A Wheat β-Patchoulene Synthase Confers Resistance against Herbivory in Transgenic Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Fungus
2.2. Gene Cloning
2.3. Recombinant Expression
2.4. GC-MS Analysis
2.5. Gene Expression Analysis and Terpene Detection in Wheat
2.6. Overexpression of TaPS in Arabidopsis
2.7. Anti-Herbivory Assays
2.8. Homology Modeling and Site-Directed Mutagenesis
2.9. Statistic Analysis
3. Results
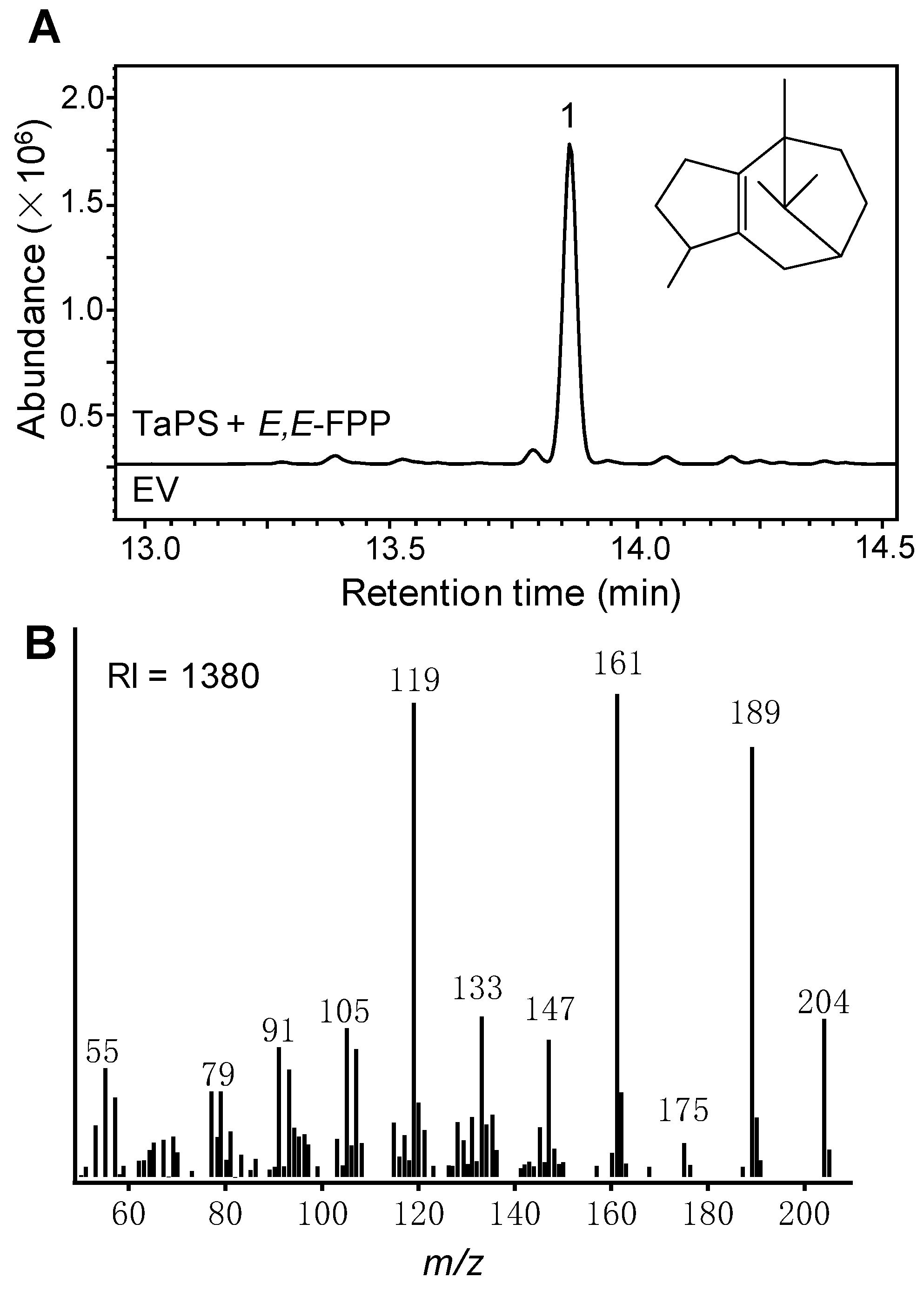
3.1. Identification of TaPS as a Sesquiterpene Synthase
3.2. Inducible TaPS Gene Expression and β-Patchoulene Accumulation
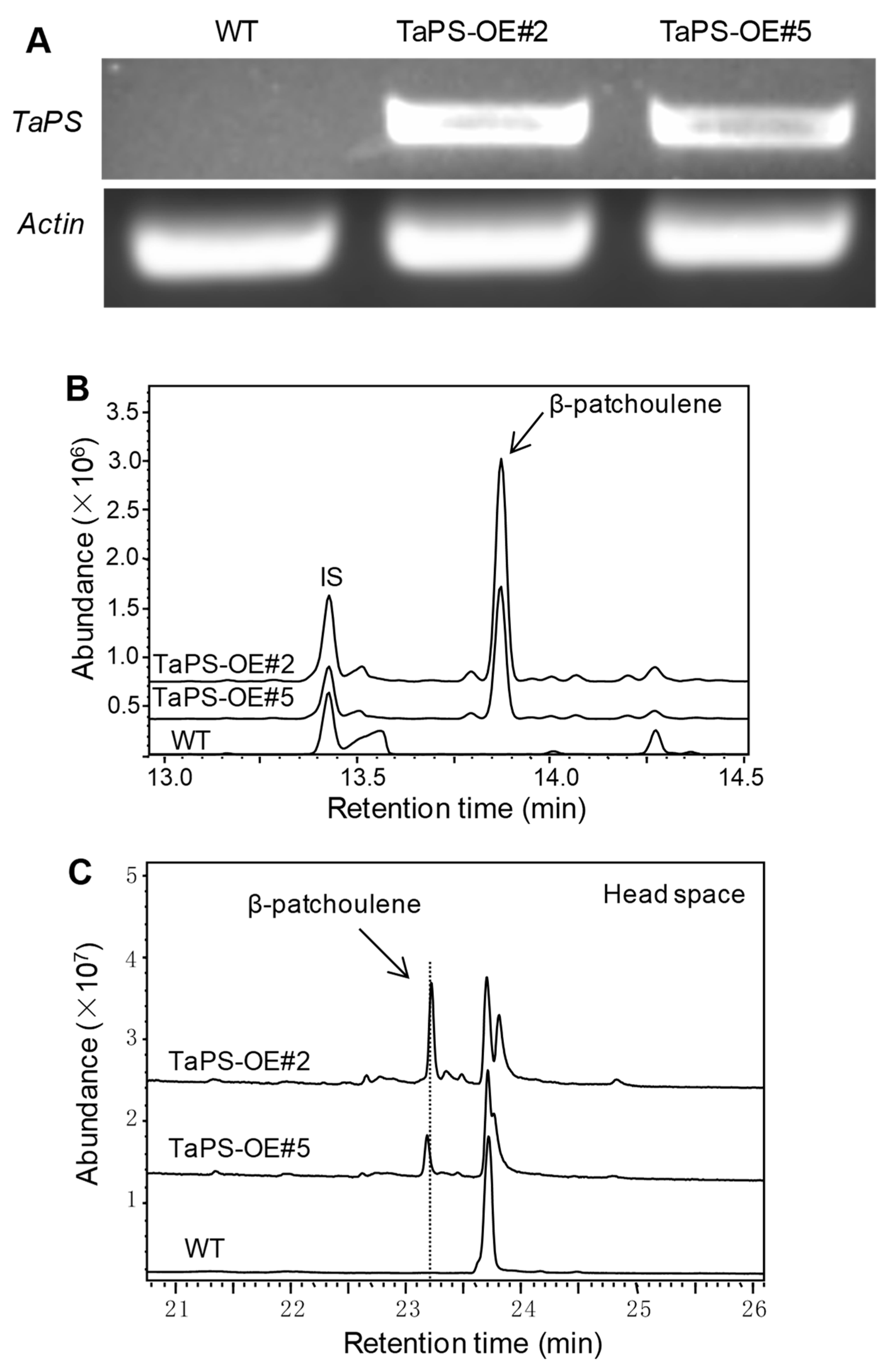
3.3. Arabidopsis Overexpressing TaPS Produced β-Patchoulene to Repel S. Exigua Larvae
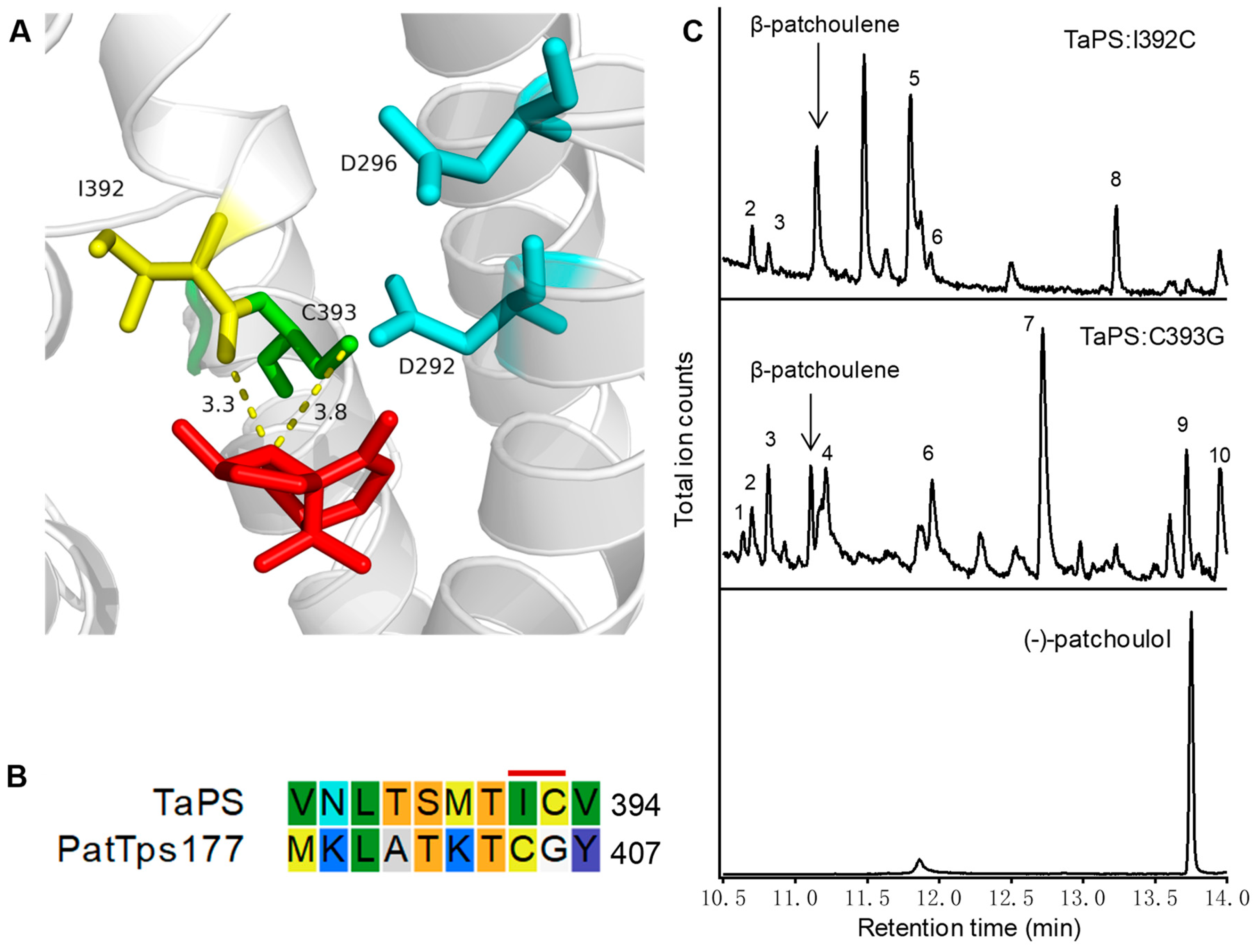
3.4. Exploring TaPS Catalytic Mechanism by Homology Modeling and Mutagenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Neilson, E.H.; Goodger, J.Q.; Woodrow, I.E.; Moller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Aljbory, Z.; Chen, M.S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.X.; Xiang, C.Y.; Li, J.X.; Yang, C.Q.; Hu, W.L.; Wang, L.J.; Lou, Y.G.; Chen, X.Y. The rice (E)-beta-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 2007, 68, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Imai, T.; Koga, J.; Okada, K.; Yamane, H.; Ohashi, Y. Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol. Plant Microbe Interact. 2010, 23, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Kodama, O.; Xin Li, W.; Tamogami, S.; Akatsuka, T. Oryzalexin S, a novel stemarane-type diterpene rice phytoalexin. Biosci. Biotechnol. Biochem. 1992, 56, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, A.; Kaplan, F.; Vaughan, M.M.; Dafoe, N.J.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E.; Schmelz, E.A. Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol. 2011, 156, 2082–2097. [Google Scholar] [CrossRef]
- Christensen, S.A.; Huffaker, A.; Sims, J.; Hunter, C.T.; Block, A.; Vaughan, M.M.; Willett, D.; Romero, M.; Mylroie, J.E.; Williams, W.P.; et al. Fungal and herbivore elicitation of the novel maize sesquiterpenoid, zealexin A4, is attenuated by elevated CO2. Planta 2018, 247, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Kaplan, F.; Huffaker, A.; Dafoe, N.J.; Vaughan, M.M.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Sci. USA 2011, 108, 5455–5460. [Google Scholar] [CrossRef]
- Mafu, S.; Ding, Y.; Murphy, K.M.; Yaacoobi, O.; Addison, J.B.; Wang, Q.; Shen, Z.; Briggs, S.P.; Bohlmann, J.; Castro-Falcon, G.; et al. Discovery, biosynthesis and stress-related accumulation of dolabradiene-derived defenses in maize. Plant Physiol. 2018, 176, 2677–2690. [Google Scholar] [CrossRef]
- Kuc, J. Phytoalexins, stress metabolism, and disease resistance in plants. Annu. Rev. Phytopathol. 1995, 33, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; San Andres, V.; Cervera, M.; Redondo, A.; Alquezar, B.; Shimada, T.; Gadea, J.; Rodrigo, M.; Zacarias, L.; Palou, L.; et al. The monoterpene limonene in orange peels attracts pests and microorganisms. Plant Signal Behav. 2011, 6, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from arabidopsis thaliana flowers, the sesquiterpene (E)-beta-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, M.X.; Guo, H.; Campos-Herrera, R.; Roder, G.; Imperiali, N.; Keel, C.; Maurhofer, M.; Turlings, T.C.J. Root-colonizing bacteria enhance the levels of (E)-beta-caryophyllene produced by maize roots in response to rootworm feeding. Oecologia 2018, 187, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kunert, G.; Reinhold, C.; Gershenzon, J. Constitutive emission of the aphid alarm pheromone, (E)-beta-farnesene, from plants does not serve as a direct defense against aphids. BMC Ecol. 2010, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Kollner, T.G.; Gershenzon, J.; Degenhardt, J. Molecular and biochemical evolution of maize terpene synthase 10, an enzyme of indirect defense. Phytochemistry 2009, 70, 1139–1145. [Google Scholar] [CrossRef]
- Kollner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.; Gershenzon, J.; Degenhardt, J. A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most american maize varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Brillada, C.; Nishihara, M.; Shimoda, T.; Garms, S.; Boland, W.; Maffei, M.E.; Arimura, G. Metabolic engineering of the C16 homoterpene TMTT in Lotus japonicus through overexpression of (E,E)-geranyllinalool synthase attracts generalist and specialist predators in different manners. New Phytol. 2013, 200, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, R.; Huh, J.H.; Badieyan, S.; Rakotondraibe, L.H.; Kliebenstein, D.J.; Sobrado, P.; Tholl, D. In planta variation of volatile biosynthesis: An alternative biosynthetic route to the formation of the pathogen-induced volatile homoterpene DMNT via triterpene degradation in Arabidopsis roots. Plant Cell 2015, 27, 874–890. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.; Lin, F.Y. Terpene biosynthesis: Modularity rules. Angew. Chem. 2012, 51, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Hemmerlin, A.; Harwood, J.L.; Fau-Bach, T.J.; Bach, T.J. A raison d’etre for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 2012, 51, 95–148. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D.; Lee, S. Terpene specialized metabolism in Arabidopsis thaliana. Arabidopsis Book 2011, 9, e0143. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Liu, J.; Ren, F.; Peters, R.J.; Wang, Q. Characterization of CYP71Z18 indicates a role in maize zealexin biosynthesis. Phytochemistry 2016, 121, 4–10. [Google Scholar] [CrossRef]
- Duba, A.; Goriewa-Duba, K.; Wachowska, U. A review of the interactions between wheat and wheat pathogens: Zymoseptoria tritici, Fusarium spp. and Parastagonospora nodorum. Int. J. Mol. Sci. 2018, 19, 1138. [Google Scholar] [CrossRef]
- Bahrini, I.; Ogawa, T.; Kobayashi, F.; Kawahigashi, H.; Handa, H. Overexpression of the pathogen-inducible wheat TAWRKY45 gene confers disease resistance to multiple fungi in transgenic wheat plants. Breed. Sci. 2011, 61, 319–326. [Google Scholar] [CrossRef]
- Wang, F.; Lin, R.; Feng, J.; Chen, W.; Qiu, D.; Xu, S. TANAC1 acts as a negative regulator of stripe rust resistance in wheat, enhances susceptibility to Pseudomonas syringae, and promotes lateral root development in transgenic Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 108. [Google Scholar] [CrossRef]
- Juliana, P.; Singh, R.P.; Singh, P.K.; Poland, J.A.; Bergstrom, G.C.; Huerta-Espino, J.; Bhavani, S.; Crossa, J.; Sorrells, M.E. Genome-wide association mapping for resistance to leaf rust, stripe rust and tan spot in wheat reveals potential candidate genes. Theor. Appl. Genet. 2018, 131, 1405–1422. [Google Scholar] [CrossRef]
- Drakulic, J.; Ajigboye, O.; Swarup, R.; Bruce, T.; Ray, R.V. Aphid infestation increases Fusarium langsethiae and T-2 and HT-2 mycotoxins in wheat. Appl. Environ. Microbiol. 2016, 82, 6548–6556. [Google Scholar] [CrossRef]
- Martyniuk, S.; Stochmal, A.; Macias, F.A.; Marin, D.; Oleszek, W. Effects of some benzoxazinoids on in vitro growth of Cephalosporium gramineum and other fungi pathogenic to cereals and on Cephalosporium stripe of winter wheat. J. Agric. Food Chem. 2006, 54, 1036–1039. [Google Scholar] [CrossRef]
- Stochmal, A.; Kus, J.; Martyniuk, S.; Oleszek, W. Concentration of benzoxazinoids in roots of field-grown wheat (Triticum aestivum L.) varieties. J. Agric. Food Chem. 2006, 54, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, K.; Toyomasu, T.; Sugawara, C.; Oku, M.; Abe, S.; Usui, M.; Mitsuhashi, W.; Chono, M.; Chandler, P.M.; et al. Functional characterization of wheat copalyl diphosphate synthases sheds light on the early evolution of labdane-related diterpenoid metabolism in the cereals. Phytochemistry 2012, 84, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Xu, M.; Tiernan, M.; Xie, Q.; Toyomasu, T.; Sugawara, C.; Oku, M.; Usui, M.; Mitsuhashi, W.; Chono, M.; et al. Functional characterization of wheat ent-kaurene(-like) synthases indicates continuing evolution of labdane-related diterpenoid metabolism in the cereals. Phytochemistry 2012, 84, 47–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.X.; Yu, X.D.; Fan, J.; Pickett, J.A.; Jones, H.D.; Zhou, J.J.; Birkett, M.A.; Caulfield, J.; Napier, J.A.; et al. Molecular characterization of two isoforms of a farnesyl pyrophosphate synthase gene in wheat and their roles in sesquiterpene synthesis and inducible defence against aphid infestation. New Phytol. 2015, 206, 1101–1115. [Google Scholar] [CrossRef]
- Cyr, A.; Wilderman, P.R.; Determan, M.; Peters, R.J. A modular approach for facile biosynthesis of labdane-related diterpenes. J. Am. Chem. Soc. 2007, 129, 6684–6685. [Google Scholar] [CrossRef] [PubMed]
- Reiling, K.; Yoshikuni, Y.; Martin, V.; Newman, J.; Bohlmann, J.; Keasling, J. Mono and diterpene production in Escherichia coli. Biotechnol. Bioeng. 2004, 87, 200–212. [Google Scholar] [CrossRef]
- Fu, J.; Liu, Q.; Wang, C.; Liang, J.; Liu, L.; Wang, Q. ZMWRKY79 positively regulates maize phytoalexin biosynthetic gene expression and is involved in stress response. J. Exp. Bot. 2018, 69, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Morrone, D.; Lowry, L.; Determan, M.K.; Hershey, D.M.; Xu, M.; Peters, R.J. Increasing diterpene yield with a modular metabolic engineering system in E. coli: Comparison of MEV and MEP isoprenoid precursor pathway engineering. Appl. Microbiol. Biotechnol. 2010, 85, 1893–1906. [Google Scholar] [CrossRef]
- Starks, C.M.; Back, K.; Chappell, J.; Noel, J.P. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science 1997, 277, 1815–1820. [Google Scholar] [CrossRef]
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. The diverse sesquiterpene profile of patchouli, pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch. Biochem. Biophys. 2006, 454, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Huffaker, A.; Kollner, T.G.; Weckwerth, P.; Robert, C.A.M.; Spencer, J.L.; Lipka, A.E.; Schmelz, E.A. Selinene volatiles are essential precursors for maize defense promoting fungal pathogen resistance. Plant Physiol. 2017, 175, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Schnee, C.; Kollner, T.G.; Held, M.; Turlings, T.C.; Gershenzon, J.; Degenhardt, J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA 2006, 103, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, X.; Ning, Y.; Jing, W.; Bruce, T.J.; Qi, F.; Xu, Q.; Wu, K.; Zhang, Y.; Guo, Y. TPS46, a Rice Terpene Synthase Conferring Natural Resistance to Bird Cherry-Oat Aphid, Rhopalosiphum padi (Linnaeus). Front. Plant Sci. 2017, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Herde, M.; Gartner, K.; Kollner, T.G.; Fode, B.; Boland, W.; Gershenzon, J.; Gatz, C.; Tholl, D. Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT. Plant Cell 2008, 20, 1152–1168. [Google Scholar] [CrossRef]
- Vaughan, M.M.; Wang, Q.; Webster, F.X.; Kiemle, D.; Tantillo, D.; Coates, R.M.; Wray, A.; Askew, W.; O’Donnell, C.; Tokuhisa, J.G.; et al. Formation of the unusual semivolatile diterpene rhizathalene by the arabidopsis class I terpene synthase TPS08 in the root stele is involved in defense against belowground herbivory. Plant Cell 2013, 25, 1108–1125. [Google Scholar] [CrossRef]
- Degenhardt, J.; Gershenzon, J.; Baldwin, I.T.; Kessler, A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 2003, 14, 169–176. [Google Scholar] [CrossRef]
- Arimura, G.; Shiojiri, K.; Karban, R. Acquired immunity to herbivory and allelopathy caused by airborne plant emissions. Phytochemistry 2010, 71, 1642–1649. [Google Scholar] [CrossRef]
- Zhou, K.; Peters, R.J. Electrostatic effects on (di)terpene synthase product outcome. Chem. Commun. 2011, 47, 4074–4080. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Q.; Liang, J.; Shen, Q.; Fu, J.; Pu, Z.; Liu, J.; Wang, X.; Wang, Q. A Wheat β-Patchoulene Synthase Confers Resistance against Herbivory in Transgenic Arabidopsis. Genes 2019, 10, 441. https://doi.org/10.3390/genes10060441
Pu Q, Liang J, Shen Q, Fu J, Pu Z, Liu J, Wang X, Wang Q. A Wheat β-Patchoulene Synthase Confers Resistance against Herbivory in Transgenic Arabidopsis. Genes. 2019; 10(6):441. https://doi.org/10.3390/genes10060441
Chicago/Turabian StylePu, Qingyu, Jin Liang, Qinqin Shen, Jingye Fu, Zhien Pu, Jiang Liu, Xuegui Wang, and Qiang Wang. 2019. "A Wheat β-Patchoulene Synthase Confers Resistance against Herbivory in Transgenic Arabidopsis" Genes 10, no. 6: 441. https://doi.org/10.3390/genes10060441
APA StylePu, Q., Liang, J., Shen, Q., Fu, J., Pu, Z., Liu, J., Wang, X., & Wang, Q. (2019). A Wheat β-Patchoulene Synthase Confers Resistance against Herbivory in Transgenic Arabidopsis. Genes, 10(6), 441. https://doi.org/10.3390/genes10060441





