Exploring Molecular Signs of Sex in the Marine Diatom Skeletonema marinoi
Abstract
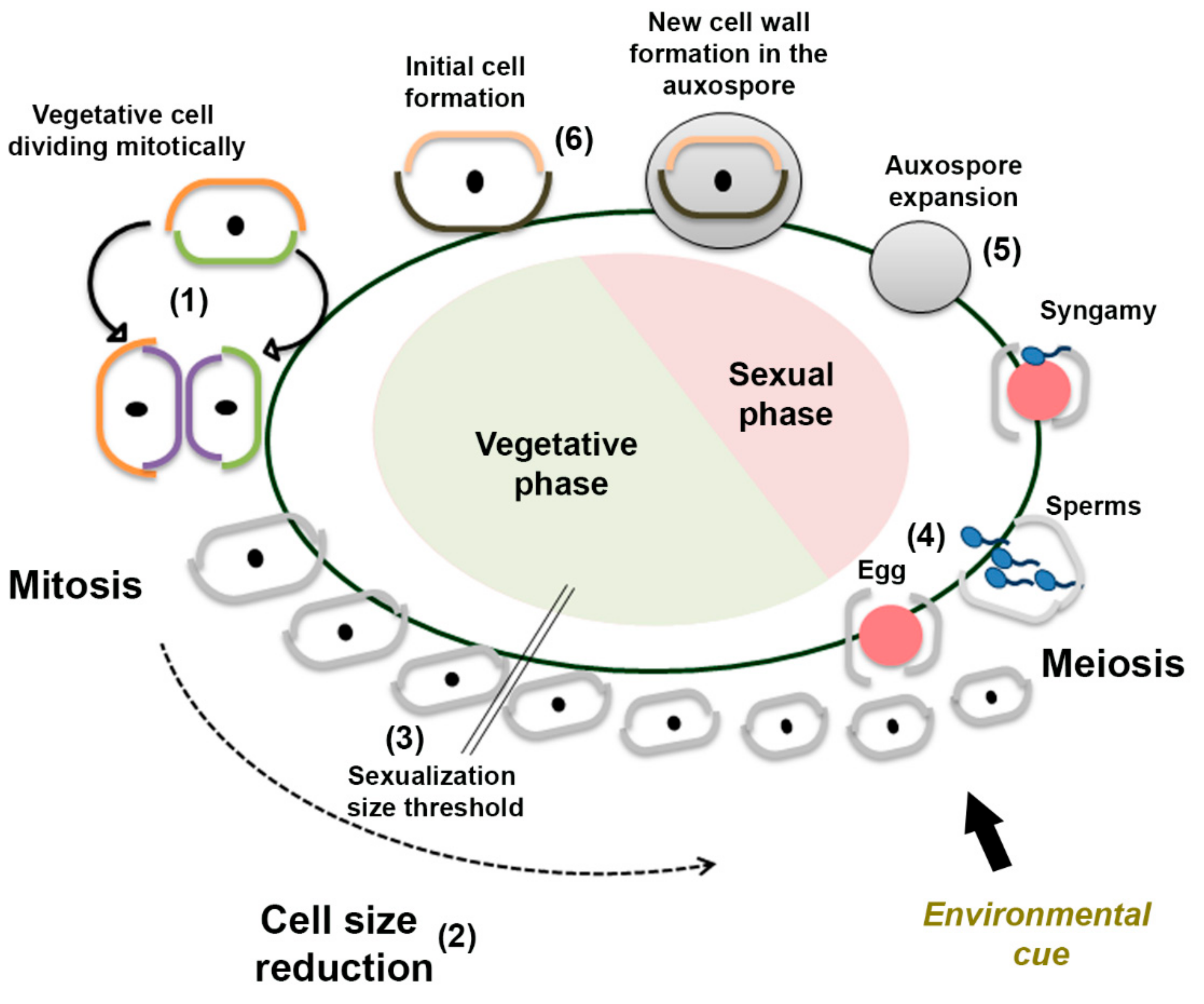
1. Introduction
2. Materials and Methods
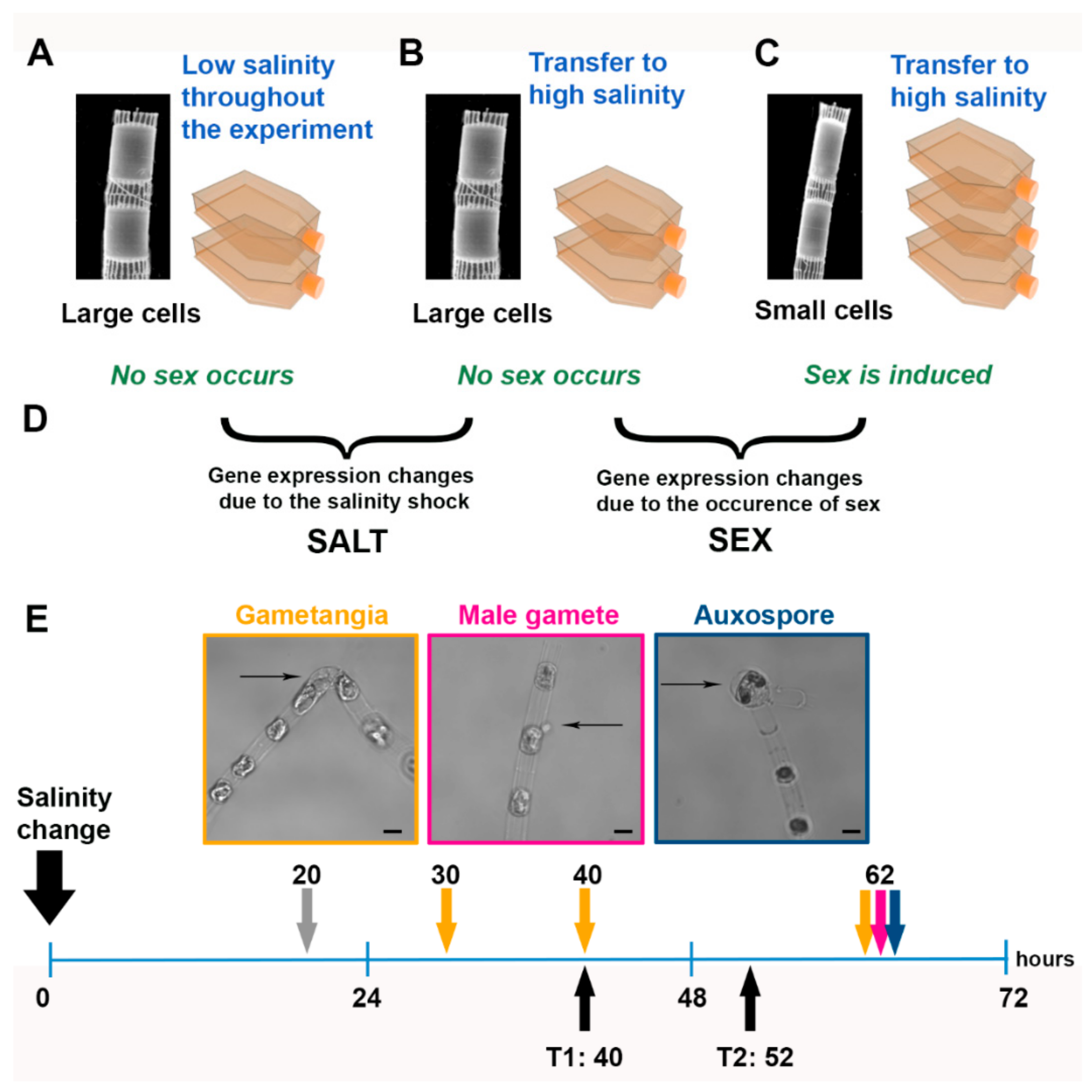
2.1. Experimental Setup
2.2. RNA Extraction and RNA-Seq
2.3. Transcriptome Assembly and Annotation
2.4. Differential Expression Analysis
2.5. GO Enrichment Analysis
2.6. Searching for Sex-Related Genes in S. marinoi
2.7. Data Deposition
3. Results
3.1. Sexual Reproduction Induced by a Change in Salinity
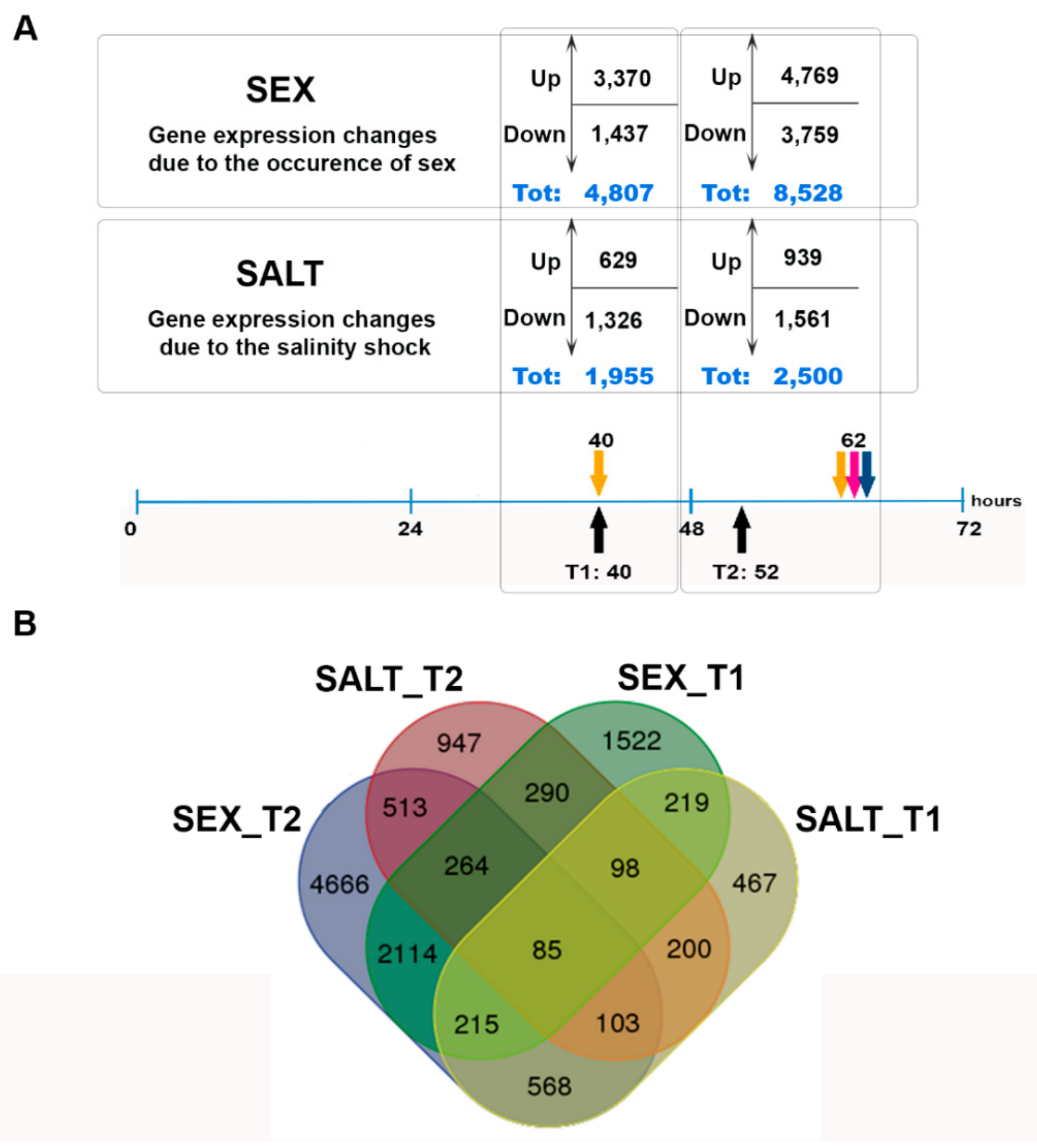
3.2. De Novo Assembly, Differential Gene Expression Analysis, and GO Enrichment Analysis
3.3. Meiosis and Flagellar Genes in the Sexualized Cultures
3.4. Homologies with Sex-Related Genes of the Pennate Diatom Pseudo-nitzschia multistriata and of the Centric Diatom Thalassiosira weissflogii
3.5. Other Major Transcriptomic Changes
4. Discussion
4.1. Meiosis-Related Genes
4.2. Flagellar Genes
4.3. Other Sex Related Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Speijer, D.; Lukeš, J.; Eliáš, M. Sex is a ubiquitous, ancient, and inherent attribute of eukaryotic life. Proc. Natl. Acad. Sci. USA 2015, 112, 8827–8834. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, U.; Heitman, J. Origins of eukaryotic sexual reproduction. Cold Spring Harbor Perspect. Biol. 2014, 6, a016154. [Google Scholar] [CrossRef] [PubMed]
- Speijer, D. What can we infer about the origin of sex in early eukaryotes? Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150530. [Google Scholar] [CrossRef] [PubMed]
- Treguer, P.; Bowler, C.; Moriceau, B.; Dutkiewicz, S.; Gehlen, M.; Aumont, O.; Bittner, L.; Dugdale, R.; Finkel, Z.; Iudicone, D.; et al. Influence of diatom diversity on the ocean biological carbon pump. Nat. Geosci. 2018, 11, 27–37. [Google Scholar] [CrossRef]
- Montresor, M.; Vitale, L.; D‘Alelio, D.; Ferrante, M.I. Sex in marine planktonic diatoms: Insights and challenges. Perspect. Phycol. 2016, 3, 61–75. [Google Scholar] [CrossRef]
- Kooistra, W.H.C.F.; Gersonde, R.; Medlin, L.K.; Mann, D.G. The origin and evolution of the diatoms: Their adaptation to a planktonic existence. In Evolution of Primary Producers in the Sea; Falkowski, P.G., Knoll, A.H., Eds.; Elsevier Academic Press: Burlington, VT, USA, 2007; pp. 207–250. [Google Scholar]
- Chepurnov, V.A.; Mann, D.G.; Sabbe, K.; Vyverman, W. Experimental studies on sexual reproduction in diatoms. Int. Rev. Cytol. 2004, 237, 91–154. [Google Scholar]
- Godhe, A.; Kremp, A.; Montresor, M. Genetic and microscopic evidence for sexual reproduction in the centric diatom Skeletonema marinoi. Protist 2014, 165, 401–416. [Google Scholar] [CrossRef]
- Gillard, J.; Frenkel, J.; Devos, V.; Sabbe, K.; Paul, C.; Rempt, M.; Inze, D.; Pohnert, G.; Vuylsteke, M.; Vyverman, W. Metabolomics enables the structure elucidation of a diatom sex pheromone. Angew. Chem. 2013, 52, 854–857. [Google Scholar] [CrossRef]
- Moeys, S.; Frenkel, J.; Lembke, C.; Gillard, J.T.F.; Devos, V.; Van den Berge, K.; Bouillon, B.; Huysman, M.J.J.; De Decker, S.; Scharf, J.; et al. A sex-inducing pheromone triggers cell cycle arrest and mate attraction in the diatom Seminavis robusta. Sci. Rep. 2016, 6, 19252. [Google Scholar] [CrossRef]
- Scalco, E.; Stec, K.; Iudicone, D.; Ferrante, M.I.; Montresor, M. The dynamics of sexual phase in the marine diatom Pseudo-nitzschia multistriata (Bacillariophyceae). J. Phycol. 2014, 50, 817–828. [Google Scholar] [CrossRef]
- Basu, S.; Patil, S.; Mapleson, D.; Russo, M.T.; Vitale, L.; Fevola, C.; Maumus, F.; Casotti, R.; Mock, T.; Caccamo, M.; et al. Finding a partner in the ocean: Molecular and evolutionary bases of the response to sexual cues in a planktonic diatom. New Phytol. 2017, 215, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Moeys, S.; von Dassow, P.; Huysman, M.J.J.; Mapleson, D.; De Veylder, L.; Sanges, R.; Vyverman, W.; Montresor, M.; Ferrante, M.I. Identification of the meiotic toolkit in diatoms and exploration of meiosis-specific SPO11 and RAD51 homologs in the sexual species Pseudo-nitzschia multistriata and Seminavis robusta. BMC Genom. 2015, 16, 930. [Google Scholar] [CrossRef] [PubMed]
- Nanjappa, D.; Sanges, R.; Ferrante, M.I.; Zingone, A. Diatom flagellar genes and their expression during sexual reproduction in Leptocylindrus danicus. BMC Genom. 2017, 18, 813. [Google Scholar] [CrossRef] [PubMed]
- Von Dassow, P.; Ogata, H.; Probert, I.; Wincker, P.; Da Silva, C.; Audic, S.; Claverie, J.M.; de Vargas, C. Transcriptome analysis of functional differentiation between haploid and diploid cells of Emiliania huxleyi, a globally significant photosynthetic calcifying cell. Genome Biol. 2009, 10, R114. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E.V. Identification of a new gene family expressed during the onset of sexual reproduction in the centric diatom Thalassiosira weissflogii. Appl. Environ. Microbiol. 1999, 65, 3121–3128. [Google Scholar] [PubMed]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Andrews, S. FastQC: A quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 7 March 2014).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 11, 1658–1659. [Google Scholar] [CrossRef]
- Smith-Unna, R.D.; Boursnell, C.; Patro, R.; Hibberd, J.M.; Kelly, S. TransRate: Reference free quality assessment of de-novo transcriptome assemblies. Genome Res. 2016, 11, 1134–1144. [Google Scholar] [CrossRef]
- Armbrust, E.V.; Berges, J.A.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.; Allen, A.E.; Apt, K.E.; Bechner, M.; et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 2004, 306, 79–86. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Colin, N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2012, 12, 323. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, e121. [Google Scholar] [CrossRef] [PubMed]
- Katoh, S. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Von Dassow, P.; John, U.; Ogata, H.; Probert, I.; Bendif, E.M.; Kegel, J.U.; Audic, S.; Wincker, P.; Da Silva, C.; Claverie, J.-M.; et al. Life-cycle modification in open oceans accounts for genome variability in a cosmopolitan phytoplankton. ISME J. 2014, 9, 1365–1377. [Google Scholar] [CrossRef]
- Malviya, S.; Scalco, E.; Audic, S.; Veluchamy, A.; Bittner, L.; Vincent, F.; Poulain, J.; Wincker, P.; Iudicone, D.; de Vargas, C.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 113, E1516–E1525. [Google Scholar] [CrossRef]
- Shodhan, A.; Lukaszewicz, A.; Novatchkova, M.; Loidl, J. Msh4 and Msh5 function in SC-independent chiasma formation during the streamlined meiosis of Tetrahymena. Genetics 2014, 198, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Soucek, R.; Börner, G.V. ZMM proteins during meiosis: Crossover artists at work. Chromosome Res. 2007, 15, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Saro, D.; Hammel, M.; Kwon, Y.; Xu, Y.; Rambo, R.P.; Williams, G.J.; Chi, P.; Lu, L.; Pezza, R.J. Mechanistic insights into the role of Hop2–Mnd1 in meiotic homologous DNA pairing. Nucleic Acids Res. 2013, 42, 906–917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsubouchi, H.; Roeder, G.S. The Mnd1 protein forms a complex with Hop2 to promote homologous chromosome pairing and meiotic double-strand break repair. Mol. Cell. Biol. 2002, 22, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Gerton, J.L.; Scott-Hawley, R. Homologous chromosome interactions in meiosis: Diversity amidst conservation. Nat. Rev. Genet. 2005, 6, 477. [Google Scholar] [CrossRef]
- Ginger, M.L.; Portman, N.; McKean, P.G. Swimming with protists: Perception, motility and flagellum assembly. Nat. Rev. Microbiol. 2008, 6, 838. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Chrétiennot-Dinet, M.-J.; Medlin, L.K.; Claustre, H.; Goër, S.L.d.; Vaulot, D. Bolidomonas: A new genus with two species belonging to a new algal class, the Bolidophyceae (Heterokonta). J. Phycol. 1999, 35, 368–381. [Google Scholar] [CrossRef]
- Manton, I.; von Stoch, H.A. Observations on the fine structure of the male gamete of the marine centric diatom Lithodesmium undulatum. J. R. Microsc. Soc. 1966, 85, 119–134. [Google Scholar] [CrossRef]
- Heath, I.B.; Darley, W.M. Observations on the ultrastructure of the male gametes of Biddulphia levis Ehr. J. Phycol. 1972, 8, 51–59. [Google Scholar] [CrossRef]
- Jensen, K.G.; Moestrup, Ø.; Schmid, A.-M.M. Ultrastructure of the male gametes from two centric diatoms, Chaetoceros laciniosus and Coscinodiscus wailesii (Bacillariophyceae). Phycologia 2003, 42, 98–105. [Google Scholar] [CrossRef]
- Idei, M.; Osada, K.; Sato, S.; Nakayama, T.; Nagumo, T.; Mann, D.G. Sperm ultrastructure in the diatoms Melosira and Thalassiosira and the significance of the 9+0 configuration. Protoplasma 2013, 250, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, T.J.; Townsend, M.J.; Turk, M.; Schlessinger, A.; Sali, A.; Field, M.C.; Huynen, M.A. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc. Natl. Acad. Sci. USA 2013, 110, 6943–6948. [Google Scholar] [CrossRef] [PubMed]
- Montsant, A.; Allen, A.E.; Coesel, S.; Martino, A.D.; Falciatore, A.; Mangogna, M.; Siaut, M.; Heijde, M.; Jabbari, K.; Maheswari, U.; et al. Identification and comparative genomic analysis of signaling and regulatory components in the diatom Thalassiosira pseudonana. J. Phycol. 2007, 43, 585–604. [Google Scholar] [CrossRef]
- French, F.W., III; Hargraves, P.E. Spore formation in the life cycles of the diatoms Chaetoceros diadema and Leptocylindrus danicus. J. Phycol. 1985, 21, 477–483. [Google Scholar] [CrossRef]
- Honda, D.; Shono, T.; Kimura, K.; Fujita, S.; Iseki, M.; Makino, Y.; Murakami, A. Homologs of the sexually induced gene 1 (sig1) product constitute the stramenopile mastigonemes. Protist 2007, 158, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Schurko, A.M.; Logsdon, J.M. Using a meiosis detection toolkit to investigate ancient asexual “scandals” and the evolution of sex. BioEssays 2008, 30, 579–589. [Google Scholar] [CrossRef]
| Function | Protein Name | Query ID (a) | S. marinoi Hit ID | Hit ID Annotation | SEX_T1 logFC | SEX_T2 logFC | SALT_T1 logFC | SALT_T2 logFC |
|---|---|---|---|---|---|---|---|---|
| Accessory proteins required during meiosis | BRCA1 | AAO39850.1 | MTRINITY_DN15891_c0_g1_i1 | Protein breast cancer susceptibility 1 homolog | - | 1.54 | - | - |
| BRCA2 (b) | AEE81814.1 | MTRINITY_DN15273_c1_g3_i3 | Breast cancer type 2 susceptibility protein homolog | - | 1.65 | - | - | |
| DNA2 (b) | NP_001184943.1 | MTRINITY_DN16883_c0_g1_i2 | DNA replication ATP-dependent helicase/nuclease JHS1 | - | 1.51 | - | - | |
| EXO1 (b) | Q8L6Z7.2 | Pc10978_g1_i1 | Exonuclease 1 | - | 7.18 | - | - | |
| Crossover regulation | MSH4 (b) | AAT70180.1 | Pc32435_g1_i6 | DNA mismatch repair protein MSH4 | 4.44 | 6.64 | - | - |
| MSH5 (b) | NP_188683.3 | Pc31899_g1_i1 | MutS protein homolog 5 | 2.76 | 1.95 | - | - | |
| DNA damage sensing and response | RAD50 (b) | AEC08614.1 | MTRINITY_DN15782_c0_g2_i2 | DNA repair protein RAD50 | - | 2.89 | - | - |
| MRE11 (b) | NP_200237.1 | Pc25688_g1_i2 | Double-strand break repair protein MRE11 | - | 1.74 | - | - | |
| DNA replication and chromosome maintenance | SCC3 (b) | AEC10920.1 | MTRINITY_DN16741_c0_g1_i1 | Cohesin subunit SA-3 | - | 3.45 | - | - |
| SMC5 (b) | AED92224.1 | Pc30152_g1_i1 | Structural maintenance of chromosomes protein 5 | - | 2.24 | - | - | |
| MCM6 | AED95141.1 | MTRINITY_DN14495_c0_g1_i2 | DNA replication licensing factor MCM6 | - | 1.84 | - | - | |
| SMC4 (b) | AED95695.1 | MTRINITY_DN13431_c0_g1_i1 | Structural maintenance of chromosomes protein 4 | 3.24 | - | −1.59 | −1.27 | |
| SMC1 (b) | AEE79265.1 | Pc27448_g3_i1 | Structural maintenance of chromosomes protein 1 | - | 2.71 | - | - | |
| MCM2 (b) | NP_001185154.1 | Pc31350_g2_i1 | DNA replication licensing factor MCM2 | - | 1.66 | 1.17 | - | |
| MCM5 (b) | NP_001189521.1 | MTRINITY_DN18289_c0_g1_i1 | DNA replication licensing factor MCM5 | - | 2.16 | - | - | |
| PDS5 (b) | NP_177883.5 | MTRINITY_DN17063_c0_g1_i3 | Sister chromatid cohesion protein PDS5 homolog A | 1.16 | 2.51 | - | - | |
| MCM4 | NP_179236.3 | MTRINITY_DN16417_c4_g1_i2 | DNA replication licensing factor MCM4 | - | 2.43 | - | - | |
| SMC3 (b) | NP_180285.4 | MTRINITY_DN4029_c0_g2_i1 | Structural maintenance of chromosomes protein 3 | 1.06 | 3.00 | - | - | |
| MCM8 (b) | NP_187577.1 | MTRINITY_DN16028_c0_g1_i1 | Probable DNA helicase MCM8 | - | 1.13 | - | - | |
| SMC2 (b) | NP_201047.1 | Pc22708_g1_i1 | Structural maintenance of chromosomes protein 2-1 | 3.33 | - | −1.41 | −1.31 | |
| Double-strand break formation | SPO11-2 (b) | AEE34178.1 | MTRINITY_DN15680_c0_g1_i1 | Meiotic recombination protein SPO11-2 | 11.92 | 8.42 | - | - |
| SPO11-3/TOPVI | NP_195902.1 | Pc22702_g1_i1 | Topoisomerase 6 subunit A3 | - | 1.58 | - | - | |
| Double-strand break repair (recombinational repair) | PMS1 (b) | AAM00563.1 | Pc31460_g3_i1 | DNA mismatch repair protein PMS1 | 1.38 | - | - | |
| RAD51-A (b) | BAE99388.1 | STRINITY_DN8677_c0_g1_i2 | DNA repair protein RAD51 homolog 1 | 4.10 | 2.28 | - | −1.52 | |
| RAD51-C (b) | CAC14091.1 | Pc29686_g1_i1 | DNA repair protein RAD51 homolog 3 | - | 1.43 | - | - | |
| MSH6 (b) | NP_001190656.1 | MTRINITY_DN17310_c0_g1_i2 | DNA mismatch repair protein Msh6 | 1.36 | 2.10 | - | - | |
| RAD51-B (b) | NP_180423.3 | MTRINITY_DN12845_c1_g1_i3 | DNA repair and recombination protein RadA | - | 1.29 | - | - | |
| XRCC3 | NP_200554.1 | Pc31108_g1_i2 | DNA repair protein XRCC3 homolog | 1.54 | 1.23 | - | - | |
| MLH1 (b) | NP_567345.2 | MTRINITY_DN12931_c0_g1_i1 | DNA mismatch repair protein MLH1 | - | 1.56 | - | - |
| Uniprot Description | Uniprot ID | S. marinoi Hit ID | Hit ID Annotation | SEX_T1 logFC | SEX_T2 logFC | SALT_T1 logFC | SALT_T2 logFC |
|---|---|---|---|---|---|---|---|
| Dynein β chain, flagellar outer arm | DYHB_CHLRE | MTRINITY_DN17388_c0_g1_i3 | Dynein β chain flagellar outer arm | - | 3.58 | - | - |
| Dynein, 70 kDa intermediate chain, flagellar outer arm | DYI3_CHLRE | STRINITY_DN18690_c0_g1_i1 | Dynein 70 kDa intermediate chain flagellar outer arm | - | 8.40 | - | - |
| Dynein heavy chain | Q5H9M7_BOVIN | Pc32863_g3_i1 | Dynein heavy chain 8 axonemal | 4.39 | 4.61 | - | - |
| Dynein light chain roadblock | Q7ZUX8_DANRE | STRINITY_DN2071_c0_g1_i1 | Dynein light chain roadblock-type 1 | - | 8.55 | - | - |
| Tctex1 domain-containing protein 1-B | TC1DB_XENLA | MTRINITY_DN20279_c0_g1_i1 | Tctex1 domain-containing protein 1 | - | 7.64 | - | - |
| Nucleoside diphosphate kinase 7 | NDK7_RAT | STRINITY_DN12961_c0_g1_i1 | Nucleoside diphosphate kinase 7 | - | 2.38 | - | - |
| Dynein light chain Tctex-type 1 | DYLT1_MOUSE | STRINITY_DN18525_c0_g1_i1 | Dynein light chain Tctex-type 1 | - | 7.05 | - | - |
| Uracil phosphoribosyltransferase | UPP_ROSS1 | MTRINITY_DN17186_c0_g1_i2 | ATP-dependent D- helicase Q-like 5 | - | 1.95 | - | - |
| EF-hand domain-containing protein 1 | EFHC1_MOUSE | STRINITY_DN11650_c0_g1_i2 | EF-hand domain-containing family member C2 | 2.49 | 4.56 | - | - |
| Dynein regulatory complex subunit 4 | GAS8_CHLRE | STRINITY_DN15037_c0_g1_i1 | Dynein regulatory complex subunit 4 | 5.38 | 7.09 | - | - |
| Dynein regulatory complex subunit 2 | CCD65_BOVIN | STRINITY_DN9819_c0_g1_i1 | Coiled-coil-domain-containing-protein-65 | 9.26 | 8.97 | - | - |
| Tubulin α-2 chain | TBA2_PELFA | STRINITY_DN11650_c0_g2_i3 | Tubulin α-2 chain | - | 2.16 | - | −1.56 |
| Tubulin β chain | TBB_TETTH | STRINITY_DN3540_c0_g1_i1 | Tubulin β chain | 6.63 | 8.51 | - | - |
| Actin. non-muscle 6.2 | ACT_HYDVU | STRINITY_DN3928_c0_g1_i1 | Actin | - | 0.98 | - | −1.17 |
| Caltractin | CATR_SCHDU | MTRINITY_DN2103_c0_g1_i1 | Caltractin | - | 1.89 | - | - |
| Glycogen synthase kinase-3 α | GSK3A_RAT | MTRINITY_DN14448_c0_g1_i1 | Glycogen synthase kinase-3 β | - | 1.18 | - | - |
| Microtubule-associated protein RP/EB family member 1 | MARE1_XENTR | Pc31703_g4_i1 | Microtubule-associated protein RP/EB family member 1B | 1.40 | - | - | −1.47 |
| Serine/threonine-protein phosphatase 4 regulatory subunit 1 | PP4R1_RAT | MTRINITY_DN15306_c0_g1_i1 | Serine/threonine-protein-phosphatase-4-regulatory-subunit-1 | - | -1.56 | - | - |
| S. marinoi Contig Id | Contig Annotation | SEX_T1 logFC | SEX_T2 logFC | P. multistriata Homolog | P. multistriata Annotation | P. multistriata logFC | Proposed Gene Name |
|---|---|---|---|---|---|---|---|
| MTRINITY_DN14053_c0_g1_i4 | - | 8.46 | 5.68 | 0002850.1 | - | 4.03 | SIG4 |
| MTRINITY_DN1615_c0_g1_i1 | - | 6.96 | 6.35 | 0059370.1 | - | 2.92 | SIG5 |
| MTRINITY_DN10312_c0_g1_i1 | Multidrug resistance protein 1 | 5.85 | 2.67 | 0056660.1 | ABC transporter B family member 3 | 2.14 | |
| Pc26735_g2_i3 | - | 5.70 | 3.53 | 0079640.1 | - | 5.85 | SIG6 |
| STRINITY_DN12692_c0_g1_i1 | - | 5.68 | 5.70 | 0010180.1 | - | 1.85 | SIG7 |
| STRINITY_DN10082_c0_g1_i1 | - | 4.73 | 6.47 | 0055440.1 | - | 1.74 | SIG8 |
| Pc19236_g1_i3 | Anaphase-promoting complex subunit cdc20 | 4.49 | 2.32 | 0095480.1 | Anaphase-promoting complex subunit cdc20 | −1.44 (a) | |
| Pc15065_g1_i1 | - | 4.10 | 2.19 | 0009930.1 | - | 4.89 | SIG9 |
| MTRINITY_DN9343_c0_g1_i2 | - | 3.42 | 2.68 | 0061540.1 | - | 2.47 | SIG10 |
| Pc26832_g1_i1 | - | 3.23 | 3.50 | 0093610.1 | - | 4.12 | SIG11 |
| MTRINITY_DN8414_c0_g2_i1 | - | 2.09 | 1.69 | 0076960.1 | Dynamin-like protein A | −1.70 (a) | |
| MTRINITY_DN15107_c0_g1_i1 | - | 1.67 | 3.14 | 0000630.1 | - | −1.91 (a) | |
| MTRINITY_DN17063_c0_g1_i3 | Sister chromatid cohesion protein PDS5 homolog A | 1.16 | 2.51 | 0089060.1 | - | 1.95 | |
| STRINITY_DN5368_c0_g1_i1 | - | 1.12 | 2.95 | 0100360.1 | - | 1.52 | |
| MTRINITY_DN4029_c0_g2_i1 | Structural maintenance of chromosomes protein 3 | 1.06 | 3.00 | 0079810.1 | Structural maintenance of chromosomes protein 3 | 1.67 | |
| Pc28241_g1_i1 | mRNA decay activator protein ZFP36L1 | 0.98 | 1.39 | 0076260.1 | - | 3.70 | |
| Pc29361_g2_i1 | - | 1.46 | 2.68 | 0074970.1 | - | 1.47 | |
| MTRINITY_DN12258_c1_g1_i2 | Translation initiation factor IF-2 | −2.05 | −2.10 | 0047520.1 | Translation initiation factor IF-2 | −2.14 | |
| MTRINITY_DN7716_c0_g1_i1 | Putative elongation factor TypA-like SVR3 | −1.46 | −0.87 | 0044870.1 | GTP-binding protein TypA/BipA homolog | −1.37 | |
| MTRINITY_DN17106_c0_g1_i1 | Acetyl-CoA carboxylase 1 | −1.21 | −0.77 | 0084140.1 | Acetyl-CoA carboxylase 1 | −1.86 | |
| MTRINITY_DN12117_c0_g1_i1 | Leucine-tRNA ligase | −1.06 | −1.05 | 0031170.1 | Leucine--tRNA ligase | −1.60 | |
| STRINITY_DN8561_c3_g1_i1 | - | −1.00 | −1.39 | 0040850.1 | Glucose-repressible alcohol dehydrogese transcriptional effector | −2.13 | |
| STRINITY_DN13193_c0_g1_i1 | Pleckstrin homology domain-containing family A member 8 | −0.94 | −1.11 | 0005620.1 | - | −1.81 | |
| MTRINITY_DN16739_c0_g1_i5 | Bifunctional purine biosynthetic protein ADE1 | −0.80 | −1.11 | 0025170.1 | Phosphoribosylformylglycimidine cyclonaligase | −1.28 | |
| MTRINITY_DN9740_c0_g1_i1 | Arogenate dehydrogenase 2 chloroplastic | −0.78 | −1.03 | 0020750.1 | Arogenate dehydrogenase 2 chloroplastic | −2.34 | |
| MTRINITY_DN17191_c0_g2_i1 | Ribosomal RNA processing protein 1 homolog B | −0.78 | −1.61 | 0119100.1 | - | −1.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrante, M.I.; Entrambasaguas, L.; Johansson, M.; Töpel, M.; Kremp, A.; Montresor, M.; Godhe, A. Exploring Molecular Signs of Sex in the Marine Diatom Skeletonema marinoi. Genes 2019, 10, 494. https://doi.org/10.3390/genes10070494
Ferrante MI, Entrambasaguas L, Johansson M, Töpel M, Kremp A, Montresor M, Godhe A. Exploring Molecular Signs of Sex in the Marine Diatom Skeletonema marinoi. Genes. 2019; 10(7):494. https://doi.org/10.3390/genes10070494
Chicago/Turabian StyleFerrante, Maria Immacolata, Laura Entrambasaguas, Mathias Johansson, Mats Töpel, Anke Kremp, Marina Montresor, and Anna Godhe. 2019. "Exploring Molecular Signs of Sex in the Marine Diatom Skeletonema marinoi" Genes 10, no. 7: 494. https://doi.org/10.3390/genes10070494
APA StyleFerrante, M. I., Entrambasaguas, L., Johansson, M., Töpel, M., Kremp, A., Montresor, M., & Godhe, A. (2019). Exploring Molecular Signs of Sex in the Marine Diatom Skeletonema marinoi. Genes, 10(7), 494. https://doi.org/10.3390/genes10070494





