Functional Research on Three Presumed Asparagine Synthetase Family Members in Poplar
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Environment and Sampling Method
2.2. Search and Analysis of Gene Families
2.3. Escherichia coli Complementation Experiment
2.4. Nitrogen Treatment
2.5. RNA Extraction and Quantitative Real-Time PCR Detection
2.6. Statistical Analysis
3. Results
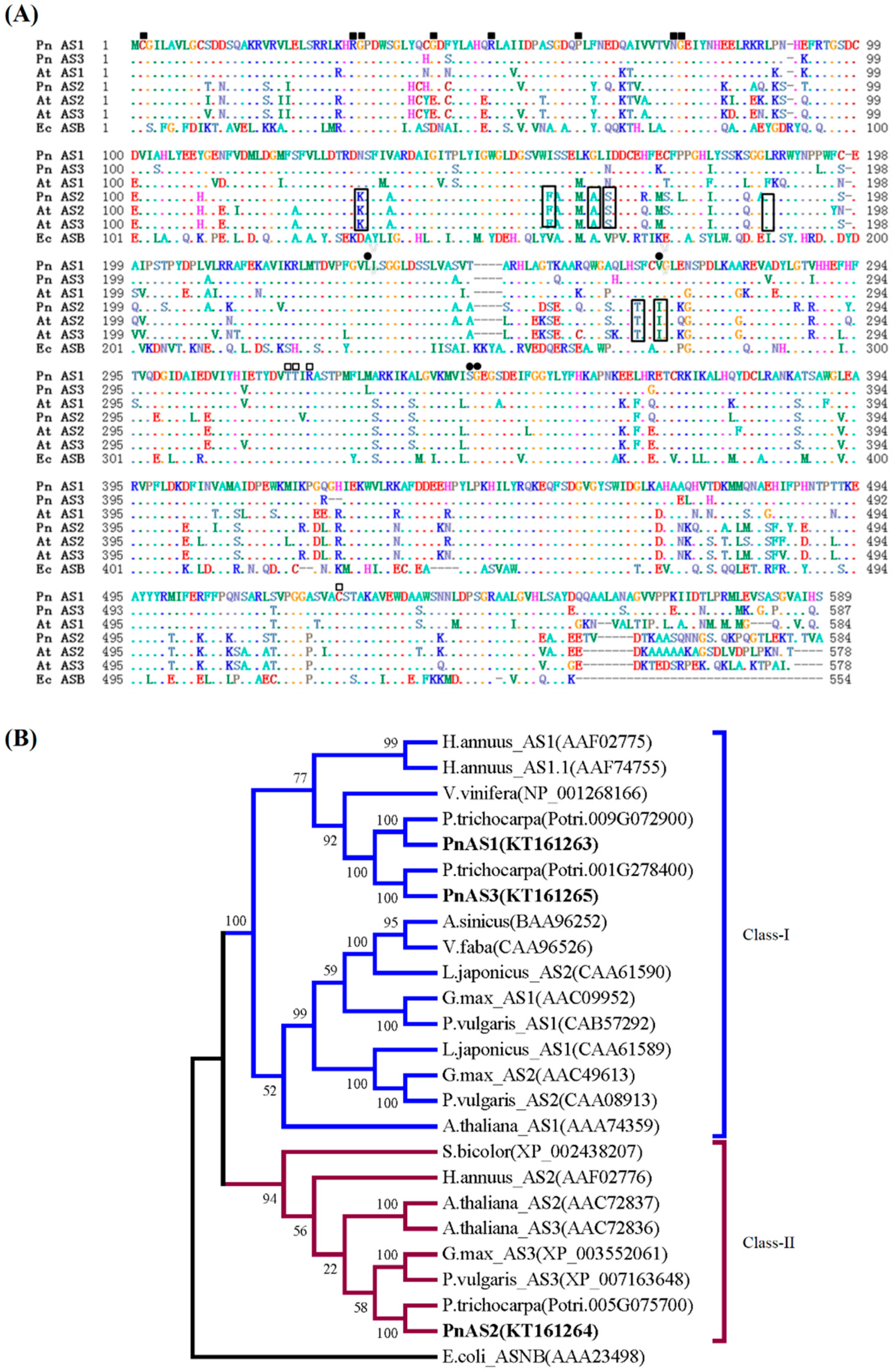
3.1. Sequence Analysis of Asparagine Synthetase Family Members in Populus simonii × P. nigra
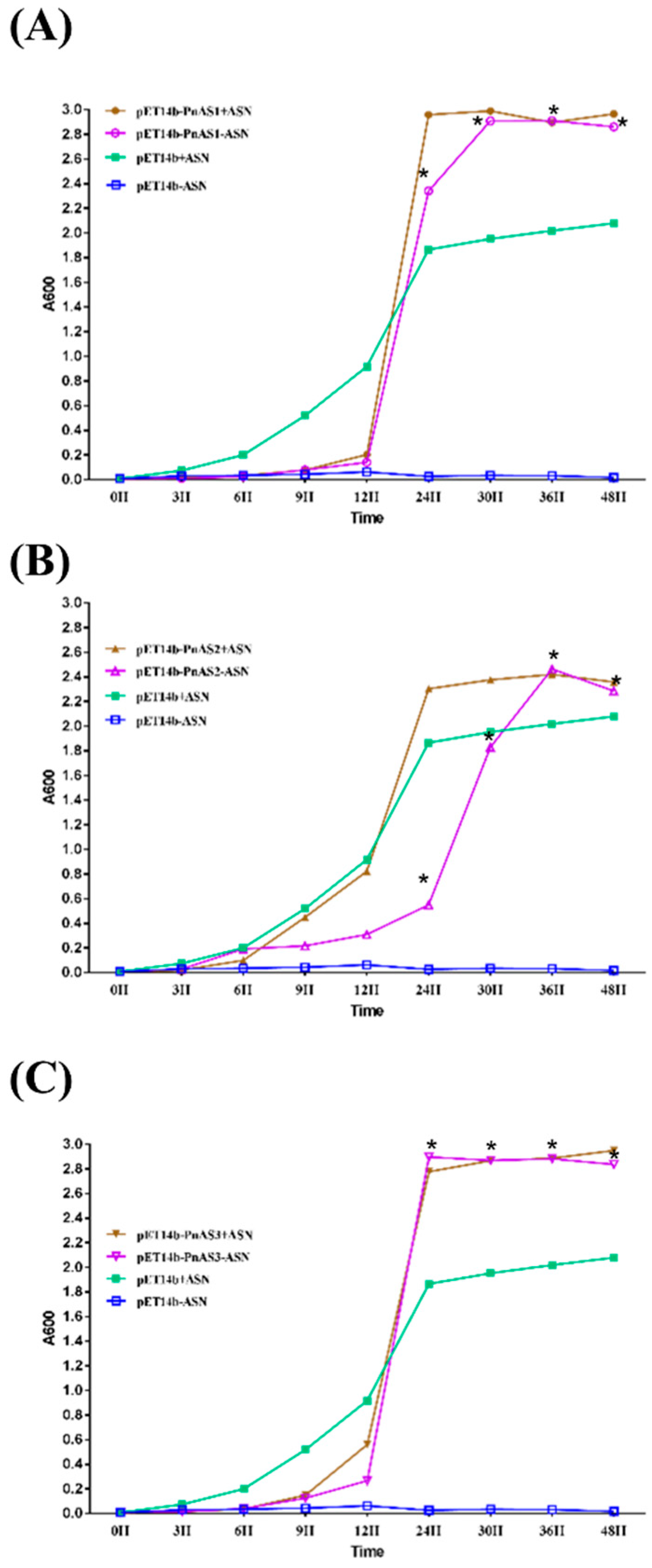
3.2. Asn-Deficient Escherichia coli Complementation Experiment
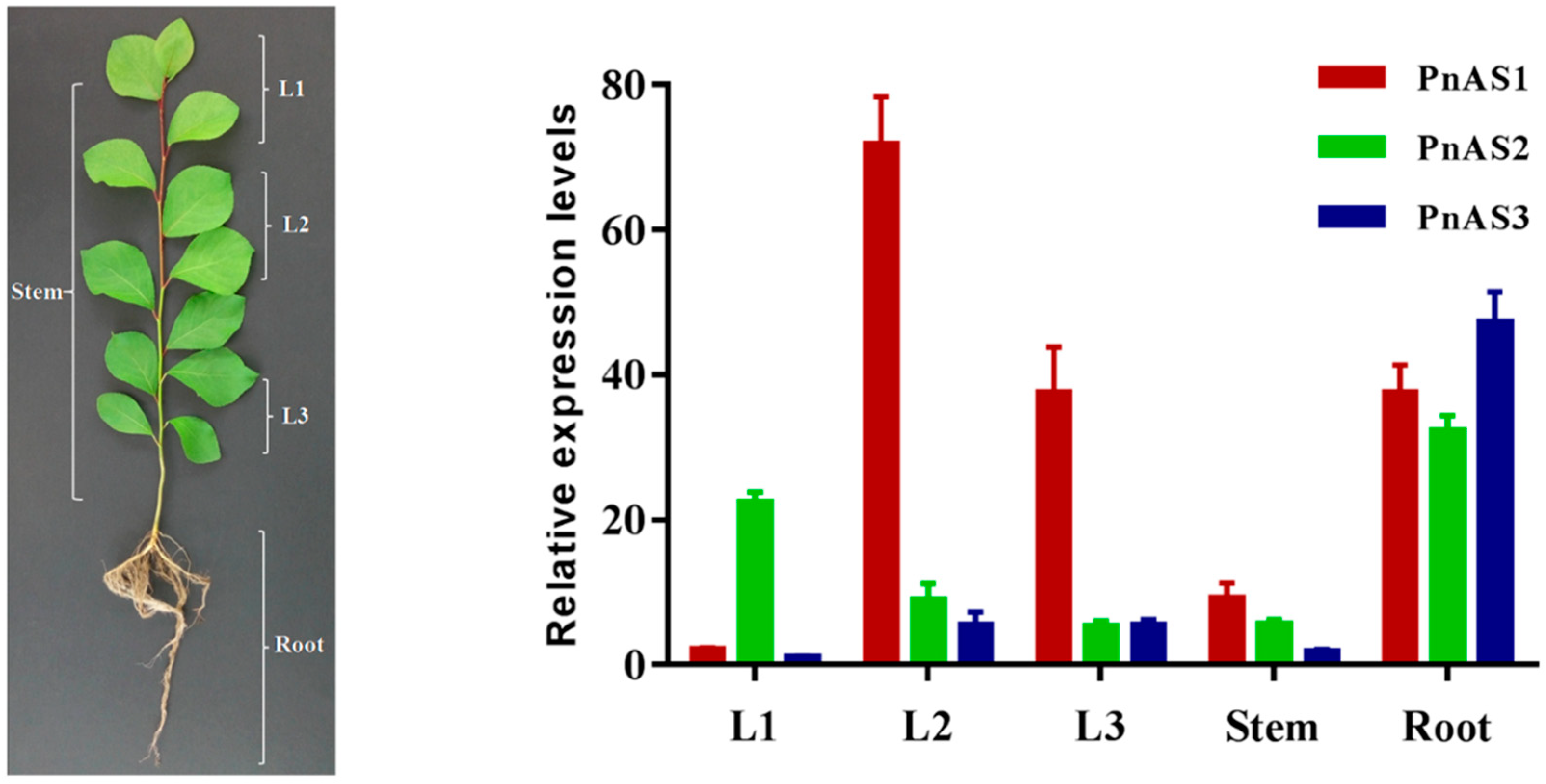
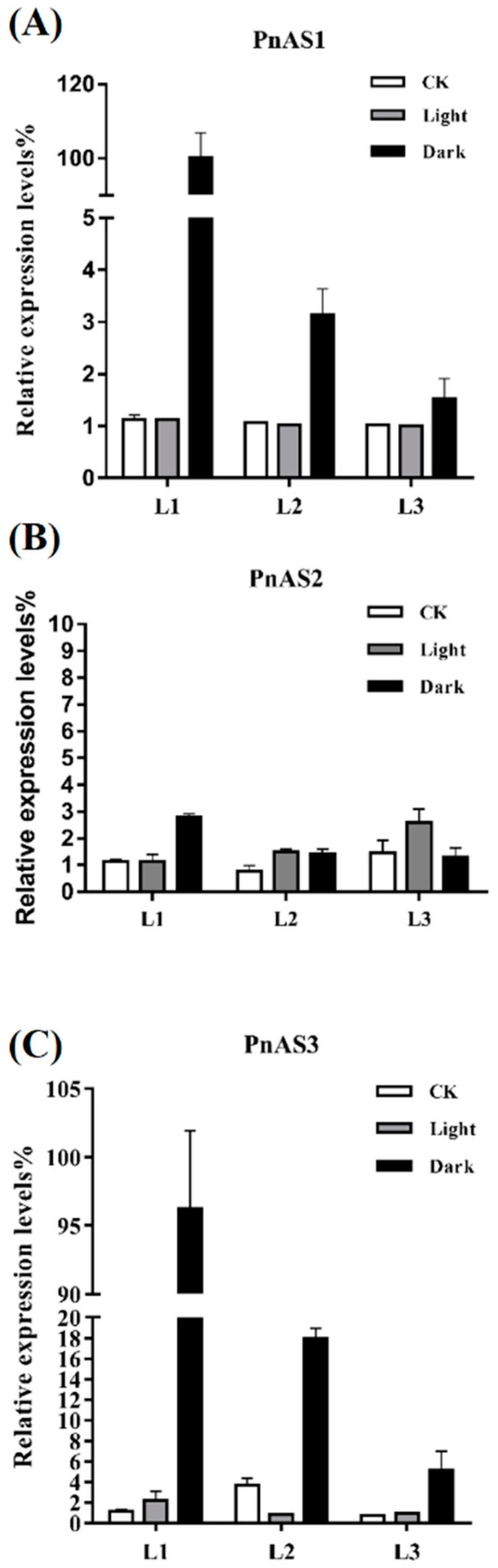
3.3. Spatiotemporal Specific Expression Patterns of the Three AS Genes in Populus simonii × P. nigra
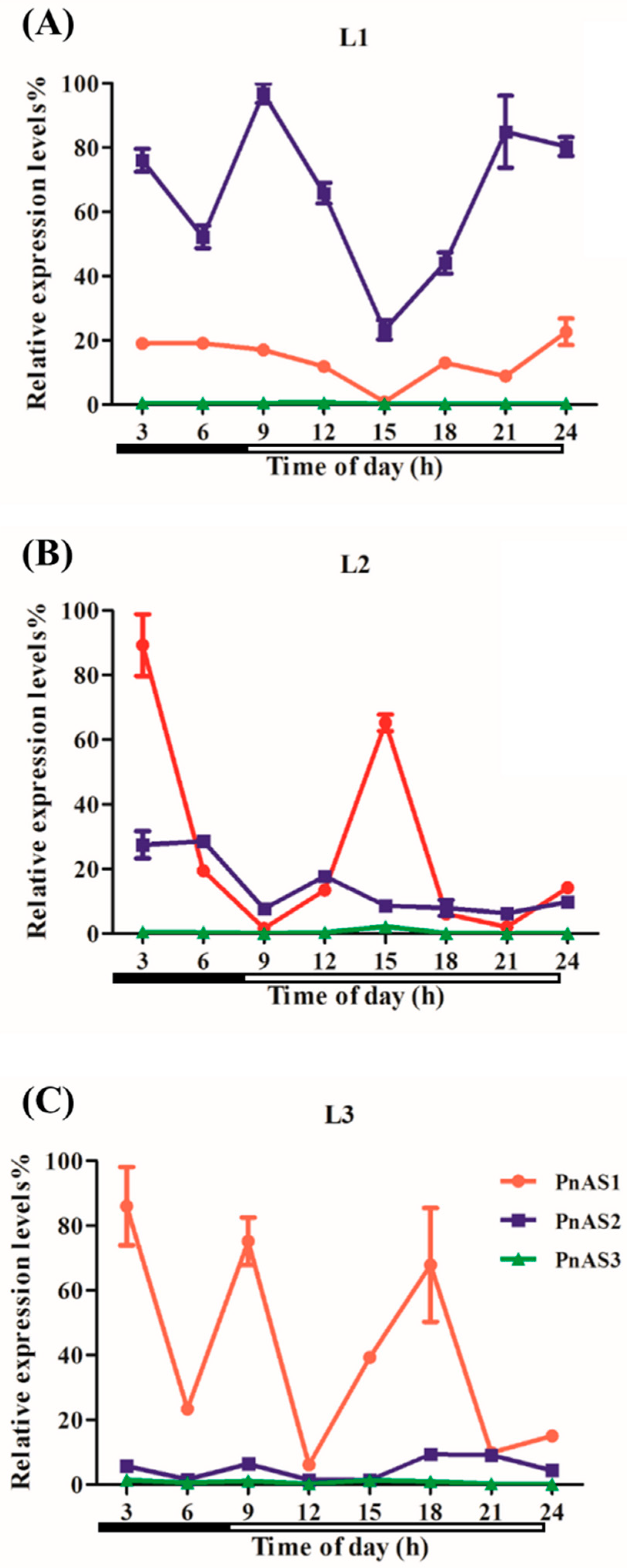
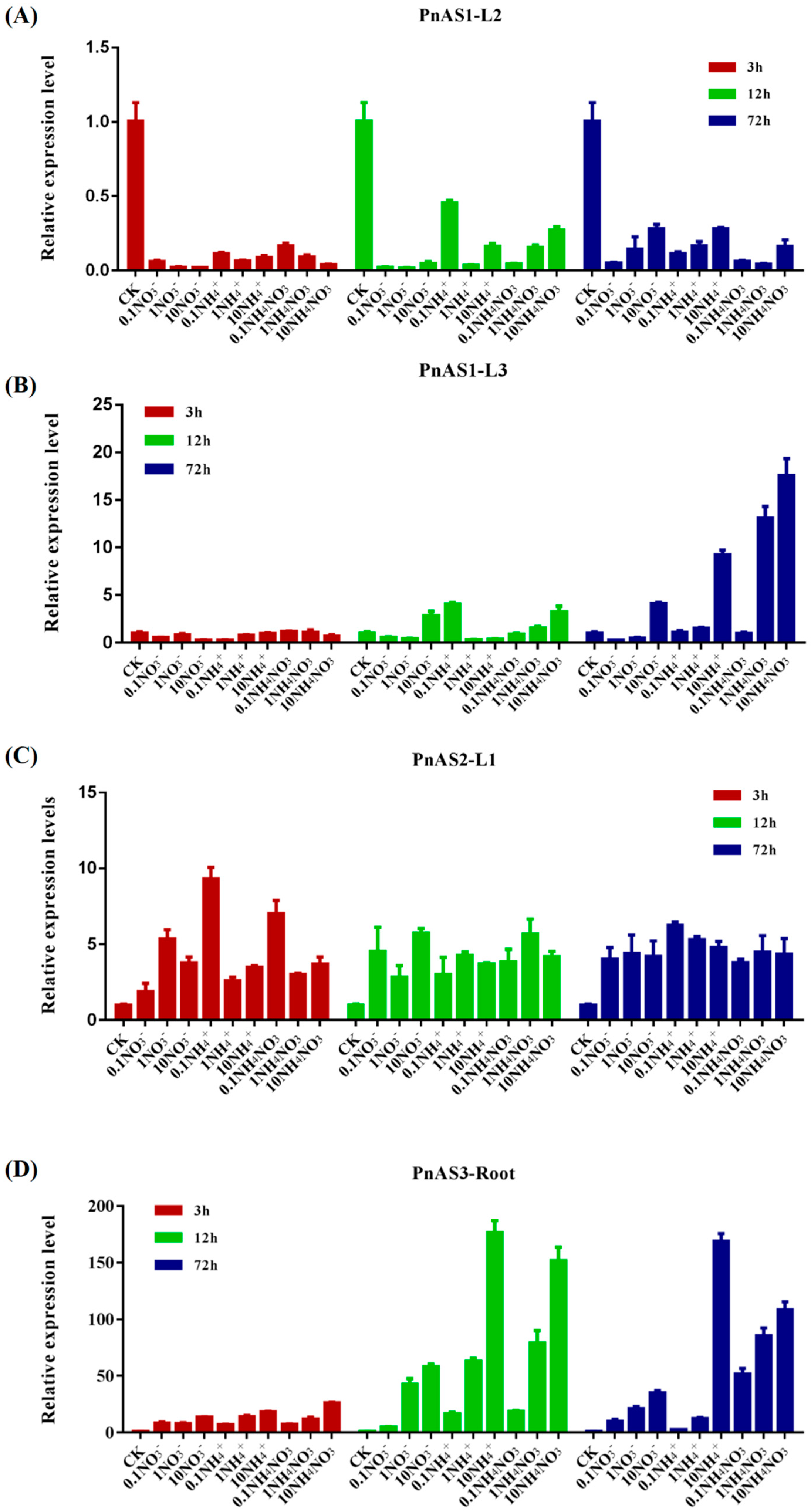
3.4. The Effect of Exogenous Nitrogen on Patterns of Asparagine Synthetase Gene Expression
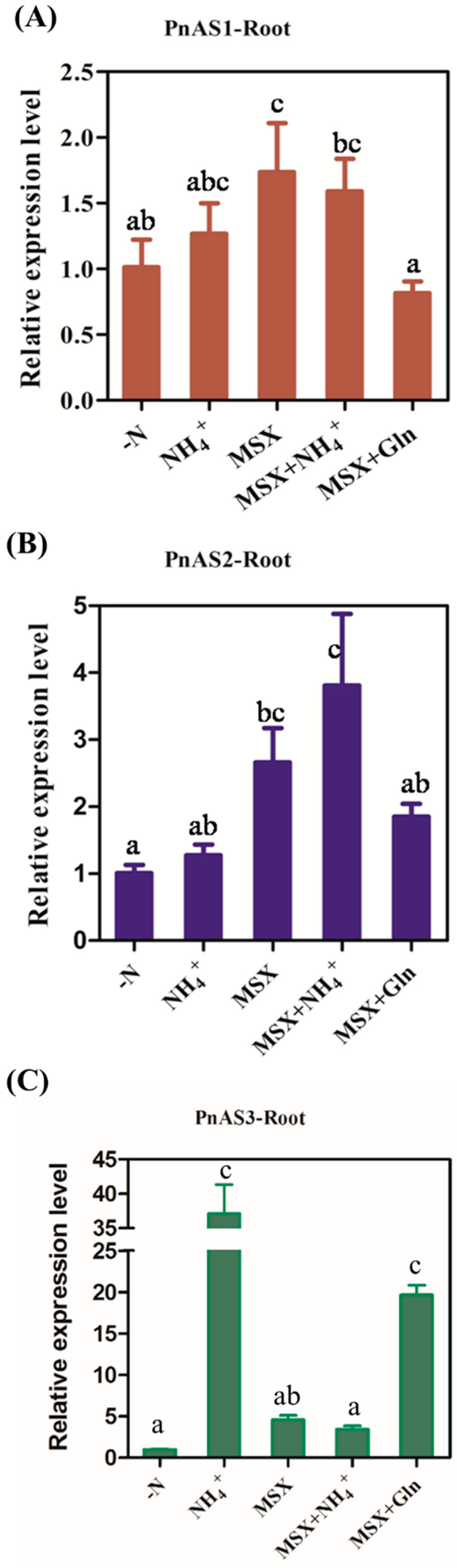
3.5. Expression Trend of PnAS under MSX Treatment
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Fukumorita, T.; Chino, M. Sugar, amino acid and inorganic contents in rice phloem sap. Plant Cell Physiol. 1982, 23, 273–283. [Google Scholar]
- Antunes, F.; Aguilar, M.; Pineda, M.; Sodek, L. Nitrogen stress and the expression of asparagine synthetase in roots and nodules of soybean (glycine max). Physiol. Plant. 2008, 133, 736–743. [Google Scholar] [CrossRef]
- Diaz-Leal, J.L.; Galvez-Valdivieso, G.; Fernandez, J.; Pineda, M.; Alamillo, J.M. Developmental effects on ureide levels are mediated by tissue-specific regulation of allantoinase in phaseolus vulgaris L. J. Exp. Bot. 2012, 63, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- Funayama, K.; Kojima, S.; Tabuchi-Kobayashi, M.; Sawa, Y.; Nakayama, Y.; Hayakawa, T.; Yamaya, T. Cytosolic glutamine synthetase1;2 is responsible for the primary assimilation of ammonium in rice roots. Plant Cell Physiol. 2013, 54, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Chino, M. Chemical composition of phloem sap from the uppermost internode of the rice plant. Plant Cell Physiol. 1990, 31, 247–251. [Google Scholar]
- Puiatti, M.; Sodek, L. Waterlogging affects nitrogen transport in the xylem of soybean. Plant Physiol. Biochem. 1999, 37, 767–773. [Google Scholar] [CrossRef]
- Van Heeke, G.; Schuster, S.M. Expression of human asparagine synthetase in escherichia coli. J. Biol. Chem. 1989, 264, 5503–5509. [Google Scholar]
- Davies, K.M.; King, G.A. Isolation and characterization of a cdna clone for a harvest-induced asparagine synthetase from asparagus officinalis L. Plant Physiol. 1993, 102, 1337–1340. [Google Scholar] [CrossRef]
- Lam, H.M.; Peng, S.S.; Coruzzi, G.M. Metabolic regulation of the gene encoding glutamine-dependent asparagine synthetase in arabidopsis thaliana. Plant Physiol. 1994, 106, 1347–1357. [Google Scholar] [CrossRef]
- Tsai, F.Y.; Coruzzi, G.M. Dark-induced and organ-specific expression of two asparagine synthetase genes in pisum sativum. EMBO J. 1990, 9, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.; Zalkin, H. A cysteine-histidine-aspartate catalytic triad is involved in glutamine amide transfer function in purf-type glutamine amidotransferases. J. Biol. Chem. 1989, 264, 16613–16619. [Google Scholar]
- Mantsala, P.; Zalkin, H. Cloning and sequence of bacillus subtilis pura and guaa, involved in the conversion of imp to amp and gmp. J. Bacteriol. 1992, 174, 1883–1890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, L.; Twary, S.N.; Yoshioka, H.; Gregerson, R.G.; Miller, S.S.; Samac, D.A.; Gantt, J.S.; Unkefer, P.J.; Vance, C.P. Nitrogen assimilation in alfalfa: Isolation and characterization of an asparagine synthetase gene showing enhanced expression in root nodules and dark-adapted leaves. Plant Cell 1997, 9, 1339–1356. [Google Scholar] [CrossRef]
- Galvez-Valdivieso, G.; Alamillo, J.M.; Fernandez, J.; Pineda, M. Molecular characterization of pvas3: An asparagine synthetase gene from common bean prevailing in developing organs. J. Plant Physiol. 2013, 170, 1484–1490. [Google Scholar] [CrossRef]
- Herrera-Rodríguez, M.B.; Carrasco-Ballesteros, S.; Maldonado, J.M.; Pineda, M.; Aguilar, M.; Pérez-Vicente, R. Three genes showing distinct regulatory patterns encode the asparagine synthetase of sunflower (helianthus annuus). New Phytol. 2002, 155, 33–45. [Google Scholar] [CrossRef]
- Gaufichon, L.; Reisdorf-Cren, M.; Rothstein, S.J.; Chardon, F.; Suzuki, A. Biological functions of asparagine synthetase in plants. Plant Sci. 2010, 179, 141–153. [Google Scholar] [CrossRef]
- Parra-Peralbo, E.; Pineda, M.; Aguilar, M. Pvas3, a class-ii ubiquitous asparagine synthetase from the common bean (phaseolus vulgaris). Mol. Biol. Rep. 2009, 36, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Canales, J.; Rueda-Lopez, M.; Craven-Bartle, B.; Avil, C.; Canovas, F.M. Novel insights into regulation of asparagine synthetase in conifers. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef]
- Lam, H.M.; Hsieh, M.H.; Coruzzi, G. Reciprocal regulation of distinct asparagine synthetase genes by light and metabolites in arabidopsis thaliana. Plant J. 1998, 16, 345–353. [Google Scholar] [CrossRef]
- Osuna, D.; Galvez-Valdivieso, G.; Piedras, P.; Pineda, M.; Aguilar, M. Cloning, characterization and mrna expression analysis of pvas1, a type i asparagine synthetase gene from phaseolus vulgaris. Planta 2001, 213, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Osuna, D.; Galvez, G.; Pineda, M.; Aguilar, M. Rt-pcr cloning, characterization and mrna expression analysis of a cdna encoding a type ii asparagine synthetase in common bean. BBA 1999, 1445, 75–85. [Google Scholar] [CrossRef]
- Wong, H.K.; Chan, H.K.; Coruzzi, G.M.; Lam, H.M. Correlation of asn2 gene expression with ammonium metabolism in arabidopsis. Plant Physiol. 2004, 134, 332–338. [Google Scholar] [CrossRef]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef]
- Brunner, A.M.; Busov, V.B.; Strauss, S.H. Poplar genome sequence: Functional genomics in an ecologically dominant plant species. Trends Plant Sci. 2004, 9, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. Mega5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Wu, X.Y.; Yang, H.; Qu, C.P.; Xu, Z.R.; Li, W.; Hao, B.Q.; Yang, C.P.; Sun, G.Y.; Liu, G.J. Sequence and expression analysis of the amt gene family in poplar. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2− δδct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Felton, J.; Michaelis, S.; Wright, A. Mutations in two unlinked genes are required to produce asparagine auxotrophy in escherichia coli. J. Bacteriol. 1980, 142, 221–228. [Google Scholar]
- Waterhouse, R.N.; Smyth, A.J.; Massonneau, A.; Prosser, I.M.; Clarkson, D.T. Molecular cloning and characterisation of asparagine synthetase from lotus japonicus: Dynamics of asparagine synthesis in n-sufficient conditions. Plant Mol. Biol. 1996, 30, 883–897. [Google Scholar] [CrossRef]
- Herrera-Rodriguez, M.B.; Maldonado, J.M.; Perez-Vicente, R. Light and metabolic regulation of has1, has1.1 and has2, three asparagine synthetase genes in helianthus annuus. Plant Physiol. Biochem. PPB 2004, 42, 511–518. [Google Scholar] [CrossRef]
- Avila-Ospina, L.; Marmagne, A.; Talbotec, J.; Krupinska, K.; Masclaux-Daubresse, C. The identification of new cytosolic glutamine synthetase and asparagine synthetase genes in barley (hordeum vulgare l.), and their expression during leaf senescence. J. Exp. Bot. 2015, 66, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Escher, P.; Eiblmeier, M.; Hetzger, I.; Rennenberg, H. Spatial and seasonal variation in amino compounds in the xylem sap of a mistletoe (viscum album) and its hosts (populus spp. And abies alba). Tree Physiol. 2004, 24, 639–650. [Google Scholar] [CrossRef]
- Kato, T. Major nitrogen compounds transported in xylem vessels from roots to top in citrus trees. Physiol. Plant. 2010, 52, 275–279. [Google Scholar] [CrossRef]
- Muttucumaru, N.; Keys, A.J.; Parry, M.A.; Powers, S.J.; Halford, N.G. Photosynthetic assimilation of (1)(4)c into amino acids in potato (solanum tuberosum) and asparagine in the tubers. Planta 2014, 239, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, V.; Garcia-Gutierrez, A.; Canales, J.; Avila, C.; Kirby, E.G.; Canovas, F.M. The glutamine synthetase gene family in populus. BMC Plant Biol. 2011, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Valdivieso, G.; Osuna, D.; Maldonado, J.M.; Pineda, M.; Aguilar, M. Purification of a functional asparagine synthetase (pvas2) from common bean (phaseolus vulgaris), a protein predominantly found in root tissues. Plant Sci. 2005, 168, 89–94. [Google Scholar] [CrossRef]
- Gaufichon, L.; Masclaux-Daubresse, C.; Tcherkez, G.; Reisdorf-Cren, M.; Sakakibara, Y.; Hase, T.; Clement, G.; Avice, J.C.; Grandjean, O.; Marmagne, A.; et al. Arabidopsis thaliana asn2 encoding asparagine synthetase is involved in the control of nitrogen assimilation and export during vegetative growth. Plant Cell Environ. 2013, 36, 328–342. [Google Scholar] [CrossRef]
- Lima, J.E.; Kojima, S.; Takahashi, H.; von Wiren, N. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. Plant Cell 2010, 22, 3621–3633. [Google Scholar] [CrossRef]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef]
- Gao, R.; Curtis, T.Y.; Powers, S.J.; Xu, H.; Huang, J.; Halford, N.G. Food safety: Structure and expression of the asparagine synthetase gene family of wheat. J. Cereal Sci. 2016, 68, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.H.; Prosser, I.; Muttucumaru, N.; Curtis, T.Y.; Wingler, A.; Powers, S.; Halford, N.G. Overexpression of gcn2-type protein kinase in wheat has profound effects on free amino acid concentration and gene expression. Plant Biotechnol. J. 2012, 10, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ball, G.; Hodgman, C.; Coules, A.; Zhao, H.; Lu, C.G. Analysis of gene regulatory networks of maize in response to nitrogen. Genes Basel 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.; Screen, S.; Crowley, J.; Peng, J.X.; Andersen, S.; Brown, T.; Qi, Q.G.; Fabbri, B.; Duff, S.M.G. Identification and characterization of four distinct asparagine synthetase (asns) genes in maize (zea mays l.). Plant Sci. 2008, 175, 799–808. [Google Scholar] [CrossRef]
| Name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| PnAS1-HF | CATGCCATGGGCATGTGTGGGATA | ATAGTTTAGCGGCCGCCTAACTGTG |
| PnAS2-HF | CCGCTCGAGATGTGCGGCATCCT | ATAGTTTAGCGGCCGCTCAAGCAACT |
| PnAS3-HF | CATGCCATGGGCATGTGTGGGATA | ATAGTTTAGCGGCCGCCTAACTTTG |
| Gene | Accession No | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|---|
| PnAS1 | KT161263 | TGTTGGAAGTTAGTGCTTCGG | GACAACACACGACTTCAAAGGA |
| PnAS2 | KT161264 | ACCAAGGCTGCCAGTCAGAATAA | CGTACCCTAACTAAAGCGAACGAAA |
| PnAS3 | KT161265 | GAGGACCGAAGTACATGCC | CAACAAGGTGCCAACACTACT |
| Actin | XM_002298946 | CACAACTGCTGAACGGGAAAT | CAGGGCAACGGAAACACTCT |
| Name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| PnAS1 | ATGTGTGGGATACTTGCTG | CTAACTGTGGATCGCAAC |
| PnAS2 | ATGTGCGGCATCCTCGCTG | TCAAGCAACTGTTGCAGT |
| PnAS3 | ATGTGTGGGATACTTGCTGTTT | CTAACTTTGGATTGCAACTCCTG |
| PnAS1 | PnAS3 | PnAS2 | |
|---|---|---|---|
| PnAS1 | — | 91% | 68.80% |
| PnAS3 | 94% | — | 69.50% |
| PnAS2 | 80% | 77.80% | — |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, C.; Hao, B.; Xu, X.; Wang, Y.; Yang, C.; Xu, Z.; Liu, G. Functional Research on Three Presumed Asparagine Synthetase Family Members in Poplar. Genes 2019, 10, 326. https://doi.org/10.3390/genes10050326
Qu C, Hao B, Xu X, Wang Y, Yang C, Xu Z, Liu G. Functional Research on Three Presumed Asparagine Synthetase Family Members in Poplar. Genes. 2019; 10(5):326. https://doi.org/10.3390/genes10050326
Chicago/Turabian StyleQu, Chunpu, Bingqing Hao, Xiuyue Xu, Yuchen Wang, Chengjun Yang, Zhiru Xu, and Guanjun Liu. 2019. "Functional Research on Three Presumed Asparagine Synthetase Family Members in Poplar" Genes 10, no. 5: 326. https://doi.org/10.3390/genes10050326
APA StyleQu, C., Hao, B., Xu, X., Wang, Y., Yang, C., Xu, Z., & Liu, G. (2019). Functional Research on Three Presumed Asparagine Synthetase Family Members in Poplar. Genes, 10(5), 326. https://doi.org/10.3390/genes10050326




