491–499. [Google Scholar] [CrossRef]
- Shiby, V.K.; Mishra, H.N. Fermented milks and milk products as functional foods—A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takada, T.; Shimizu, K.; Moriyama, K.; Kawakami, K.; Hirano, K.; Kajimoto, O.; Nomoto, K. Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. J. Biosci. Bioeng. 2010, 110, 547–552. [Google Scholar] [CrossRef]
- Bell, V.; Ferrao, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One health, fermented foods, and gut microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Napoli, S.; Alessandri, G.; Mancabelli, L.; Anzalone, R.; Longhi, G.; Viappiani, A.; Mangifesta, M.; Lugli, G.A.; et al. Colonization of the human gut by bovine bacteria present in parmesan cheese. Nat. Commun 2019, 10, 1286. [Google Scholar] [CrossRef]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The ketogenic diet and sport: A possible marriage? Exerc. Sport Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef]
- Nakatani, A.; Li, X.; Miyamoto, J.; Igarashi, M.; Watanabe, H.; Sutou, A.; Watanabe, K.; Motoyama, T.; Tachibana, N.; Kohno, M.; et al. Dietary mung bean protein reduces high-fat diet-induced weight gain by modulating host bile acid metabolism in a gut microbiota-dependent manner. Biochem. Biophys. Res. Commun. 2018, 501, 955–961. [Google Scholar] [CrossRef]
- Meddah, A.T.; Yazourh, A.; Desmet, I.; Risbourg, B.; Verstraete, W.; Romond, M.B. The regulatory effects of whey retentate from bifidobacteria fermented milk on the microbiota of the simulator of the human intestinal microbial ecosystem (SHIME). J. Appl. Microbiol. 2001, 91, 1110–1117. [Google Scholar] [CrossRef]
- Swiatecka, D.; Narbad, A.; Ridgway, K.P.; Kostyra, H. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The colonic microbiome and epithelial transcriptome are altered in rats fed a high-protein diet compared with a normal-protein diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Li, C.; Zhou, G. Erratum: Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci. Rep. 2015, 5, 16546. [Google Scholar] [CrossRef]
- Griffin, N.W.; Ahern, P.P.; Cheng, J.; Heath, A.C.; Ilkayeva, O.; Newgard, C.B.; Fontana, L.; Gordon, J.I. Prior dietary practices and connections to a human gut microbial metacommunity alter responses to diet interventions. Cell Host Microbe 2017, 21, 84–96. [Google Scholar] [CrossRef]
- Healey, G.R.; Murphy, R.; Brough, L.; Butts, C.A.; Coad, J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 2017, 75, 1059–1080. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Mancabelli, L.; Mangifesta, M.; Viappiani, A.; Lugli, G.A.; Ferrario, C.; Gioiosa, L.; Ferrarini, A.; et al. Deciphering bifidobacterial-mediated metabolic interactions and their impact on gut microbiota by a multi-omics approach. ISME J. 2016, 10, 1656–1668. [Google Scholar] [CrossRef]
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project: Dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe 2014, 16, 276–289. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).