Increased Overall Mortality Even after Risk Reducing Surgery for BRCA-Positive Women in Western Sweden
Abstract
1. Introduction
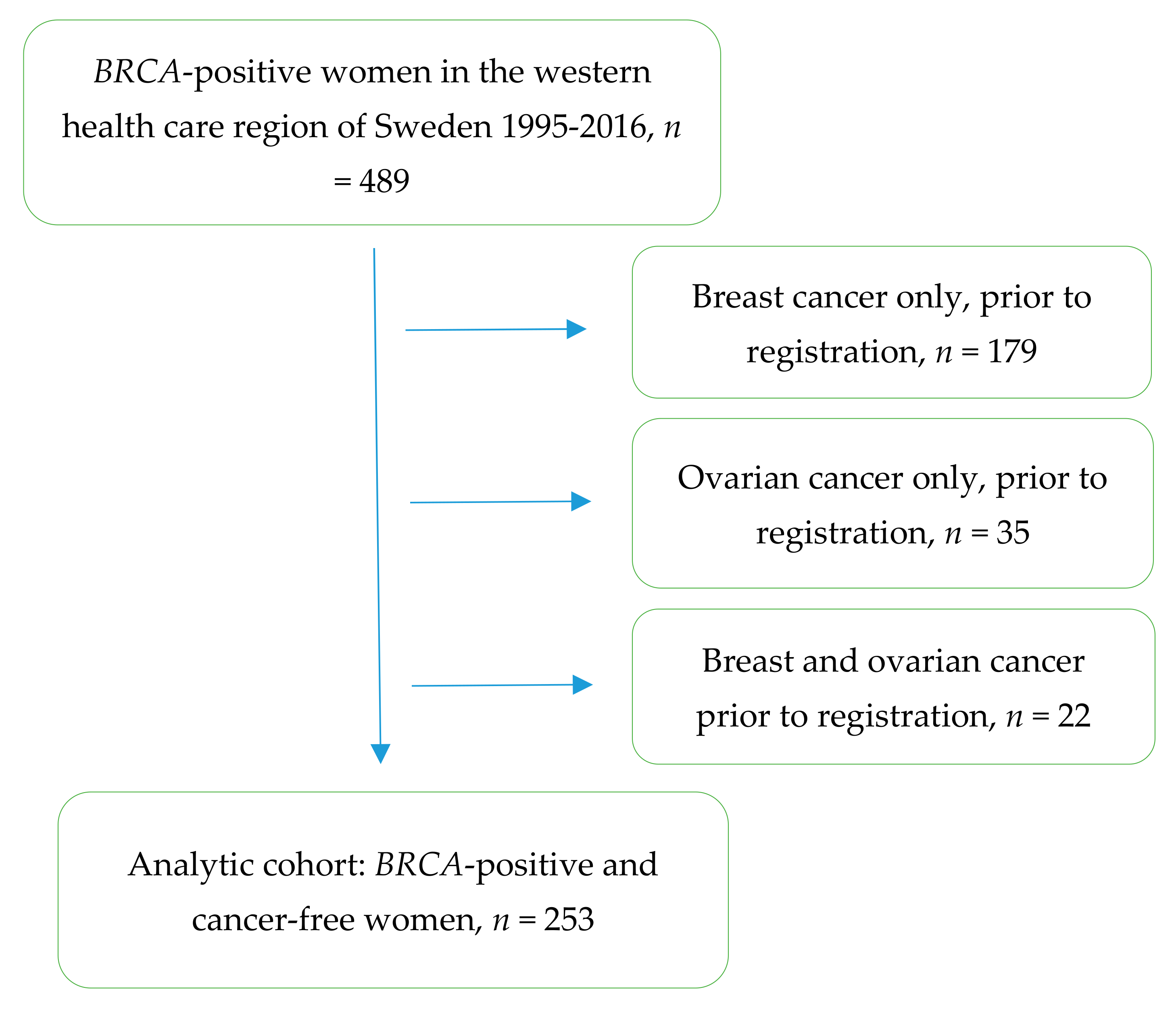
2. Materials and Methods
3. Results
3.1. Breast Cancer Incidence
3.2. Ovarian Cancer Incidence
3.3. Overall Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Wooster, R.; Neuhausen, S.L.; Mangion, J.; Quirk, Y.; Ford, D.; Collins, N.; Nguyen, K.; Seal, S.; Tran, T.; Averill, D.; et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science 1994, 265, 2088–2090. [Google Scholar] [CrossRef] [PubMed]
- Einbeigi, Z.; Bergman, A.; Kindblom, L.G.; Martinsson, T.; Meis-Kindblom, J.M.; Nordling, M.; Suurküla, M.; Wahlström, J.; Wallgren, A.; Karlsson, P. A founder mutation of the BRCA1 gene in Western Sweden associated with a high incidence of breast and ovarian cancer. Eur. J. Cancer 2001, 37, 1904–1909. [Google Scholar] [CrossRef]
- Bergman, A.; Einbeigi, Z.; Olofsson, U.; Taib, Z.; Wallgren, A.; Karlsson, P.; Wahlström, J.; Martinsson, T.; Nordling, M. The western Swedish BRCA1 founder mutation 3171ins5; a 3.7 cM conserved haplotype of today is a reminiscence of a 1500-year-old mutation. Eur. J. Hum. Genet. 2001, 9, 787–793. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.K.; Neuner, J.; Butler, A.; Geurts, J.L.; Kong, A.L. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am. J. Surg. 2016, 212, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Casey, M.J.; Synder, C.; Bewtra, C.; Narod, S.A.; Watson, P.; Lynch, H.T. Intra-abdominal carcinomatosis after prophylactic oophorectomy in women of hereditary breast ovarian cancer syndrome kindreds associated with BRCA1 and BRCA2 mutations. Gynecol. Oncol. 2005, 97, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.P.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Cybulski, C.; et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2014, 32, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Gareth Evans, D.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, N.M.; Mourits, M.J.E.; Arts, H.J.G.; de Vries, J.; Leegte, B.K.; Dijkhuis, G.; Oosterwijk, J.C.; de Bock, G.H. Time to stop ovarian cancer screening in BRCA1/2 mutation carriers? Int. J. Cancer 2009, 124, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Danckert, B.; Ferlay, J.; Engholm, G.; Hansen, H.L.; Johannesen, T.B.; Khan, S.; Køtlum, J.E.; Ólafsdóttir, E.; Schmidt, L.K.H.; Virtanen, A.; et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.2 (26.03.2019). 2019. Available online: http: //www.ancr.nu (accessed on 2 December 2019).
- Working Group Report. International Association of Cancer Registries (IARC). Internal Report No. 2004/02. International rules for multiple primary cancers (ICD-0 third edition). Eur. J. Cancer Prev. 2005, 14, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Stata Statistical Software. Stata Statistical Software. Stata: Release 15 Statistical Software; StataCorp LLC: College Station, TX, USA, 2017. [Google Scholar]
- Mavaddat, N.; Michailidou, K.; Dennis, J.; Lush, M.; Fachal, L.; Lee, A.; Tyrer, J.P.; Chen, T.H.; Wang, Q.; Bolla, M.K.; et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am. J. Hum. Genet. 2019, 104, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Crum, C.P.; Drapkin, R.; Kindelberger, D.; Medeiros, F.; Miron, A.; Lee, Y. Lessons from BRCA: The tubal fimbria emerges as an origin for pelvic serous cancer. Clin. Med. Res. 2007, 5, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Guney Eskiler, G. Talazoparib to treat BRCA-positive breast cancer. Drugs Today Barc. 2019, 55, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.M.; Grossardt, B.R.; Rhodes, D.J.; Brown, R.D., Jr.; Roger, V.L.; Joseph Melton, L., III; Rocca, W.A. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Gordhandas, S.; Norquist, B.M.; Pennington, K.P.; Yung, R.L.; Laya, M.B.; Swisher, E.M. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol. Oncol. 2019, 153, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.; Chen, D.; Lang, J.E. The current status of the clinical utility of liquid biopsies in cancer. Expert Rev. Mol. Diagn. 2019, 19, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Clatot, F. Review ctDNA and breast cancer. Recent Results Cancer Res. 2020, 215, 231–252. [Google Scholar] [PubMed]
- Thompson, D.; Easton, D. Variation in BRCA1 cancer risks by mutation position. Cancer Epidemiol Biomark. Prev. 2002, 11, 329–336. [Google Scholar]
- Mersch, J.; Jackson, M.A.; Park, M.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015, 121, 269–275. [Google Scholar] [CrossRef] [PubMed]
| Breast cancer in the cohort | |
| All women, cancer-free when registered | 253 |
| All women cancer-free and with both breasts | 249 |
| Breast cancer diagnoses | 31/249 |
| Diagnoses during surveillance | 25/31 |
| Diagnoses from final pathology report, RRM | 4/31 |
| Diagnoses after RRM | 2/31 |
| Mean age at diagnosis (range) | 46.0 (32.4–64.4) |
| Median age at RRM | 42.3 (22.0–65.3) |
| Ovarian cancer in the cohort | |
| All women, with ovaries and cancer-free when registered | 239 |
| Ovarian cancer diagnosis | 14/239 |
| Diagnosis during surveillance | 7/14 |
| Diagnosis from final pathology report, RRSO | 4/14 |
| Diagnosis after RRSO | 3/14 |
| Mean age at diagnosis (range) | 46.7 (39.1–51.5) |
| Median age at RRSO | 43.4 (28.2–79.7) |
| Breast Cancer Incidence | Follow-Up Time, Years, Median (Range) | Breast Years | Number of Breast Cancers (Expected Number) | SIR (95% CI) |
| Women before RRM (n = 249) | 3.85 (0.02–19.9) | 2721.6 | 25 (1.8) | 14.0 (9.42–20.7) |
| Women after RRM (n = 184) | 5.3 (0.3–23.8) | 1271.7 | 2 (1.04) | 1.93 (0.48–7.70) |
| Ovarian Cancer Incidence | Follow-Up Time, Years, Median (Range) | Person Years | Number of Ovarian Cancers (Expected Number) | SIR (95% CI) |
| Women before RRSO (n = 239) | 2.2 (0.0–17.3) | 887.4 | 7 (0.1) | 124.6 (59.4–261.3) |
| Women after RRSO (n = 136) | 7.6 (0.58–23.8) | 1883.9 | 3 (0.2) | 13.5 (4.34–41.8) |
| Mortality | Follow-Up Time, Tears, Median (Range) | Person Years | Number of Deaths (Expected Number) | SMR (95% CI) |
| All women in cohort (n = 253) | 7.6 (0.8–21.1) | 2160.6 | 16 (4.9) | 3.24 (1.99–5.30) |
| Women before any risk reducing surgery (n = 235) | 2.1 (0.0–17.7) | 833.6 | 4 (0.7) | 5.56 (2.09–14.8) |
| Women after RRM (n = 80) | 5.3 (0.6–19.1) | 577.6 | 4 (1.0) | 4.15 (1.56–11.0) |
| Women after RRSO (n = 136) | 7.4 (0.7–21.1) | 1144.3 | 11 (3.7) | 2.99 (1.66–5.40) |
| Women after both RRM and RRSO (n = 62) | 6.5 (0.9–19.3) | 480.5 | 4 (0.9) | 4.32 (1.62–11.5) |
| Cause of Death in the Cohort | |
|---|---|
| Breast cancer | 3/16 |
| Breast cancer after RRM | 0/3 |
| Ovarian cancer | 7/16 |
| Peritoneal cancer of ovarian origin after RRSO | 5/7 |
| Other cancer | 3/16 |
| Other cause, non-malignant | 3/16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öfverholm, A.; Einbeigi, Z.; Wigermo, A.; Holmberg, E.; Karsson, P. Increased Overall Mortality Even after Risk Reducing Surgery for BRCA-Positive Women in Western Sweden. Genes 2019, 10, 1046. https://doi.org/10.3390/genes10121046
Öfverholm A, Einbeigi Z, Wigermo A, Holmberg E, Karsson P. Increased Overall Mortality Even after Risk Reducing Surgery for BRCA-Positive Women in Western Sweden. Genes. 2019; 10(12):1046. https://doi.org/10.3390/genes10121046
Chicago/Turabian StyleÖfverholm, Anna, Zakaria Einbeigi, Antonia Wigermo, Erik Holmberg, and Per Karsson. 2019. "Increased Overall Mortality Even after Risk Reducing Surgery for BRCA-Positive Women in Western Sweden" Genes 10, no. 12: 1046. https://doi.org/10.3390/genes10121046
APA StyleÖfverholm, A., Einbeigi, Z., Wigermo, A., Holmberg, E., & Karsson, P. (2019). Increased Overall Mortality Even after Risk Reducing Surgery for BRCA-Positive Women in Western Sweden. Genes, 10(12), 1046. https://doi.org/10.3390/genes10121046





