Global Dynamics in Protein Disorder during Maize Seed Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Transcriptomic Data Acquisition
2.2. Protein Disorder Analysis and Data Clustering
2.3. Transcriptome Mining and Differential Expression Analysis
2.4. Gene Co-Expression Network Analysis
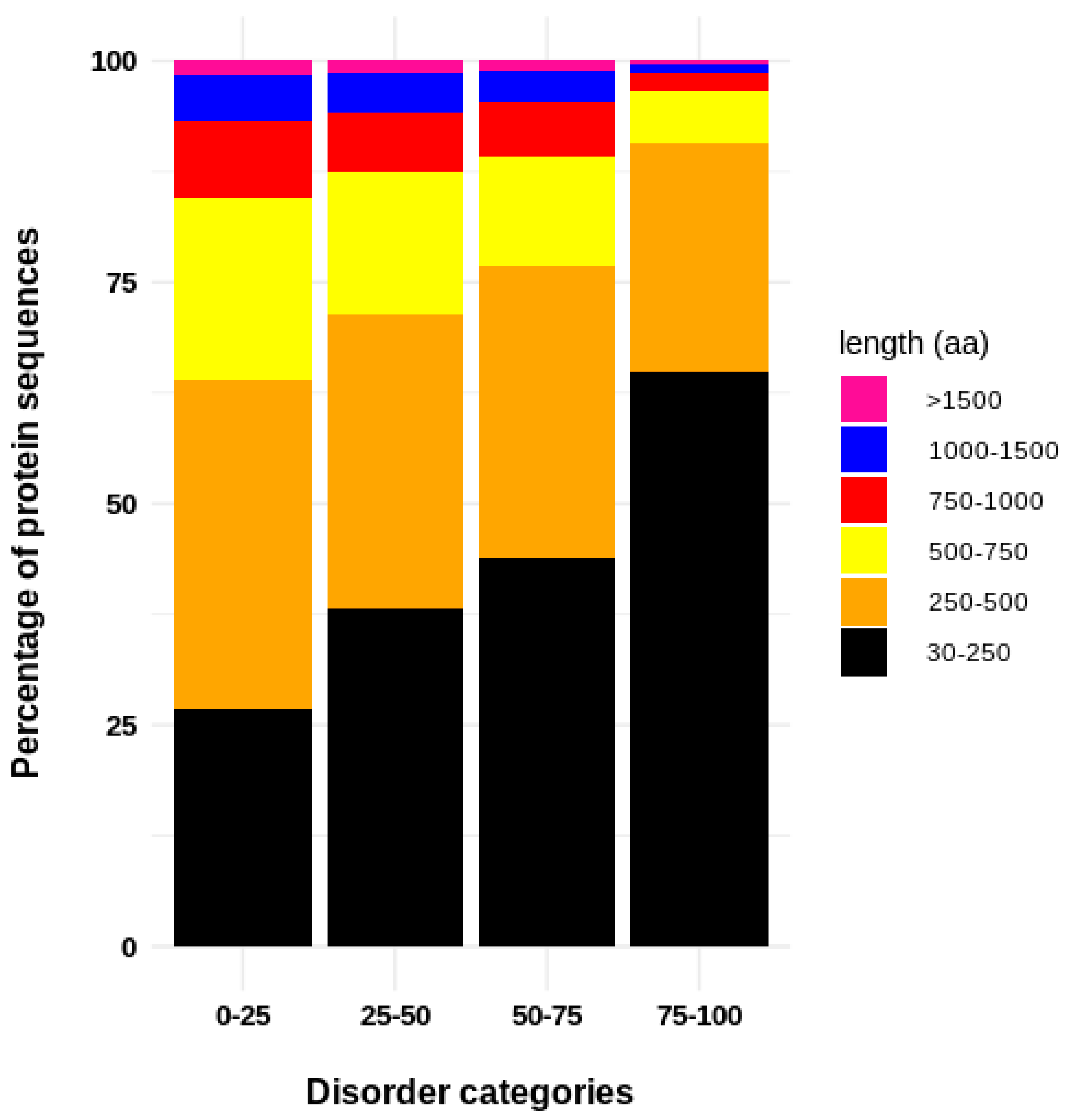
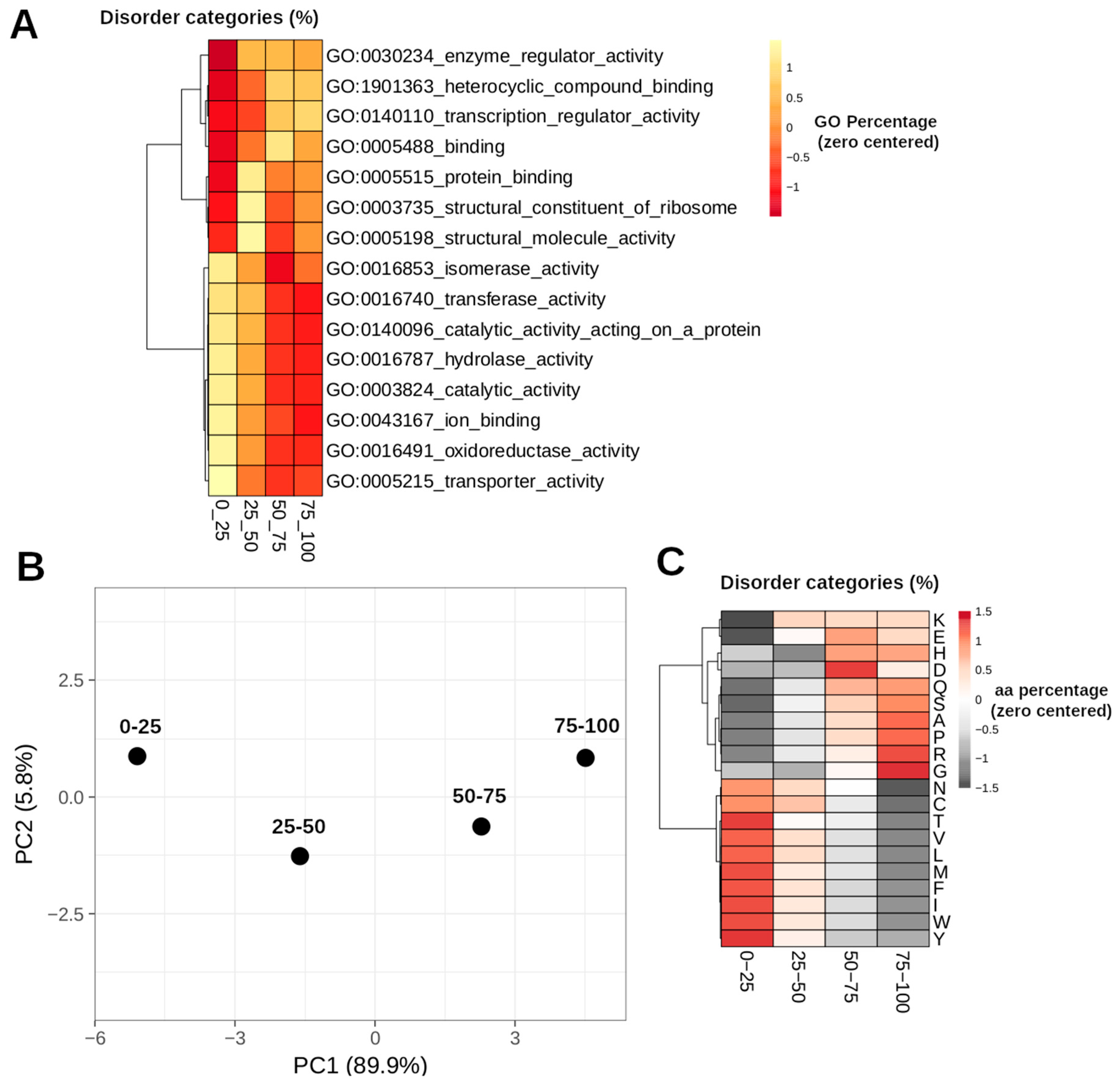
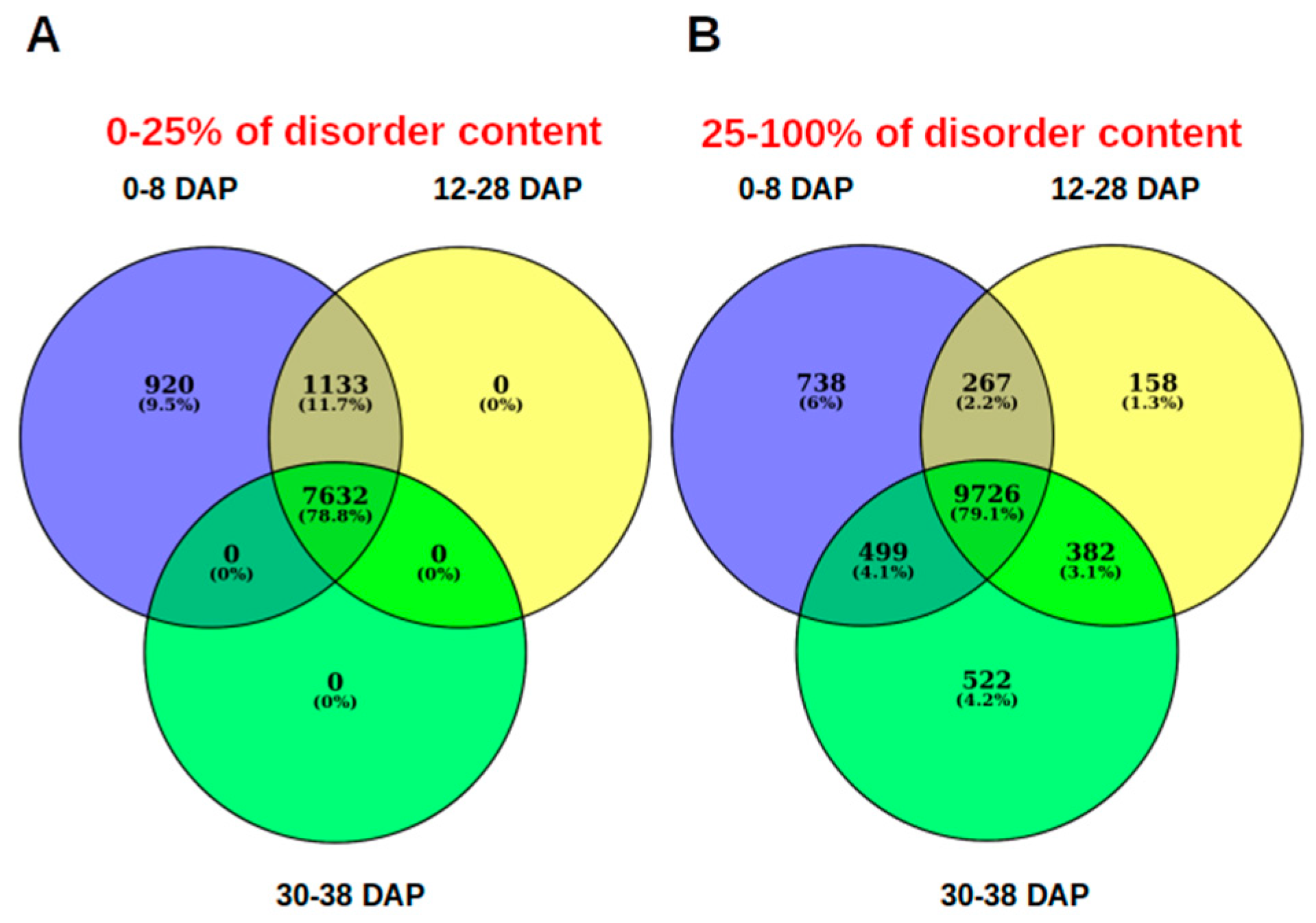
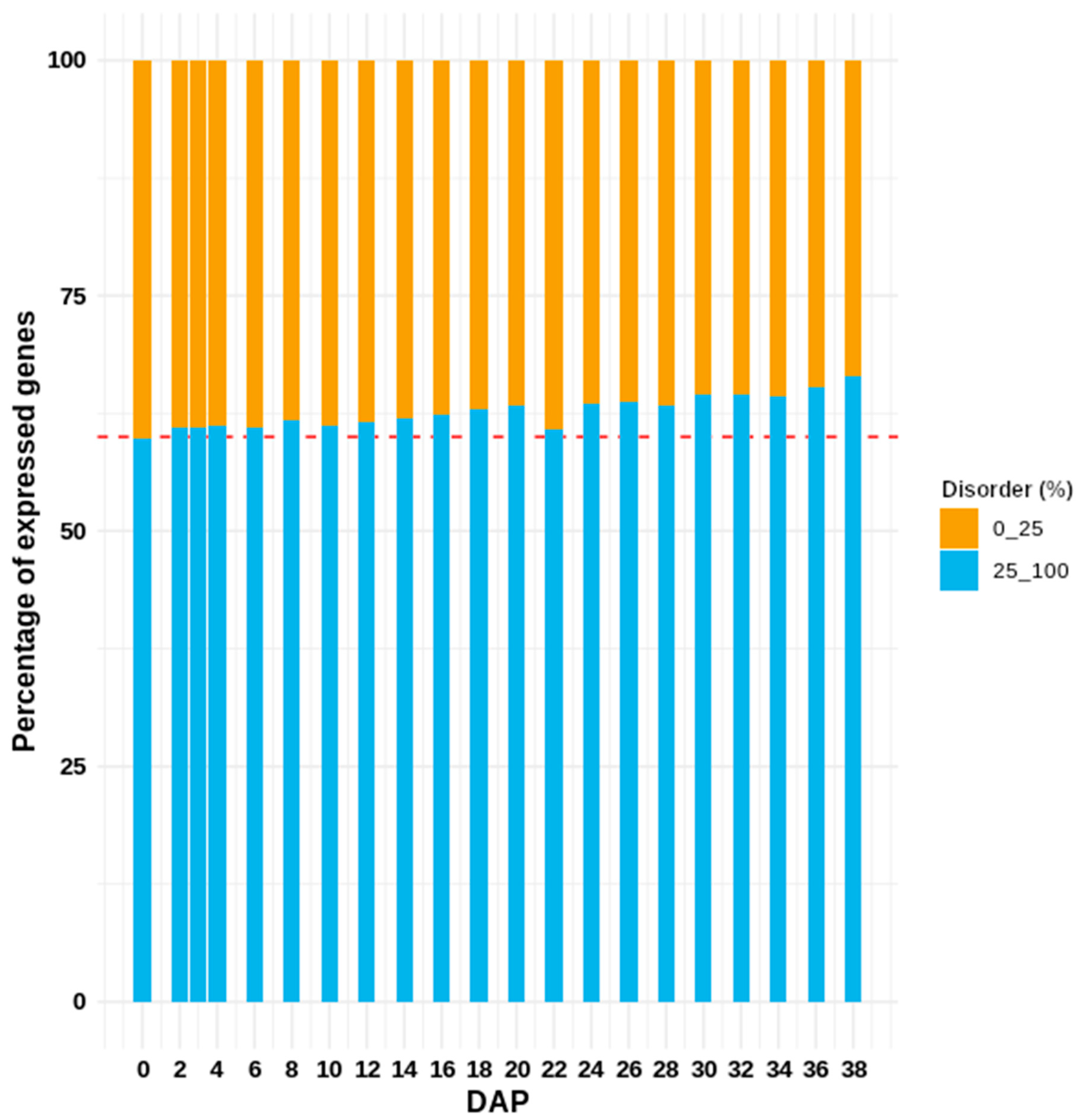
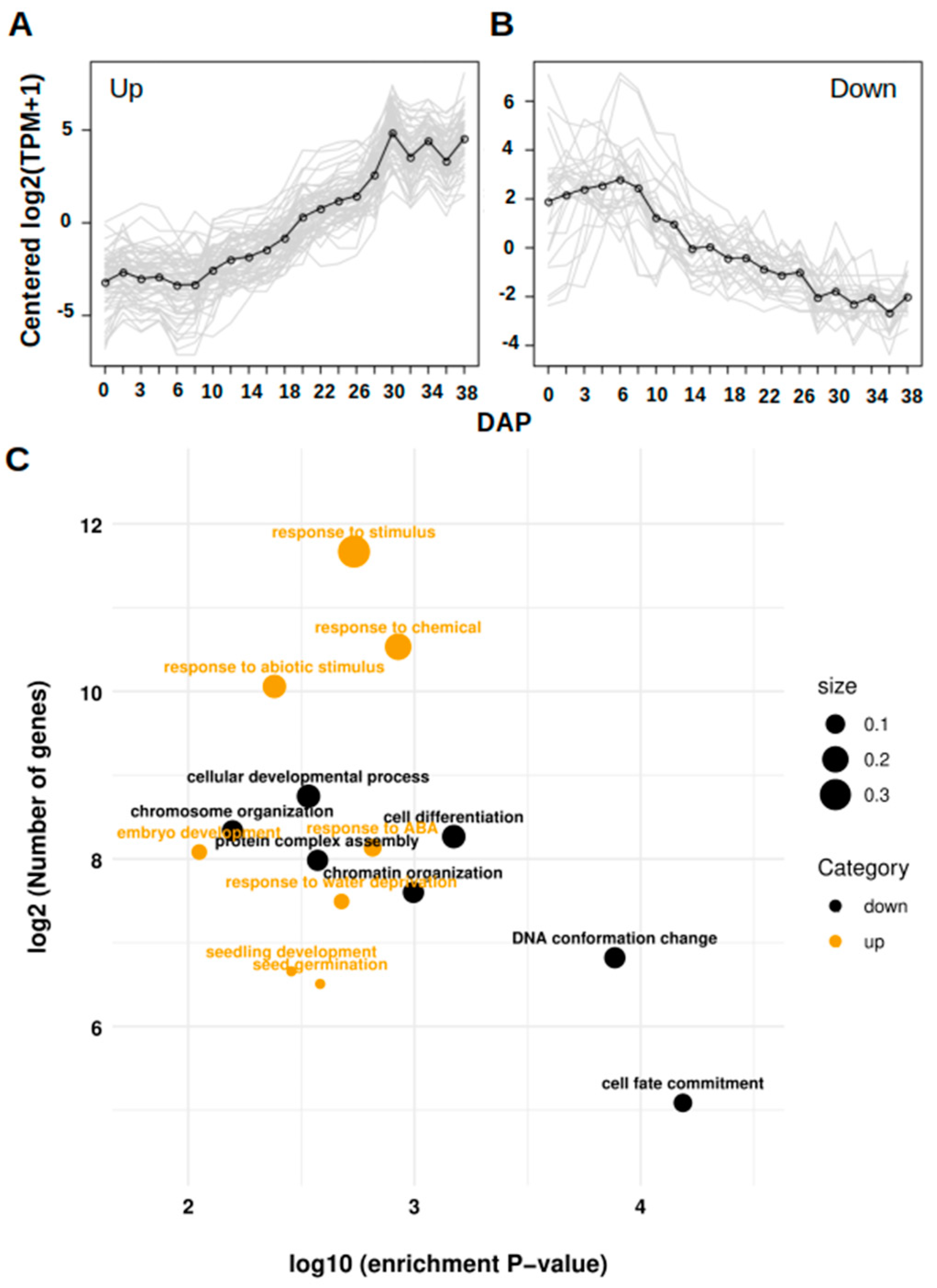
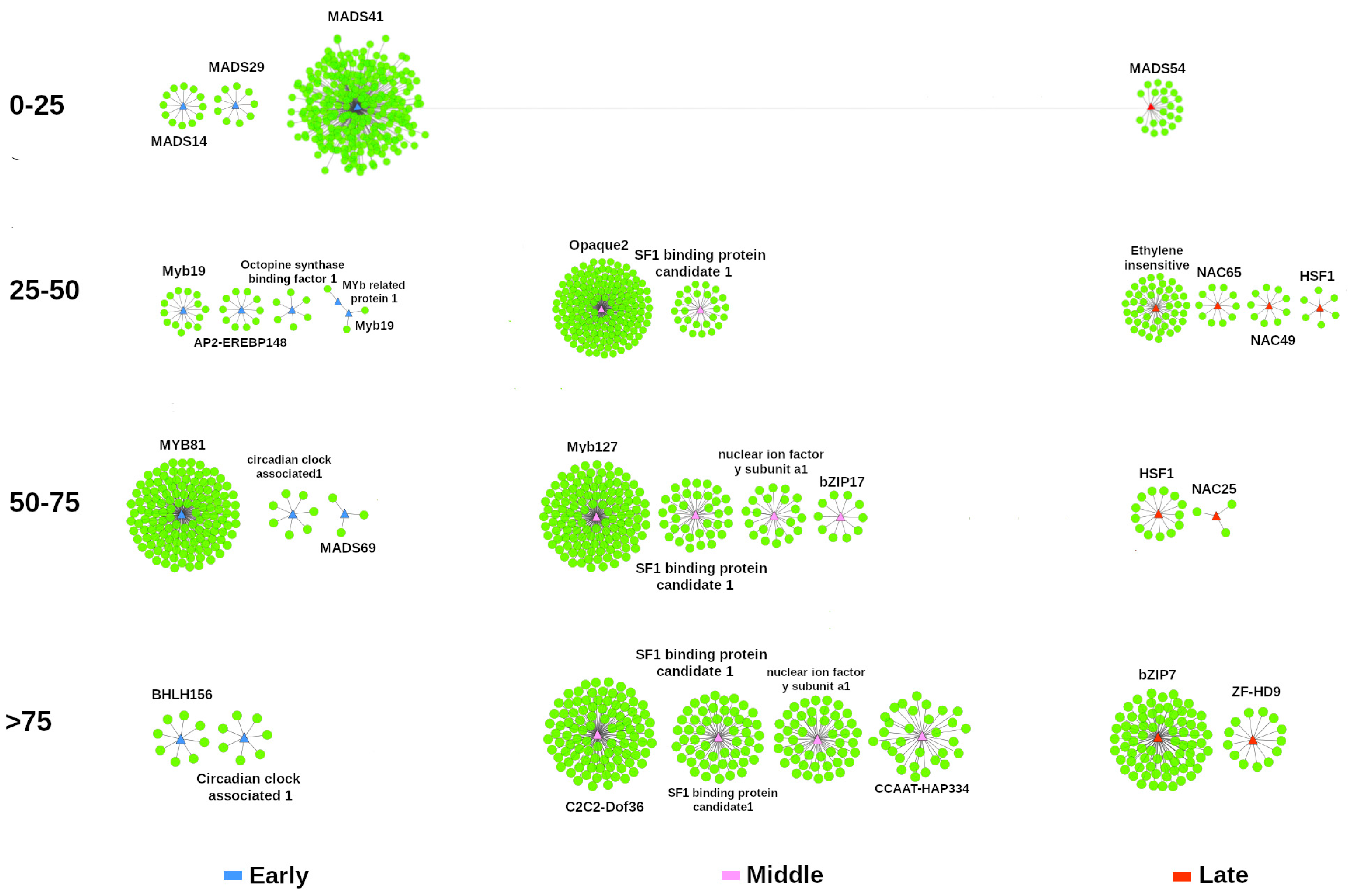
3. Results
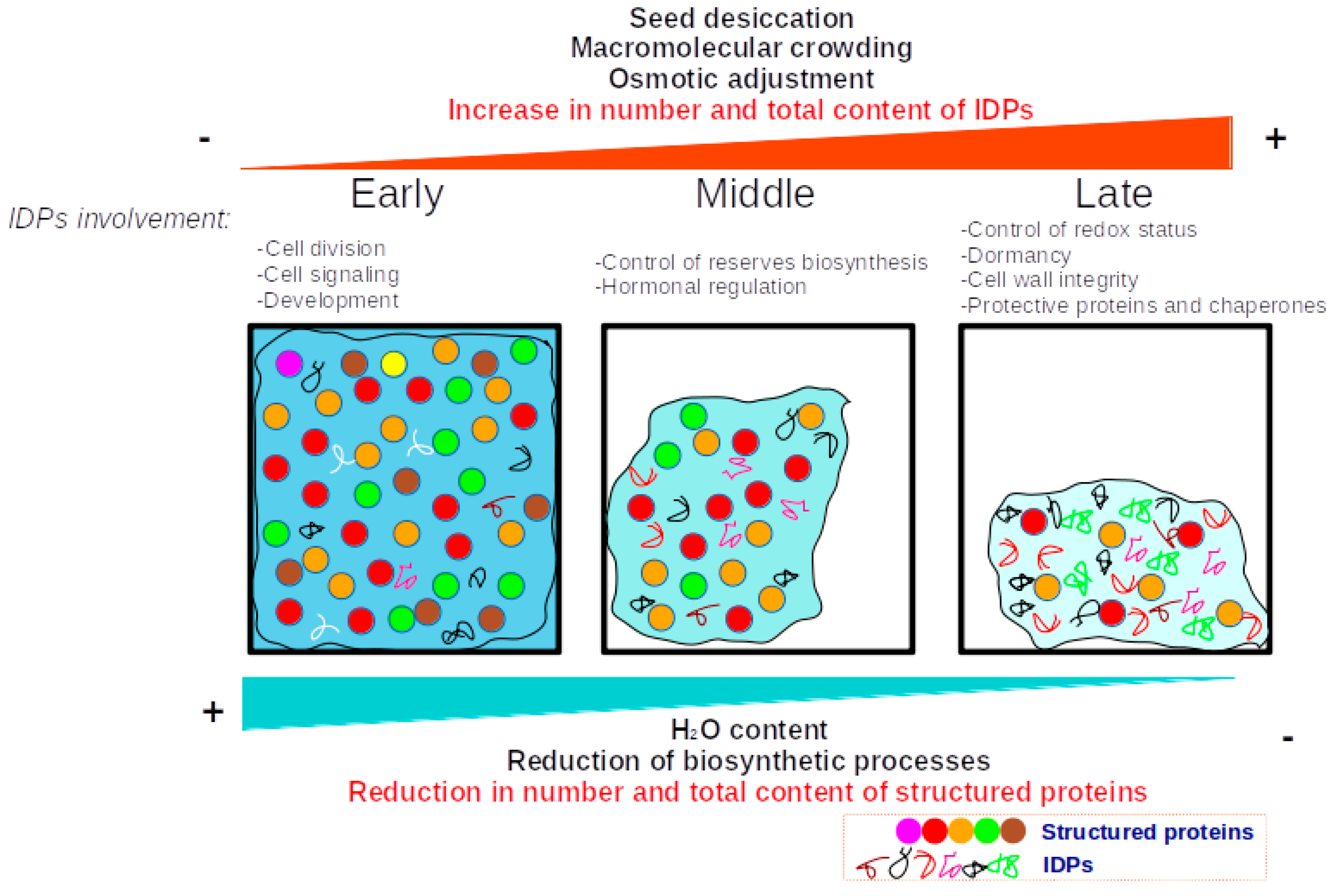
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Gillespie, J.R.; Fink, A.L. Why are ‘natively unfolded’ proteins unstructured under physiologic conditions? Proteins Struct. Funct. Genet. 2000. [Google Scholar] [CrossRef]
- Tompa, P.; Kovacs, D. Intrinsically disordered chaperones in plants and animals. Biochem. Cell Biol. 2010. [Google Scholar] [CrossRef]
- Romero, P.; Obradovic, Z.; Li, X.; Garner, E.C.; Brown, C.J.; Dunker, A.K. Sequence complexity of disordered protein. Proteins Struct. Funct. Genet. 2001. [Google Scholar] [CrossRef]
- Zamora-Briseño, J.A.; Reyes-Hernández, S.J.; Zapata, L.C.R. Does water stress promote the proteome-wide adjustment of intrinsically disordered proteins in plants? Cell Stress Chaperones 2018. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y. Advantages of proteins being disordered. Protein Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Iakoucheva, L.M.; Radivojac, P.; Brown, C.J.; O’Connor, T.R.; Sikes, J.G.; Obradovic, Z.; Dunker, A.K. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004. [Google Scholar] [CrossRef]
- Iakoucheva, L.M.; Brown, C.J.; Lawson, J.D.; Obradović, Z.; Dunker, A.K. Intrinsic disorder in cell-signaling and cancer-associated proteins. J. Mol. Biol. 2002. [Google Scholar] [CrossRef]
- Tompa, P.; Szász, C.; Buday, L. Structural disorder throws new light on moonlighting. Trends Biochem. Sci. 2005. [Google Scholar] [CrossRef]
- Pietrosemoli, N.; García-Martín, J.A.; Solano, R.; Pazos, F. Genome-wide analysis of protein disorder in Arabidopsis thaliana: Implications for plant environmental adaptation. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Alvarez-Ponce, D.; Ruiz-González, M.X.; Vera-Sirera, F.; Feyertag, F.; Perez-Amador, M.A.; Fares, M.A. Arabidopsis heat stress-induced proteins are enriched in electrostatically charged amino acids and intrinsically disordered regions. Int. J. Mol. Sci. 2018, 19, 2276. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I.; Contreras-Moreira, B. Protein disorder in plants: A view from the chloroplast. BMC Plant Biol. 2012, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Schnell, S. A collection of intrinsic disorder characterizations from eukaryotic proteomes. Sci. Data 2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Sun, N.; Tu, C.; Shi, X.; Cheng, H.; Liu, S.; Li, S.; Wang, Y.; Zheng, Y.; et al. Intrinsically disordered proteins as important players during desiccation stress of soybean radicles. J. Proteome Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pazos, F.; Pietrosemoli, N.; García-Martín, J.A.; Solano, R. Protein intrinsic disorder in plants. Front. Plant Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Kalmar, E.; Torok, Z.; Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 2008. [Google Scholar] [CrossRef]
- Kovacs, D.; Agoston, B.; Tompa, P. Disordered plant LEA proteins as molecular chaperones. Plant Signal. Behav. 2008. [Google Scholar] [CrossRef] [PubMed]
- Mouillon, J.M.; Eriksson, S.K.; Harryson, P. Mimicking the plant cell interior under water stress by macromolecular crowding: Disordered dehydrin proteins are highly resistant to structural collapse. Plant Physiol. 2008. [Google Scholar] [CrossRef]
- Manfre, A.J.; LaHatte, G.A.; Climer, C.R.; Marcotte, W.R. Seed dehydration and the establishment of desiccation tolerance during seed maturation is altered in the Arabidopsis thaliana mutant atem6-1. Plant Cell Physiol. 2009. [Google Scholar] [CrossRef]
- Chakrabortee, S.; Boschetti, C.; Walton, L.J.; Sarkar, S.; Rubinsztein, D.C.; Tunnacliffe, A. Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc. Natl. Acad. Sci. USA 2007. [Google Scholar] [CrossRef]
- Das, R.K.; Ruff, K.M.; Pappu, R.V. Relating sequence encoded information to form and function of intrinsically disordered proteins. Curr. Opin. Struct. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santana, A.; Alvarado-Robledo, E.J.; Zamora-Briseño, J.A.; Ayala-Sumuano, J.T.; Gonzalez-Mendoza, V.M.; Espadas-Gil, F.; Alcaraz, L.D.; Castaño, E.; Keb-Llanes, M.A.; Sanchez-Teyer, F.; et al. Transcriptional profiling of sugarcane leaves and roots under progressive osmotic stress reveals a regulated coordination of gene expression in a spatiotemporal manner. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Le, N.T.; Brandt, W.F.; Driouich, A.; Farrant, J.M. Towards a systems-based understanding of plant desiccation tolerance. Trends Plant Sci. 2009. [Google Scholar] [CrossRef] [PubMed]
- Nannas, N.J.; Kelly Dawe, R. Genetic and genomic toolbox of Zea mays. Genetics 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, B.; Zhang, M.; Xie, S.; Wang, G.; Hauck, A.; Lai, J. Dynamic transcriptome landscape of maize embryo and endosperm development. Plant Physiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Prioul, J.L.; Méchin, V.; Lessard, P.; Thévenot, C.; Grimmer, M.; Chateau-Joubert, S.; Coates, S.; Hartings, H.; Kloiber-Maitz, M.; Murigneux, A.; et al. A joint transcriptomic, proteomic and metabolic analysis of maize endosperm development and starch filling. Plant Biotechnol. J. 2008. [Google Scholar] [CrossRef]
- Yi, F.; Gu, W.; Chen, J.; Song, N.; Gao, X.; Zhang, X.; Zhou, Y.; Ma, X.; Song, W.; Zhao, H.; et al. High temporal-resolution transcriptome landscape of early maize seed development. Plant Cell 2019. [Google Scholar] [CrossRef]
- Silva-Sanchez, C.; Chen, S.; Li, J.; Chourey, P.S. A comparative glycoproteome study of developing endosperm in the hexose-deficient miniature1 (mn1) seed mutant and its wild type Mn1 in maize. Front. Plant Sci. 2014. [Google Scholar] [CrossRef]
- Walsh, I.; Martin, A.J.; Di Domenico, T.; Tosatto, S.C. Espritz: Accurate and fast prediction of protein disorder. Bioinformatics 2012. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using principal Component Analysis and heatmap. Nucleic Acids Res. 2015. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006. [Google Scholar] [CrossRef] [PubMed]
- Hanway, J. How a Corn Plant Develops. Sci. Technol. 1966, 38. Special Report. 38. Available online: http://lib.dr.iastate.edu/specialreports/3 (accessed on 24 June 2019).
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009. [Google Scholar] [CrossRef]
- Aibar, S.; González-Blas, C.B.; Moerman, T.; Huynh-Thu, V.; Imrichova, H.; Hulselmans, G.; Rambow, F.; Marine, J.C.; Geurts, P.; Aerts, J.; et al. SCENIC: Single-cell regulatory network inference and clustering. Nat. Methods 2017. [Google Scholar] [CrossRef] [PubMed]
- Huynh-Thu, V.A.; Irrthum, A.; Wehenkel, L.; Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 2010. [Google Scholar] [CrossRef]
- Breiman, L. Statistical Modeling: The two cultures (with comments and a rejoinder by the author). Stat. Sci. 2002. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003. [Google Scholar] [CrossRef]
- Su, G.; Kuchinsky, A.; Morris, J.H.; States, D.J.; Meng, F. GLay: Community structure analysis of biological networks. Bioinformatics 2010. [Google Scholar] [CrossRef]
- Vernoud, V.; Hajduch, M.; Khaled, A.S.; Depège, N.; Rogowsky, P.M. Maize embryogenesis. Maydica 2005, 50, 469. [Google Scholar]
- Arvidsson, G.; Wright, A.P.H. A protein intrinsic disorder approach for characterising differentially expressed genes in transcriptome data: Analysis of cell-adhesion regulated gene expression in lymphoma cells. Int. J. Mol. Sci. 2018, 19, 3101. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortee, S.; Meersman, F.; Kaminski Schierle, G.S.; Bertoncini, C.W.; McGee, B.; Kaminski, C.F.; Tunnacliffe, A. Catalytic and chaperone-like functions in an intrinsically disordered protein associated with desiccation tolerance. Proc. Natl. Acad. Sci. USA 2010. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Rikkerink EH, A.; Jones, W.T.; Uversky, V.N. Multifarious roles of intrinsic disorder in proteins illustrate its broad impact on plant biology. Plant Cell 2013. [Google Scholar] [CrossRef] [PubMed]
- Boothby, T.C.; Tapia, H.; Brozena, A.H.; Piszkiewicz, S.; Smith, A.E.; Giovannini, I.; Rebecchi, L.; Pielak, G.J.; Koshland, D.; Goldstein, B. Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell 2017. [Google Scholar] [CrossRef]
- Boothby, T.C.; Pielak, G.J. Intrinsically isordered proteins and desiccation tolerance: Elucidating functional and mechanistic underpinnings of anhydrobiosis. BioEssays 2017. [Google Scholar] [CrossRef]
- Wetzler, D.E.; Fuchs Wightman, F.; Bucci, H.A.; Rinaldi, J.; Caramelo, J.J.; Iusem, N.D.; Ricardi, M.M. Conformational plasticity of the intrinsically disordered protein asr1 modulates its function as a drought stress-responsive gene. PLoS ONE 2018. [Google Scholar] [CrossRef]
- Zamora-Briseño, J.A.; de Jiménez, E.S. A LEA 4 protein up-regulated by ABA is involved in drought response in maize roots. Mol. Biol. Rep. 2016. [Google Scholar] [CrossRef]
- Carugo, O. Amino acid composition and protein dimension. Protein Sci. 2008. [Google Scholar] [CrossRef]
- Niklas, K.J.; Bondos, S.E.; Dunker, A.K.; Newman, S.A. Rethinking gene regulatory networks in light of alternative splicing, intrinsically disordered protein domains, and post-translational modifications. Front. Cell Dev. Biol. 2015. [Google Scholar] [CrossRef]
- Minezaki, Y.; Homma, K.; Kinjo, A.R.; Nishikawa, K. Human transcription factors contain a high fraction of intrinsically disordered regions essential for transcriptional regulation. J. Mol. Biol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Yizhi, Z.; Hélène, L.; Antoine, S.; Régine, L.; Brigitte, G. Exploring intrinsically disordered proteins in chlamydomonas reinhardtii. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically disordered proteins. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Academic Press: San Diego, CA, USA, 2013. [Google Scholar] [CrossRef]
- Williams, R.M.; Obradovi, Z.; Mathura, V.; Braun, W.; Garner, E.C.; Young, J.; Takayama, S.; Brown, C.J.; Dunker, A.K. The protein non-folding problem: amino acid determinants of intrinsic order and disorder. In Proceedings of the Pacific Symposium on Biocomputing, Mauna Lani, HI, USA, 3–7 January 2001. [Google Scholar]
- Méchin, V.; Balliau, T.; Château-Joubert, S.; Davanture, M.; Langella, O.; Négroni, L.; Prioul, J.L.; Thévenot, C.; Zivy, M.; Damerval, C. A two-dimensional proteome map of maize endosperm. Phytochemistry 2004. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Ji, H.Q.; Cui, Z.T.; Wu, X.; Duan, L.J.; Feng, X.X.; Tang, J.H. QTL detected for grain-filling rate in maize using a RIL population. Mol. Breed. 2011. [Google Scholar] [CrossRef]
- Jin, X.; Fu, Z.; Ding, D.; Li, W.; Liu, Z.; Tang, J. Proteomic identification of genes associated with maize grain-filling rate. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Esen, A. A proposed nomenclature for the alcohol-soluble proteins (zeins) of maize (Zea mays L.). J. Cereal Sci. 1987. [Google Scholar] [CrossRef]
- Coleman, C.E.; Herman, E.M.; Takasaki, K.; Larkins, B.A. The Maize g-Zein Sequesters a-Zein and Stabilizes Its Accumulation in Protein Bodies of Transgenic Tobacco Endosperm. Plant Cell 2007. [Google Scholar] [CrossRef]
- Wilson, C.M. Serial analysis of Zein by isoelectric focusing and sodium dodecyl sulfate gel electrophoresis. Plant Physiol. 2008. [Google Scholar] [CrossRef]
- Woo, Y.M.; Hu, D.W.; Larkins, B.A.; Jung, R. Genomics analysis of genes expressed in maize endosperm identifies novel seed proteins and clarifies patterns of zein gene expression. Plant Cell 2001, 13, 2297–2317. [Google Scholar] [CrossRef]
- Li, J.S.; Vasal, S.K. Maize: Quality protein maize. In Encyclopedia of Food Grains, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; eBook; ISBN 9780123947864. [Google Scholar] [CrossRef]
- Meng, Y.; Cloutier, S. Gelatin and Other Proteins for Microencapsulation. In Microencapsulation in the Food Industry; Academic Press: Cambridge, MA, USA, 2014; eBook; ISBN 9780124047358. [Google Scholar] [CrossRef]
- Song, R.; Llaca, V.; Linton, E.; Messing, J. Sequence, regulation, and evolution of the maize 22-kD alpha zein gene family. Genome Res. 2001, 11, 1817–1825. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Ketudat, M.; Aukerman, M.J.; Hoschek, G. Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell 2007. [Google Scholar] [CrossRef]
- Gibbon, B.C.; Larkins, B.A. Molecular genetic approaches to developing quality protein maize. Trends Genet. 2005. [Google Scholar] [CrossRef] [PubMed]
- Devic, M.; Roscoe, T. Seed maturation: Simplification of control networks in plants. Plant Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Mizianty, M.J.; Xue, B.; Kurgan, L.; Uversky, V.N. More than just tails: Intrinsic disorder in histone proteins. Mol. Biosyst. 2012. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R. HMG nuclear proteins: Linking chromatin structure to cellular phenotype. Biochim. Biophys. Acta 2009. [Google Scholar] [CrossRef]
- Slama-Schwok, A.; Zakrzewska, K.; Léger, G.; Leroux, Y.; Takahashi, M.; Käs, E.; Debey, P. Structural changes induced by binding of the high-mobility group I protein to a mouse satellite DNA sequence. Biophys. J. 2000. [Google Scholar] [CrossRef]
- Love, J.J.; Li, X.; Chung, J.; Dyson, H.J.; Wright, P.E. The LEF-1 high-mobility group domain undergoes a disorder-to-order transition upon formation of a complex with cognate DNA. Biochemistry 2004. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Ge, J.; Xu, M.; Zhu, Q.; Wu, T.; Guo, A.; Xie, J.; Dong, H. Recessive Mutation Identifies Auxin-Repressed Protein ARP1, Which Regulates Growth and Disease Resistance in Tobacco. Mol. Plant Microbe Interact. 2014. [Google Scholar] [CrossRef]
- Shi, H.Y.; Zhang, Y.X.; Chen, L. Two pear auxin-repressed protein genes, PpARP1 and PpARP2, are predominantly expressed in fruit and involved in response to salicylic acid signaling. Plant Cell Tissue Organ Cult. 2013. [Google Scholar] [CrossRef]
- Wu, L.; Yu, M.; Holowachuk, J.; Sharpe, A.; Lydiate, D.; Dwayne, H.; Gruber, M. Evaluation of a Brassica napus auxin-repressed gene induced by flea beetle damage and Sclerotinia sclerotiorum infection. Am. J. Plant Sci. 2017. [Google Scholar] [CrossRef][Green Version]
- Borisjuk, N.; Sitailo, L.; Adler, K.; Malysheva, L.; Tewes, A.; Borisjuk, L.; Manteuffel, R. Calreticulin expression in plant cells: Developmental regulation, tissue specificity and intracellular distribution. Planta 1998. [Google Scholar] [CrossRef] [PubMed]
- High, S.; Lecomte, F.J.L.; Russell, S.J.; Abell, B.M.; Oliver, J.D. Glycoprotein folding in the endoplasmic reticulum: A tale of three chaperones? FEBS Lett. 2000. [Google Scholar] [CrossRef]
- Varricchio, L.; Falchi, M.; Dall’Ora, M.; De Benedittis, C.; Ruggeri, A.; Uversky, V.N.; Migliaccio, A.R. Calreticulin: Challenges posed by the intrinsically disordered nature of calreticulin to the study of its function. Front. Cell Dev. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xi, J.; Du, L.; Poovaiah, B.W. The function of calreticulin in plant immunity: New discoveries for an old protein. Plant Signal. Behav. 2012. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009. [Google Scholar] [CrossRef] [PubMed]
- Dure, L.; Galau, G.A. Developmental biochemistry of cottonseed embryogenesis and germination 1. Plant Physiol. 1981. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008. [Google Scholar] [CrossRef] [PubMed]
- Delahaie, J.; Hundertmark, M.; Bove, J.; Leprince, O.; Rogniaux, H.; Buitink, J. LEA polypeptide profiling of recalcitrant and orthodox legume seeds reveals ABI3-regulated LEA protein abundance linked to desiccation tolerance. J. Exp. Bot. 2013. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Carrillo, Y.; Luis Reyes, J.; Covarrubias, A.A. Late embryogenesis abundant proteins. Plant Signal. Behav. 2011. [Google Scholar] [CrossRef] [PubMed]
- Thomann, E.B.; Sollinger, J.; White, C.; Rivin, C.J. Accumulation of group 3 late embryogenesis abundant proteins in Zea mays embryos: Roles of abscisic acid and the viviparous-1 gene product. Plant Physiol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kwak, K.J.; Oh, T.R.; Kim, Y.O.; Kang, H. Cold shock domain proteins affect seed germination and growth of Arabidopsis thaliana under abiotic stress conditions. Plant Cell Physiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, J.; Yang, Z.; Zhao, C.; Zhu, M.; Ma, D.; Dong, T.; Zhou, Z.; Liu, M.; Yang, D.; et al. Genome-wide identification and expression analysis of glycine-rich RNA-binding protein family in sweet potato wild relative Ipomoea trifida. Gene 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, W.Y.; Kwak, K.J.; Oh, S.H.; Han, Y.S.; Kang, H. Glycine-rich RNA-binding proteins are functionally conserved in Arabidopsis thaliana and Oryza sativa during cold adaptation process. J. Exp. Bot. 2010. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Holá, D.; Fischer, L.; Jedelský, P.L.; Hnilička, F.; Wilhelmová, N.; Rothová, O.; Kočová, M.; Procházková, D.; Honnerová, J.; et al. The physiology and proteomics of drought tolerance in Maize: Early stomatal closure as a cause of lower tolerance to short-term dehydration? PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Checker, V.G.; Khurana, P. Molecular and functional characterization of mulberry EST encoding remorin (MiREM) involved in abiotic stress. Plant Cell Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.S.; Mittal, N.; Randhawa, G.S. Cyamopsis tetragonoloba type 1 metallothionein (CtMT1) gene is upregulated under drought stress and its protein product has an additional C-X-C motif and unique metal binding pattern. Int. J. Biol. Macromol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Theißen, G.; Gramzow, L. Chapter 8—Structure and Evolution of Plant MADS Domain Transcription Factors A2. In Plant Transcription Factors; Gonzalez, D.H., Ed.; Academic Press: Cambridge, MA, USA, 2016; eBook; ISBN 9780128011270. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010. [Google Scholar] [CrossRef] [PubMed]
- Hartings, H.; Lauria, M.; Lazzaroni, N.; Pirona, R.; Motto, M. The Zea mays mutants opaque-2 and opaque-7 disclose extensive changes in endosperm metabolism as revealed by protein, amino acid, and transcriptome-wide analyses. BMC Genom. 2011. [Google Scholar] [CrossRef] [PubMed]
- Motto, M.; Thompson, R.; Salamini, F. Genetic regulation of carbohydrate and protein accumulation in seeds. In Cellular and Molecular Biology of Plant Seed Development; Springer: Heidelberg, Germany, 1997. [Google Scholar] [CrossRef]
- Brochetto-Braga, M.R.; Leite, A.; Arruda, P. Partial purification and characterization of lysine-ketoglutarate reductase in normal and opaque-2 Maize endosperms. Plant Physiol. 2008. [Google Scholar] [CrossRef][Green Version]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santana, A.; Alcaraz, L.D.; Castaño, E.; Sanchez-Calderon, L.; Sanchez-Teyer, F.; Rodriguez-Zapata, L. Comparative genomics of NAC transcriptional factors in angiosperms: Implications for the adaptation and diversification of flowering plants. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Mochida, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; You, J.; Xie, K.; Xie, W.; Xiong, L. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol. Genet. Genom. 2008. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wei, Y.; Xia, Z.; Yan, Y.; Hou, X.; Zou, M.; Lu, C.; Wang, W.; Peng, M. Genome-wide identification and expression analysis of the NAC transcription factor family in Cassava. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tripp, J.; Winkelhaus, S.; Tschiersch, B.; Theres, K.; Nover, L.; Scharf, K.D. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev. 2002. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Mittler, R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006. [Google Scholar] [CrossRef]
- Ying, S.; Zhang, D.F.; Fu, J.; Shi, Y.S.; Song, Y.C.; Wang, T.Y.; Li, Y. Cloning and characterization of a maize bZIP transcription factor, ZmbZIP72, confers drought and salt tolerance in transgenic Arabidopsis. Planta 2012. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Briseño, J.A.; Pereira-Santana, A.; Reyes-Hernández, S.J.; Castaño, E.; Rodríguez-Zapata, L.C. Global Dynamics in Protein Disorder during Maize Seed Development. Genes 2019, 10, 502. https://doi.org/10.3390/genes10070502
Zamora-Briseño JA, Pereira-Santana A, Reyes-Hernández SJ, Castaño E, Rodríguez-Zapata LC. Global Dynamics in Protein Disorder during Maize Seed Development. Genes. 2019; 10(7):502. https://doi.org/10.3390/genes10070502
Chicago/Turabian StyleZamora-Briseño, Jesús Alejandro, Alejandro Pereira-Santana, Sandi Julissa Reyes-Hernández, Enrique Castaño, and Luis Carlos Rodríguez-Zapata. 2019. "Global Dynamics in Protein Disorder during Maize Seed Development" Genes 10, no. 7: 502. https://doi.org/10.3390/genes10070502
APA StyleZamora-Briseño, J. A., Pereira-Santana, A., Reyes-Hernández, S. J., Castaño, E., & Rodríguez-Zapata, L. C. (2019). Global Dynamics in Protein Disorder during Maize Seed Development. Genes, 10(7), 502. https://doi.org/10.3390/genes10070502




