A SIX6 Nonsense Variant in Golden Retrievers with Congenital Eye Malformations
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Selection
2.3. Clinical Examination
2.4. DNA Extraction
2.5. Whole Genome Sequencing of an Affected Golden Retriever
2.6. Variant Calling
2.7. Gene Analysis
2.8. Sanger Sequencing
3. Results
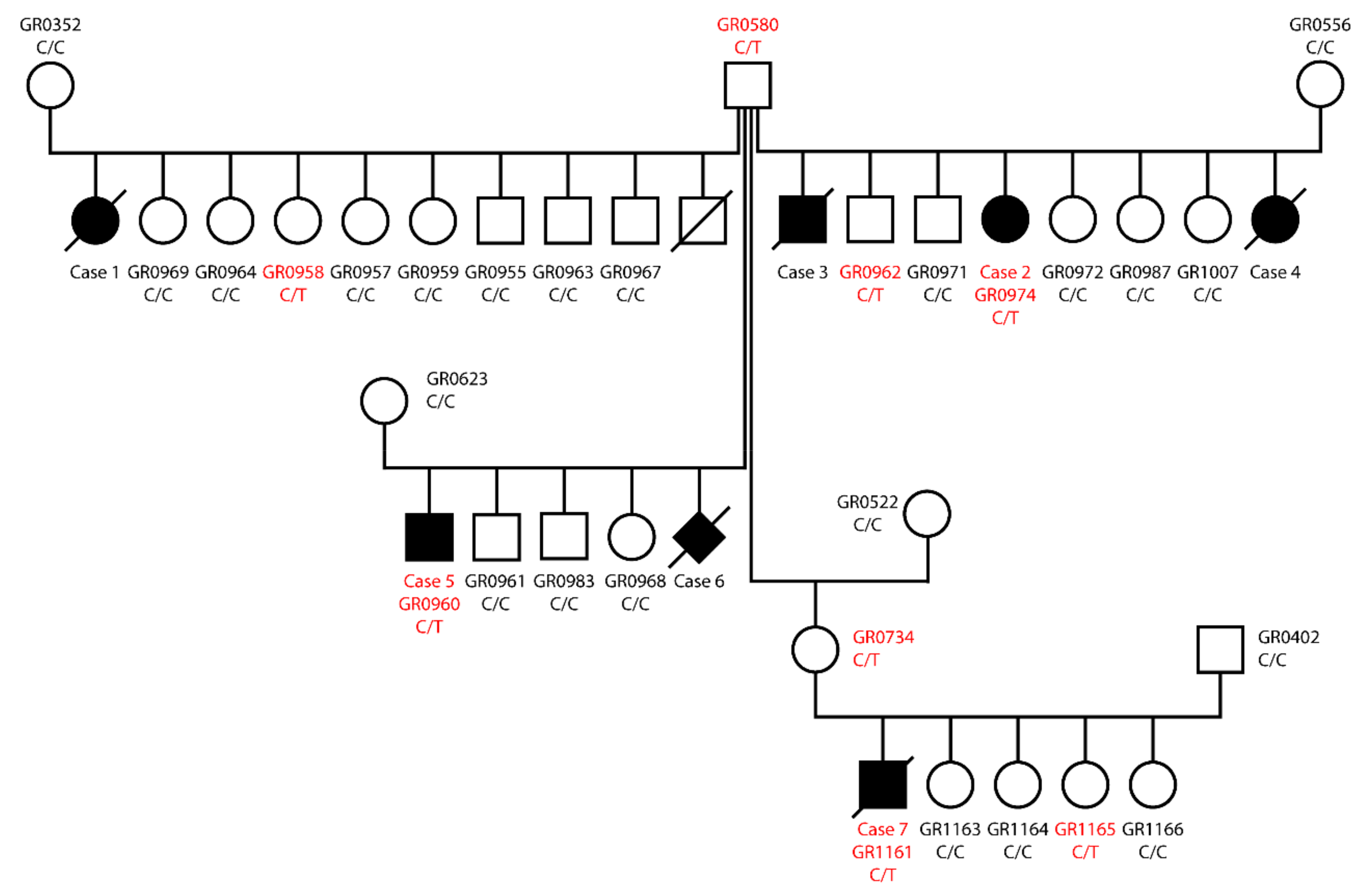
3.1. Eye Examinations
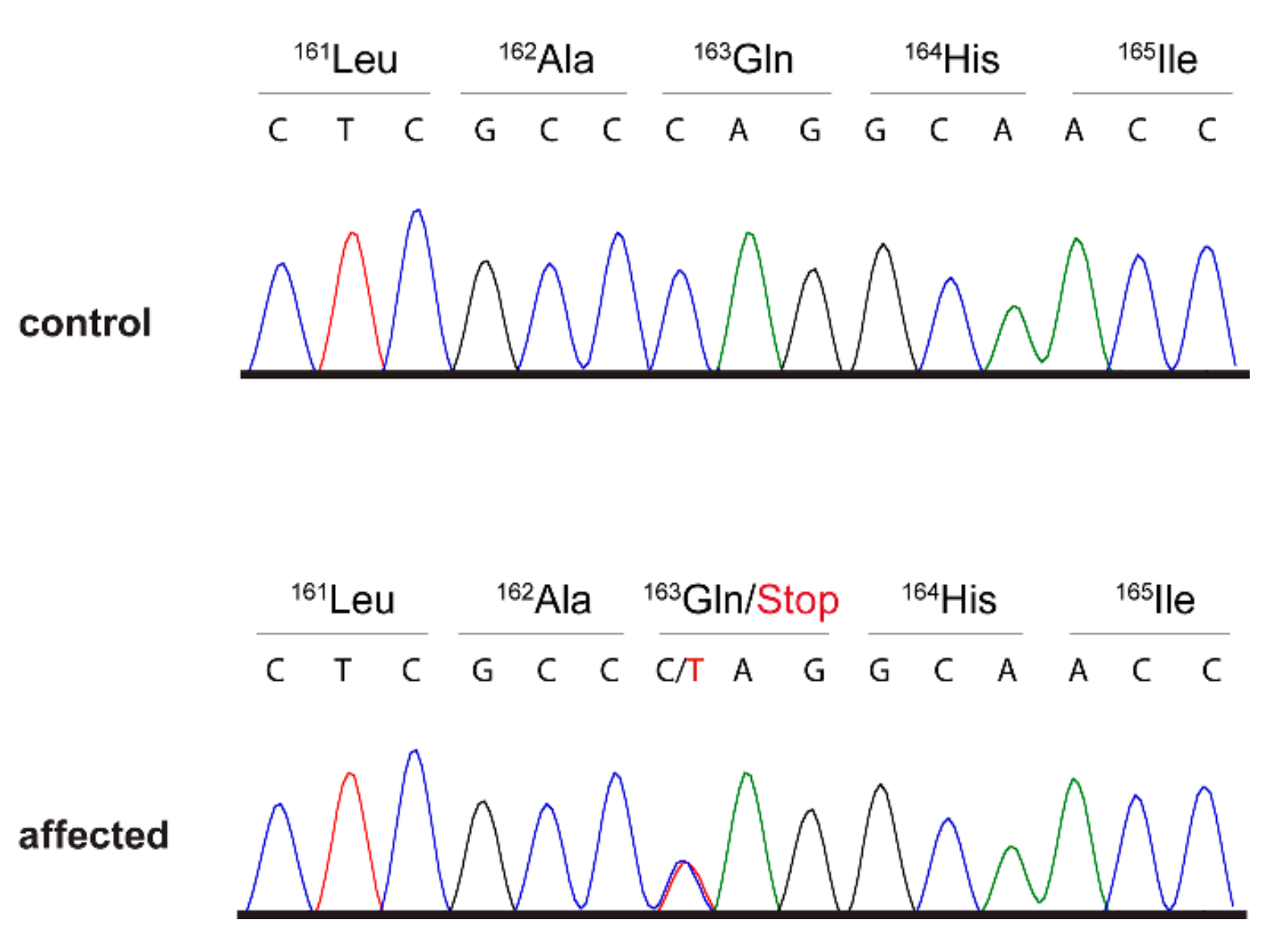
3.2. Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- FitzPatrick, D.R.; van Heyningen, V. Developmental eye disorders. Curr. Opin. Genet. Dev. 2005, 15, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Graw, J. The genetic and molecular basis of congenital eye defects. Nat. Rev. Genet. 2003, 4, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yin, J.; Lewis, R.; Schaaf, C. Genetic causes of optic nerve hypoplasia. J. Med. Genet. 2017, 54, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Pichi, F.; Limbo, A.; Serafino, M.; Nucci, P. Genetics of Congenital Cataract. Pediatr. Cataract Dev. Ophthalmol. 2016, 57, 1–14. [Google Scholar]
- Jeng-Miller, K.W.; Cestari, D.M.; Gaier, E.D. Congenital anomalies of the optic disc: Insights from optical coherence tomography imaging. Curr. Opin. Ophthalmol. 2017, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Black, L.J.; da Costa Martins, B.; Plummer, C.E.; Abbott, J.R.; Leissinger, M.K. What Is Your Diagnosis? Eyelid mass in a dog. Vet. Clin. Pathol. 2018, 47, 157–159. [Google Scholar] [CrossRef]
- Scott, E.M.; Schlesener, B.N.; Shaw, G.C.; Teixeira, L.B.C. Canine ocular and periocular snakebites requiring enucleation: A report of 19 cases. Vet. Ophthalmol. 2019, 1–8. [Google Scholar] [CrossRef]
- Hodzic, A.; Hinney, B.; König, S.; Naucke, T.J.; Duscher, G.; Joachim, A. A case of ocular infection with Onchocerca lupi in a dog from Germany. Transbound. Emerg. Dis. 2018, 65, 214–216. [Google Scholar] [CrossRef]
- Acland, G.M.; Fletcher, R.T.; Gentleman, S.; Chader, G.J.; Aguirre, G.D. Non-allelism of three genes (rcd1, rcd2 and erd) for early-onset hereditary retinal degeneration. Exp. Eye Res. 1989, 49, 983–998. [Google Scholar] [CrossRef]
- Miller, E.J.; Brines, C.M. Canine Diabetes Mellitus Associated Ocular Disease. Top. Companion Anim. Med. 2018, 33, 29–34. [Google Scholar] [CrossRef]
- Violette, N.P.; Ledbetter, E.C. Punctate retinal hemorrhage and its relation to ocular and systemic disease in dogs: 83 cases. Vet. Ophthalmol. 2018, 21, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.D. Chlorpyrifos (Dursban)-associated birth defects: Report of four cases. Arch. Environ. Health 1996, 51, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Mets, M.B. Eye manifestations of intrauterine infections. Ophthalmol. Clin. N. Am. 2001, 3, 521–531. [Google Scholar] [CrossRef]
- Mellersh, C.S. The genetics of eye disorders in the dog. Canine Genet. Epidemiol. 2014, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Crispin, S. Hereditary Eye Disease in Dogs; Canine Health Schemes: London, UK, 2016; Available online: https://www.bva.co.uk/uploadedFiles/Content/Canine_Health_Schemes/20160321%20CHS%20Eye%20leaflet%202016%20v8A.pdf (accessed on 17 April 2019).
- Mäkeläinen, S.; Gòdia, M.; Hellsand, M.; Viluma, A.; Hahn, D.; Makdoumi, K.; Zeiss, C.J.; Mellersh, C.; Ricketts, S.L.; Narfström, K.; et al. An ABCA4 loss-of-function mutation causes a canine form of Stargardt disease. PLoS Genet. 2019, 15, e1007873. [Google Scholar] [CrossRef] [PubMed]
- Kropatsch, R.; Petrasch-Parwez, E.; Seelow, D.; Schlichting, A.; Gerding, W.M.; Akkad, D.A.; Epplen, J.T.; Dekomien, G. Generalized progressive retinal atrophy in the Irish Glen of Imaal Terrier is associated with a deletion in the ADAM9 gene. Mol. Cell. Probes 2010, 24, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kuchtey, J.; Olson, L.M.; Rinkoski, T.; Mackay, E.O.; Iverson, T.M.; Gelatt, K.N.; Haines, J.L.; Kuchtey, R.W. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS Genet. 2011, 7, e1001306. [Google Scholar] [CrossRef]
- Ahonen, S.J.; Kaukonen, M.; Nussdorfer, F.D.; Harman, C.D.; Komáromy, A.M.; Lohi, H. A novel missense mutation in ADAMTS10 in Norwegian Elkhound primary glaucoma. PLoS ONE 2014, 9, e111941. [Google Scholar] [CrossRef]
- Oliver, J.A.; Forman, O.P.; Pettitt, L.; Mellersh, C.S. Two independent mutations in ADAMTS17 are associated with primary open angle glaucoma in the Basset Hound and Basset Fauve de Bretagne breeds of dog. PLoS ONE 2015, 10, e0140436. [Google Scholar] [CrossRef]
- Oliver, J.A.C.; Rustidge, S.; Pettitt, L.; Jenkins, C.A.; Farias, F.H.G.; Giuliano, E.A.; Mellersh, C.S. Evaluation of ADAMTS17 in Chinese Shar-Pei with primary open-angle glaucoma, primary lens luxation, or both. Am. J. Vet. Res. 2018, 79, 98–106. [Google Scholar] [CrossRef]
- Guziewicz, K.E.; Zangerl, B.; Lindauer, S.J.; Mullins, R.F.; Sandmeyer, L.S.; Grahn, B.H.; Stone, E.M.; Acland, G.M.; Aguirre, G.D. Bestrophin gene mutations cause canine multifocal retinopathy: A novel animal model for best disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Zangerl, B.; Wickström, K.; Slavik, J.; Lindauer, S.J.; Ahonen, S.; Schelling, C.; Lohi, H.; Guziewicz, K.E.; Aguirre, G.D. Assessment of canine BEST1 variations identifies new mutations and establishes an independent bestrophinopathy model (cmr3). Mol. Vis. 2010, 16, 2791–2804. [Google Scholar] [PubMed]
- Dekomien, G.; Vollrath, C.; Petrasch-Parwez, E.; Boevé, M.H.; Akkad, D.A.; Gerding, W.M.; Epplen, J.T. Progressive retinal atrophy in Schapendoes dogs: Mutation of the newly identified CCDC66 gene. Neurogenetics 2010, 11, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Wiik, A.C.; Ropstad, E.O.; Ekesten, B.; Karlstam, L.; Wade, C.M.; Lingaas, F. Progressive retinal atrophy in Shetland sheepdog is associated with a mutation in the CNGA1 gene. Anim. Genet. 2015, 46, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, S.J.; Arumilli, M.; Lohi, H. A CNGB1 frameshift mutation in Papillon and Phalène dogs with progressive retinal atrophy. PLoS ONE 2013, 8, e72122. [Google Scholar] [CrossRef] [PubMed]
- Sidjanin, D.J.; Lowe, J.K.; McElwee, J.L.; Milne, B.S.; Phippen, T.M.; Sargan, D.R.; Aguirre, G.D.; Acland, G.M.; Ostrander, E.A. Canine CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum. Mol. Genet. 2002, 11, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, O.; Guyon, R.; Kukekova, A.; Kuznetsova, T.N.; Pearce-Kelling, S.E.; Johnson, J.; Aguirre, G.D.; Acland, G.M. COL9A2 and COL9A3 mutations in canine autosomal recessive oculoskeletal dysplasia. Mamm. Genome 2010, 21, 398–408. [Google Scholar] [CrossRef]
- Downs, L.M.; Mellersh, C.S. An intronic SINE insertion in FAM161A that causes exon-skipping is associated with progressive retinal atrophy in Tibetan Spaniels and Tibetan Terriers. PLoS ONE 2014, 9, e93990. [Google Scholar] [CrossRef]
- Mellersh, C.S.; Pettitt, L.; Forman, O.P.; Vaudin, M.; Barnett, K.C. Identification of mutations in HSF4 in dogs of three different breeds with hereditary cataracts. Vet. Ophthalmol. 2006, 9, 369–378. [Google Scholar] [CrossRef]
- Goldstein, O.; Mezey, J.G.; Schweitzer, P.A.; Boyko, A.R.; Gao, C.; Bustamante, C.D.; Jordan, J.A.; Aguirre, G.D.; Acland, G.M. IQCB1 and PDE6B mutations cause similar early onset retinal degenerations in two closely related terrier dog breeds. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7005–7019. [Google Scholar] [CrossRef]
- Everson, R.; Pettitt, L.; Forman, O.P.; Dower-Tylee, O.; McLaughlin, B.; Ahonen, S.; Kaukonen, M.; Komáromy, A.M.; Lohi, H.; Mellersh, C.S.; et al. An intronic LINE-1 insertion in MERTK is strongly associated with retinopathy in Swedish Vallhund dogs. PLoS ONE 2017, 12, e0183021. [Google Scholar] [CrossRef] [PubMed]
- Hitti, R.J.; Oliver, J.A.C.; Schofield, E.C.; Bauer, A.; Kaukonen, M.; Forman, O.P.; Leeb, T.; Lohi, H.; Burmeister, L.M.; Sargan, D.; et al. Whole genome sequencing of Giant Schnauzer Dogs with progressive retinal atrophy establishes NECAP1 as a novel candidate gene for retinal degeneration. Genes 2019, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.G.; Kukekova, A.V.; Akey, D.T.; Goldstein, O.; Kirkness, E.F.; Baysac, K.C.; Mosher, D.S.; Aguirre, G.D.; Acland, G.M.; Ostrander, E.A. Breed relationships facilitate fine-mapping studies: A 7.8-kb deletion cosegregates with Collie eye anomaly across multiple dog breeds. Genome Res. 2007, 17, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Wiik, A.C.; Wade, C.; Biagi, T.; Ropstad, E.O.; Bjerkås, E.; Lindblad-Toh, K.; Lingaas, F. A deletion in nephronophthisis 4 (NPHP4) is associated with recessive cone-rod dystrophy in standard wire-haired dachshund. Genome Res. 2008, 18, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.A.; Farrell, L.L.; Carlisle, A.J.; Bush, S.J.; Ewing, A.; Trejo-Reveles, V.; Matika, O.; de Kloet, A.; Walsh, C.; Bishop, S.C.; et al. Arginine to glutamine variant in olfactomedin like 3 (OLFML3) is a candidate for severe goniodysgenesis and glaucoma in the Border Collie dog breed. G3 Genes Genomes Genet. 2019, 9, 943–954. [Google Scholar] [CrossRef]
- Downs, L.M.; Bell, J.S.; Freeman, J.; Hartley, C.; Hayward, L.J.; Mellersh, C.S. Late-onset progressive retinal atrophy in the Gordon and Irish Setter breeds is associated with a frameshift mutation in C2orf71. Anim. Genet. 2013, 44, 169–177. [Google Scholar] [CrossRef]
- Petersen-Jones, S.M.; Entz, D.D.; Sargan, D.R. cGMP phosphodiesterase-alpha mutation causes progressive retinal atrophy in the Cardigan Welsh corgi dog. Investig. Ophthalmol. Vis. Sci. 1999, 8, 1637–1644. [Google Scholar]
- Suber, M.L.; Pittler, S.J.; Qin, N.; Wright, G.C.; Holcombe, V.; Lee, R.H.; Craft, C.M.; Lolley, R.N.; Baehr, W.; Hurwitz, R.L. Irish setter dogs affected with rod/cone dysplasia contain a nonsense mutation in the rod cGMP phosphodiesterase beta-subunit gene. Proc. Natl. Acad. Sci. USA 1993, 90, 3968–3972. [Google Scholar] [CrossRef]
- Dekomien, G.; Runte, M.; Gödde, R.; Epplen, J.T. Generalized progressive retinal atrophy of Sloughi dogs is due to an 8-bp insertion in exon 21 of the PDE6B gene. Cytogenet. Cell Genet. 2000, 90, 261–267. [Google Scholar] [CrossRef]
- Murgiano, L.; Becker, D.; Torjman, D.; Niggel, J.K.; Milano, A.; Cullen, C.; Feng, R.; Wang, F.; Jagannathan, V.; Pearce-Kelling, S.; et al. Complex Structural PPT1 Variant Associated with Non-syndromic Canine Retinal Degeneration. G3 Genes Genomes Genet. 2019, 2, 425–437. [Google Scholar] [CrossRef]
- Zangerl, B.; Goldstein, O.; Philp, A.R.; Lindauer, S.J.; Pearce-Kelling, S.E.; Mullins, R.F.; Graphodatsky, A.S.; Ripoll, D.; Felix, J.S.; Stone, E.M.; et al. Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics 2006, 5, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Kukekova, A.V.; Goldstein, O.; Johnson, J.L.; Richardson, M.A.; Pearce-Kelling, S.E.; Swaroop, A.; Friedman, J.S.; Aguirre, G.D.; Acland, G.M. Canine RD3 mutation establishes rod-cone dysplasia type 2 (rcd2) as ortholog of human and murine rd3. Mamm. Genome 2009, 2, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Kijas, J.W.; Cideciyan, A.V.; Aleman, T.S.; Pianta, M.J.; Pearce-Kelling, S.E.; Miller, B.J.; Jacobson, S.G.; Aguirre, G.D.; Acland, G.M. Naturally occurring rhodopsin mutation in the dog causes retinal dysfunction and degeneration mimicking human dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2002, 9, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.D.; Baldwin, V.; Pearce-Kelling, S.; Narfström, K.; Ray, K.; Acland, G.M. Congenital stationary night blindness in the dog: Common mutation in the RPE65 gene indicates founder effect. Mol. Vis. 1998, 4, 23. [Google Scholar] [PubMed]
- Zhang, Q.; Acland, G.M.; Wu, W.X.; Johnson, J.L.; Pearce-Kelling, S.; Tulloch, B.; Vervoort, R.; Wright, A.F.; Aguirre, G.D. Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum. Mol. Genet. 2002, 9, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Kropatsch, R.; Akkad, D.A.; Frank, M.; Rosenhagen, C.; Altmüller, J.; Nürnberg, P.; Epplen, J.T.; Dekomien, G. A large deletion in RPGR causes XLPRA in Weimaraner dogs. Canine Genet. Epidemiol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Mellersh, C.S.; Boursnell, M.E.; Pettitt, L.; Ryder, E.J.; Holmes, N.G.; Grafham, D.; Forman, O.P.; Sampson, J.; Barnett, K.C.; Blanton, S.; et al. Canine RPGRIP1 mutation establishes cone-rod dystrophy in miniature longhaired dachshunds as a homologue of human Leber congenital amaurosis. Genomics 2006, 3, 293–301. [Google Scholar] [CrossRef]
- Forman, O.P.; Hitti, R.J.; Boursnell, M.; Miyadera, K.; Sargan, D.; Mellersh, C. Canine genome assembly correction facilitates identification of a MAP9 deletion as a potential age of onset modifier for RPGRIP1-associated canine retinal degeneration. Mamm. Genome 2016, 27, 237–245. [Google Scholar] [CrossRef]
- Goldstein, O.; Jordan, J.A.; Aguirre, G.D.; Acland, G.M. A non-stop S-antigen gene mutation is associated with late onset hereditary retinal degeneration in dogs. Mol. Vis. 2013, 19, 1871–1884. [Google Scholar]
- Downs, L.M.; Wallin-Håkansson, B.; Boursnell, M.; Marklund, S.; Hedhammar, Å.; Truvé, K.; Hübinette, L.; Lindblad-Toh, K.; Bergström, T.; Mellersh, C.S. A frameshift mutation in golden retriever dogs with progressive retinal atrophy endorses SLC4A3 as a candidate gene for human retinal degenerations. PLoS ONE 2011, 6, e21452. [Google Scholar] [CrossRef]
- Goldstein, O.; Kukekova, A.V.; Aguirre, G.D.; Acland, G.M. Exonic SINE insertion in STK38L causes canine early retinal degeneration (erd). Genomics 2010, 6, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Downs, L.M.; Wallin-Håkansson, B.; Bergström, T.; Mellersh, C.S. A novel mutation in TTC8 is associated with progressive retinal atrophy in the golden retriever. Canine Genet. Epidemiol. 2014, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Jagannathan, V.; Hogler, S.; Richter, B.; McEwan, N.A.; Thomas, A. MKLN1 splicing defect in dogs with lethal acrodermatitis. PLoS Genet. 2018, 14, e1007264. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang le, L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Zhao, W.M.; Tang, B.X.; Wang, Y.Q.; Wang, L.; Zhang, Z.; Yang, H.C.; Liu, Y.H.; Zhu, J.W.; Irwin, D.M.; et al. DoGSD: The dog and wolf genome SNP database. Nucleic Acids Res. 2015, 43, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Yariz, K.O.; Sakalar, Y.B.; Jin, X.; Hertz, J.; Sener, E.F.; Akay, H.; Özbek, M.N.; Farooq, A.; Goldberg, J.; Tekin, M. A homozygous SIX6 mutation is associated with optic disc anomalies and macular atrophy and reduces retinal ganglion cell differentiation. Clin. Genet. 2015, 87, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Aldahmesh, M.A.; Khan, A.O.; Hijazi, H.; Alkuraya, F.S. Homozygous truncation of SIX6 causes complex microphthalmia in humans. Clin. Genet. 2013, 84, 198–199. [Google Scholar] [CrossRef]
- Gallardo, M.E.; Lopez-Rios, J.; Fernaud-Espinosa, I.; Granadino, B.; Sanz, R.; Ramos, C.; Ayuso, C.; Seller, M.J.; Brunner, H.G.; Bovolenta, P.; et al. Genomic cloning and characterization of the human homeobox gene Six6 reveals a cluster of SIX genes in chromosome 14 and associates Six6 hemizygositiy with bilateral anophtalmia and pituitary anomalies. Genomics 1999, 61, 82–91. [Google Scholar] [CrossRef]
- Gallardo, M.E.; Rodriguez de Cordoba, S.; Schneider, A.S.; Dwyer, M.A.; Ayuso, C.; Bovolenta, P. Analysis of the developmental SIX6 homeobox gene in patients with anophthalmia/microphthalmia. Am. J. Med. Genet. 2004, 129A, 92–94. [Google Scholar] [CrossRef]
- Bürglin, T.R. Homeodomain subtypes and functional diversity. Subcell. Biochem. 2011, 52, 95–122. [Google Scholar]
- Holland, P.W. Evolution of homeobox genes. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.S.; Brand, A.H. Optix defines a neutroepithelial compartment the optic lobe of the Drosophila brain. Neural Dev. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Cheyette, B.N.R.; Green, P.J.; Martin, K.; Garren, H.; Hartenstein, V.; Zipursky, S.L. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entirevisual system. Neuron 1994, 12, 977–996. [Google Scholar] [CrossRef]
- Holland, P.W.; Booth, H.A.; Bruford, E.A. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Mailhos, A.; Wehr, R.; Copeland, N.G.; Jenkins, N.A.; Gruss, P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 1995, 121, 4045–4055. [Google Scholar] [PubMed]
- Li, X.; Perissi, V.; Liu, F.; Rose, D.W.; Rosenfeld, M.G. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science 2002, 297, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
| Gene | Phenotype | Inheritance | Breed | OMIA | Ref. |
|---|---|---|---|---|---|
| ABCA4 | Stargardt disease 1 | AR | Labrador Retriever | 002179-9615 | [16] |
| ADAM9 | cone-rod dystrophy 3 | AR | Glen of Imaal Terrier | 001520-9615 | [17] |
| ADAMTS10 | POAG | AR | Beagle, Nor. Elkhound | 001870-9615 | [18,19] |
| ADAMTS17 | POAG and/or PLL | AR | many | 001976-9615 | [20,21] |
| BEST1 | multifocal retinopathy 1 | AR | many | 001311-9615 | [22] |
| BEST1 | multifocal retinopathy 2 | AR | Coton de Tulear | 001553-9615 | [22] |
| BEST1 | multifocal retinopathy 3 | AR | Lapponian Herder | 001554-9615 | [23] |
| CCDC66 | generalized PRA | AR | Schapendoes | 001521-9615 | [24] |
| CNGA1 | PRA | AR | Shetland Sheepdog | 001977-9615 | [25] |
| CNGB1 | PRA | AR | Papillon | 000830-9615 | [26] |
| CNGB3 | achromatopsia | AR | many | 001365-9615 | [27] |
| COL9A2 | oculoskeletal dysplasia 2 | AR | Samoyed | 001523-9615 | [28] |
| COL9A3 | oculoskeletal dysplasia 1 | AR | Labrador Retriever | 001522-9615 | [28] |
| FAM161A | PRA, type 3 | AR | several | 001918-9615 | [29] |
| HSF4 | cataract, early onset | AR | many | 001758-9615 | [30] |
| IQCB1 | cone-rod dystrophy 2 | AR | Am. Pit Bull Terrier | 001675-9615 | [31] |
| MERTK | PRA | AR | Swedish Vallhund | 001932-9615 | [32] |
| NECAP1 | PRA | AR | Giant Schnauzer | n.a. | [33] |
| NHEJ1 | Collie eye anomaly | AR | many | 000218-9615 | [34] |
| NPHP4 | cone-rod dystrophy | AR | Dachshund | 001455-9615 | [35] |
| OLFML3 | goniodysgenesis | AR | Border Collie | 001223-9615 | [36] |
| PCARE | rod-cone dysplasia 4 | AR | many | 001575-9615 | [37] |
| PDE6A | rod-cone dysplasia 3 | AR | Cardigan Welsh Corgi. | 001314-9615 | [38] |
| PDE6B | cone-rod dystrophy 1 | AR | Am. Staff. Terrier | 001674-9615 | [31] |
| PDE6B | rod-cone dysplasia 1 | AR | Irish Setter | 000882-9615 | [39] |
| PDE6B | rod-cone dysplasia 1a | AR | Sloughi | 001669-9615 | [40] |
| PPT1 | photoreceptor dysplasia | AR | Miniature Schnauzer | 001311-9615 | [41] |
| PRCD | prog. rod-cone degeneration | AR | many | 001298-9615 | [42] |
| RD3 | rod-cone dysplasia 2 | AR | Collie | 001260-9615 | [43] |
| RHO | autosomal dominant PRA | AD | Bull & English Mastiff | 001346-9615 | [44] |
| RPE65 | Leber congenital amaurosis | AR | Briard | 001222-9615 | [45] |
| RPGR | RRA, X-linked, type 1 | X-linked | many | 000831-9615 | [46,47] |
| RPGR | PRA, X-linked, type 2 | X-linked | mixed breed dog | 001518-9615 | [46] |
| RPGRIP1 (& MAP9) | cone-rod dystrophy 4 | complex | Dachsund | 001432-9615 | [48,49] |
| SAG | PRA | AR | Basenji | 001876-9615 | [50] |
| SLC4A3 | Golden Retriever PRA 1 | AR | Golden Retriever | 001572-9615 | [51] |
| STK38L | early retinal degeneration | AR | Norwegian Elkhound | 001297-9615 | [52] |
| TTC8 | Golden Retriever PRA 2 | AR | Golden Retriever | 001984-9615 | [53] |
| Filtering Step | Heterozygous Variants | Homozygous Variants |
|---|---|---|
| variants in the whole genome | 3,024,455 | 2,913,164 |
| private variants | 8983 | 1214 |
| protein-changing private variants | 19 | 2 |
| Dogs | C/C | T/C |
|---|---|---|
| Cases (n = 3) | 0 | 3 |
| Non-affected family members (n = 22) | 17 | 5 |
| “unrelated>” Golden Retrievers (n = 464) | 464 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hug, P.; Anderegg, L.; Dürig, N.; Lepori, V.; Jagannathan, V.; Spiess, B.; Richter, M.; Leeb, T. A SIX6 Nonsense Variant in Golden Retrievers with Congenital Eye Malformations. Genes 2019, 10, 454. https://doi.org/10.3390/genes10060454
Hug P, Anderegg L, Dürig N, Lepori V, Jagannathan V, Spiess B, Richter M, Leeb T. A SIX6 Nonsense Variant in Golden Retrievers with Congenital Eye Malformations. Genes. 2019; 10(6):454. https://doi.org/10.3390/genes10060454
Chicago/Turabian StyleHug, Petra, Linda Anderegg, Nicole Dürig, Vincent Lepori, Vidhya Jagannathan, Bernhard Spiess, Marianne Richter, and Tosso Leeb. 2019. "A SIX6 Nonsense Variant in Golden Retrievers with Congenital Eye Malformations" Genes 10, no. 6: 454. https://doi.org/10.3390/genes10060454
APA StyleHug, P., Anderegg, L., Dürig, N., Lepori, V., Jagannathan, V., Spiess, B., Richter, M., & Leeb, T. (2019). A SIX6 Nonsense Variant in Golden Retrievers with Congenital Eye Malformations. Genes, 10(6), 454. https://doi.org/10.3390/genes10060454






