Genome-Wide Analysis of Members of the WRKY Gene Family and Their Cold Stress Response in Prunus mume
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of P. mume WRKY Genes
2.2. Genomic Localization and Gene Family Expansion Pattern Analysis
2.3. Phylogenetic and Conserved Domains Analysis
2.4. Gene Structure and Conserved Motif Analysis
2.5. Heat Map Analysis by Transcriptome Data
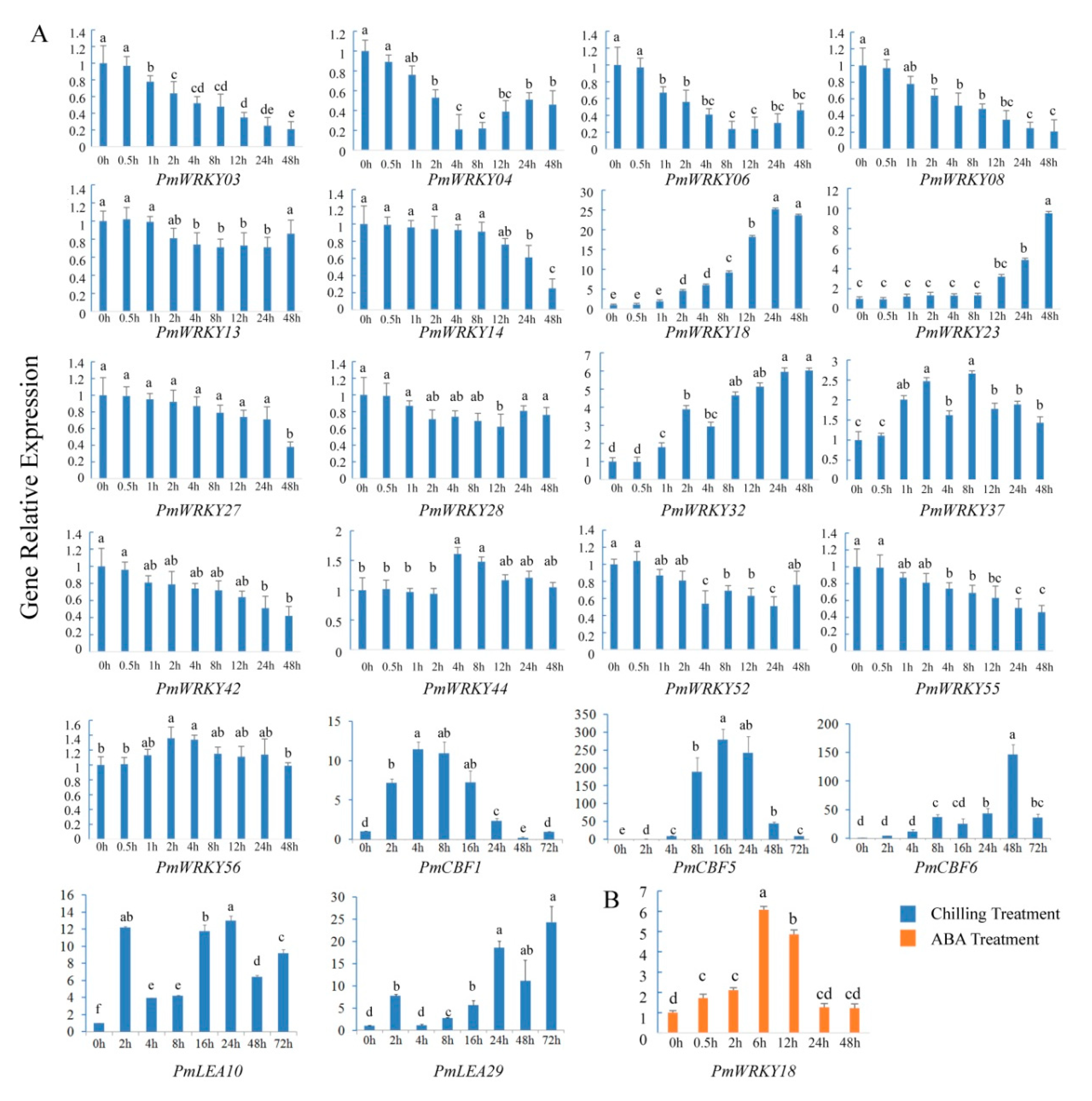
2.6. Chilling and ABA Treatments
2.7. Gene Expression Analyses
3. Results
3.1. Identification and Classification of WRKY Genes in P. mume
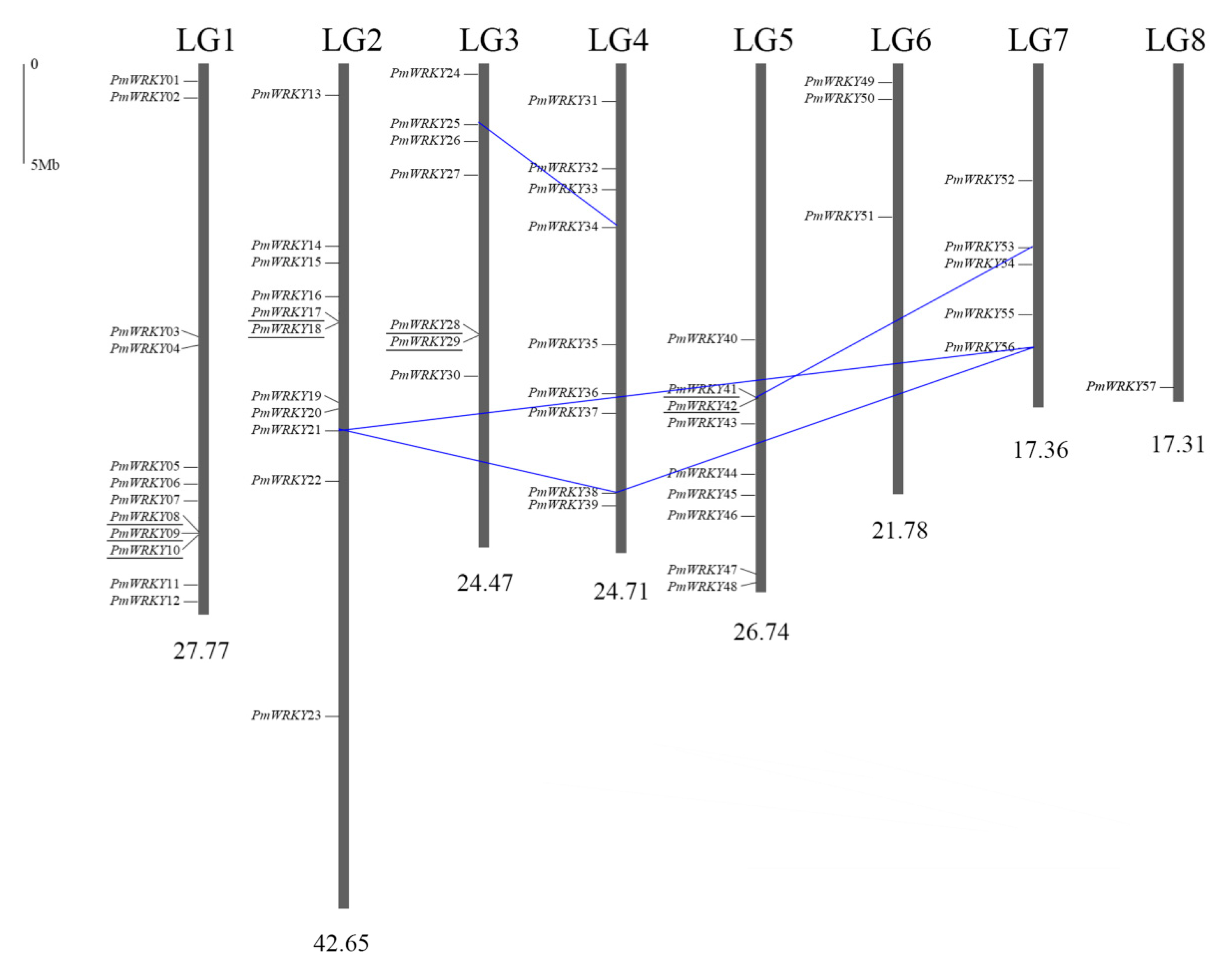
3.2. Genomic Localization and Duplication of the PmWRKY Genes
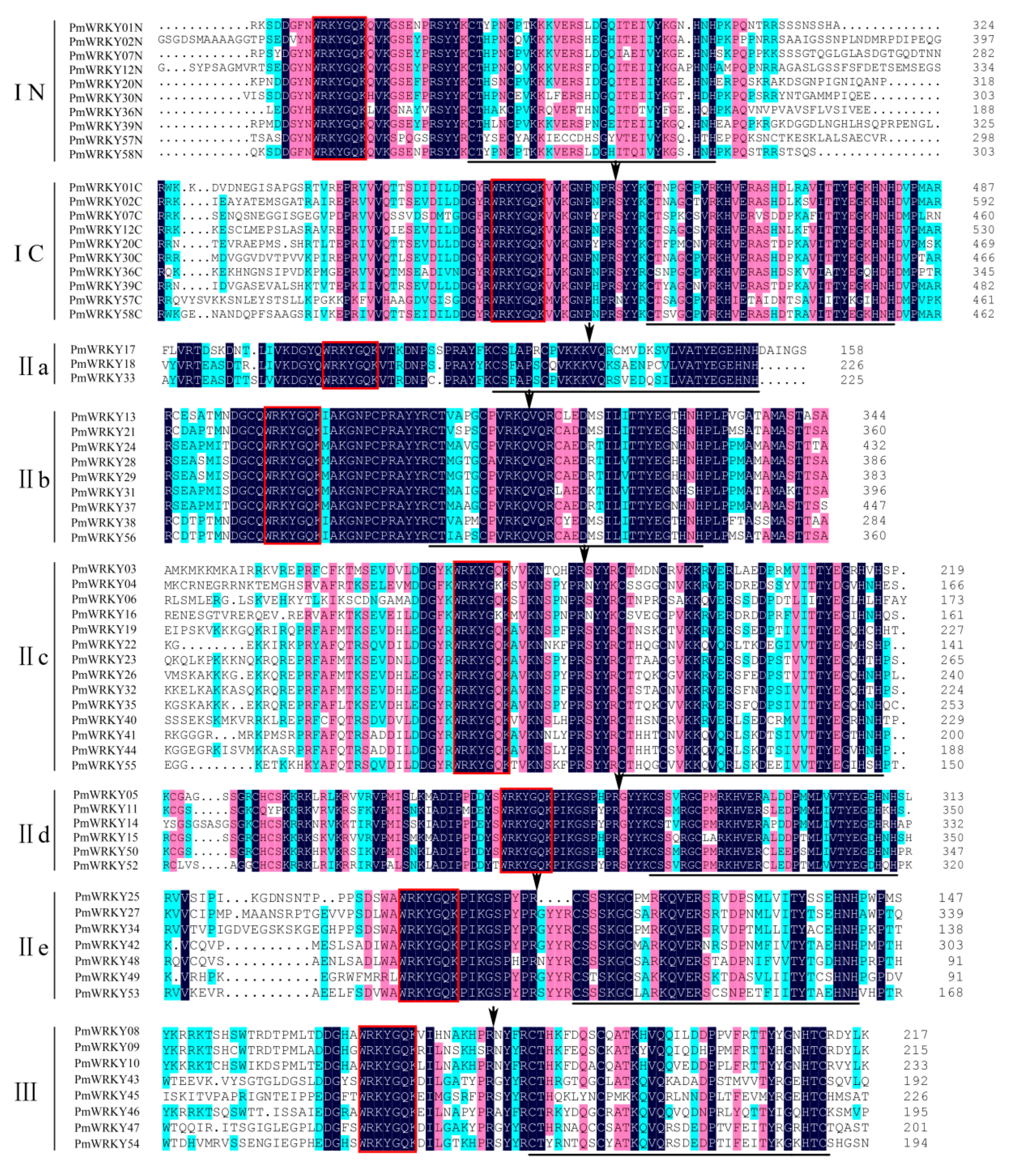
3.3. Gene Structure and Conserved Motif Analysis of PmWRKYs
3.4. Multiple Sequence Alignment of the WRKY Domains of PmWRKYs
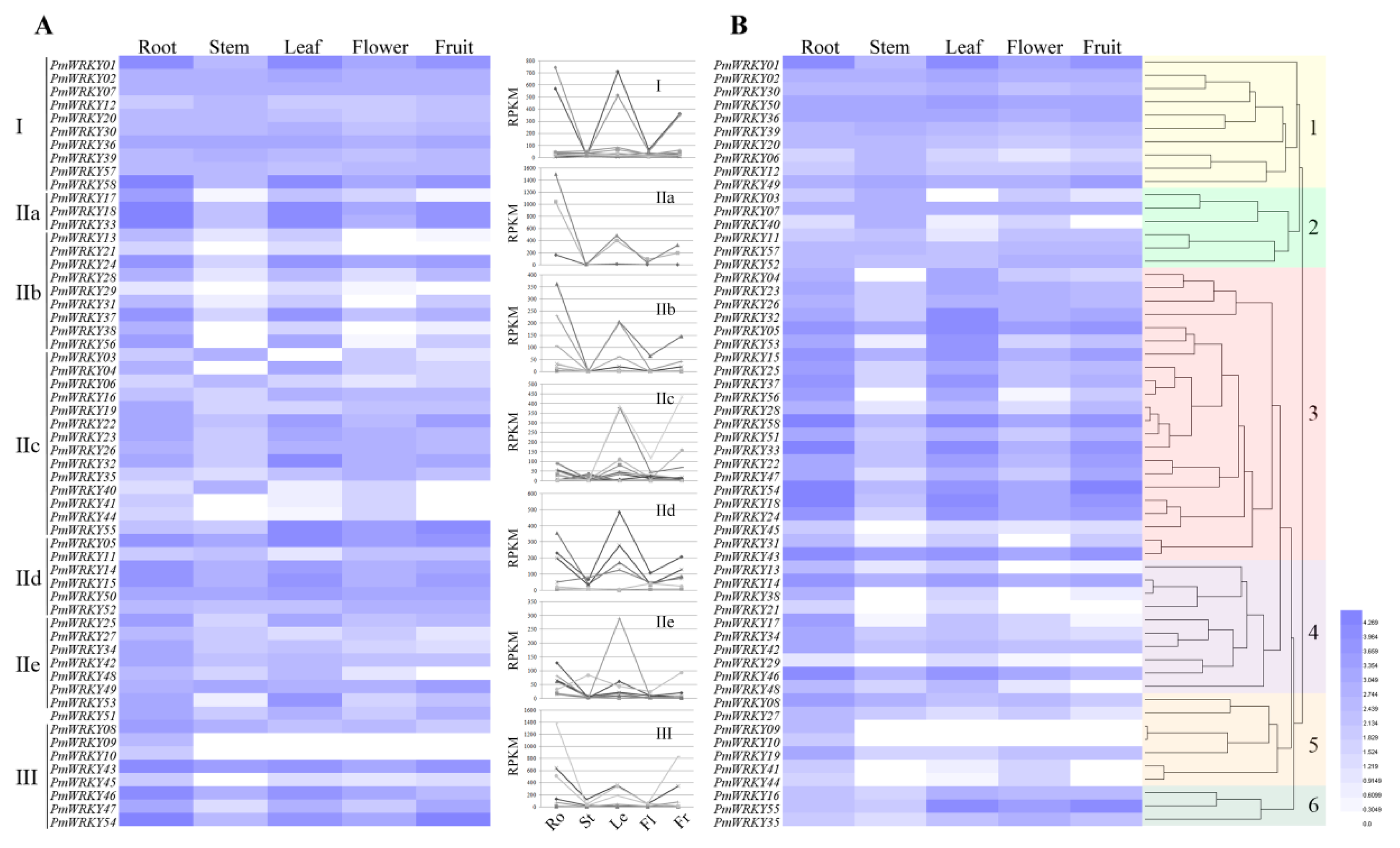
3.5. Expression Profiles of PmWRKY Genes in Different Tissues
3.6. Expression Analysis of PmWRKY Genes under Chilling Treatment
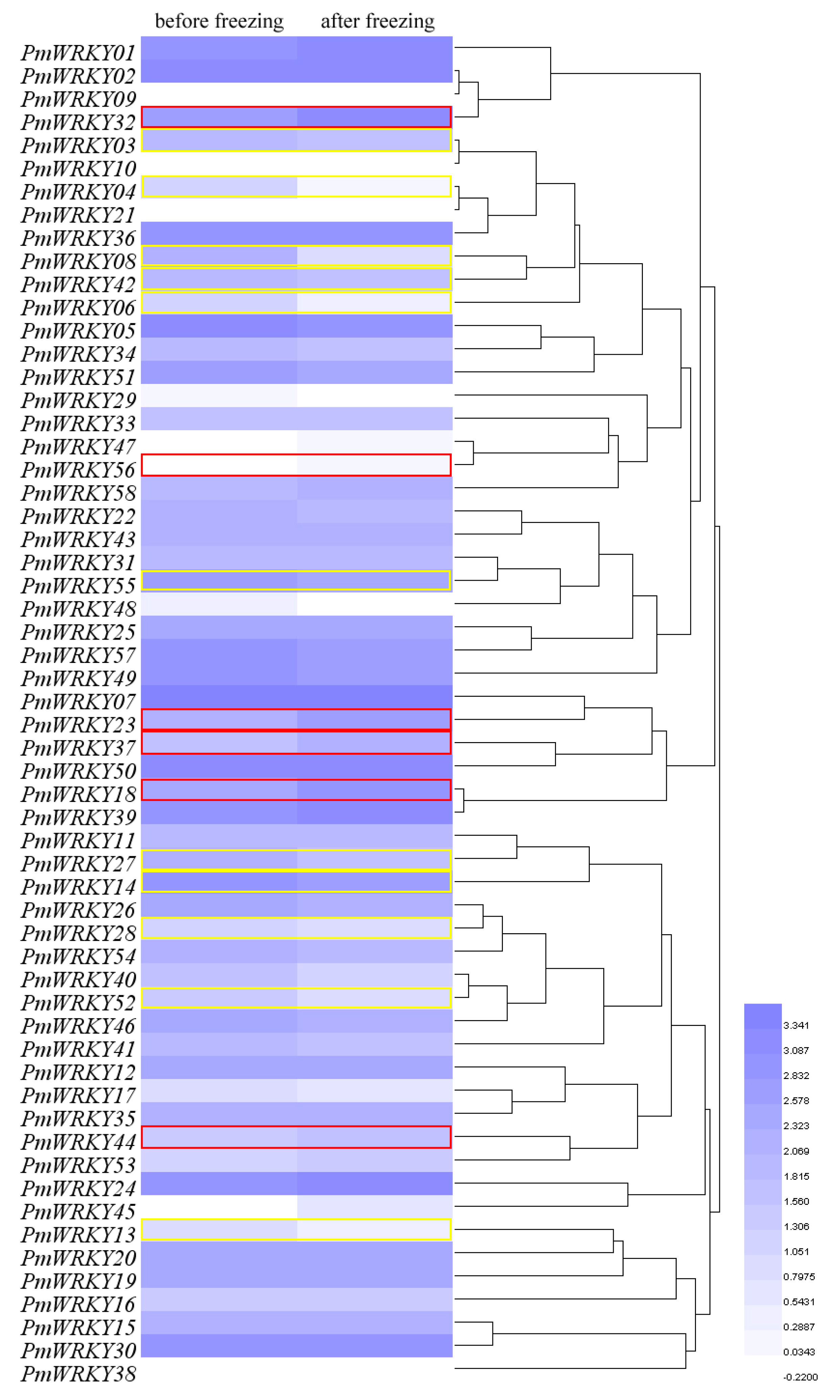
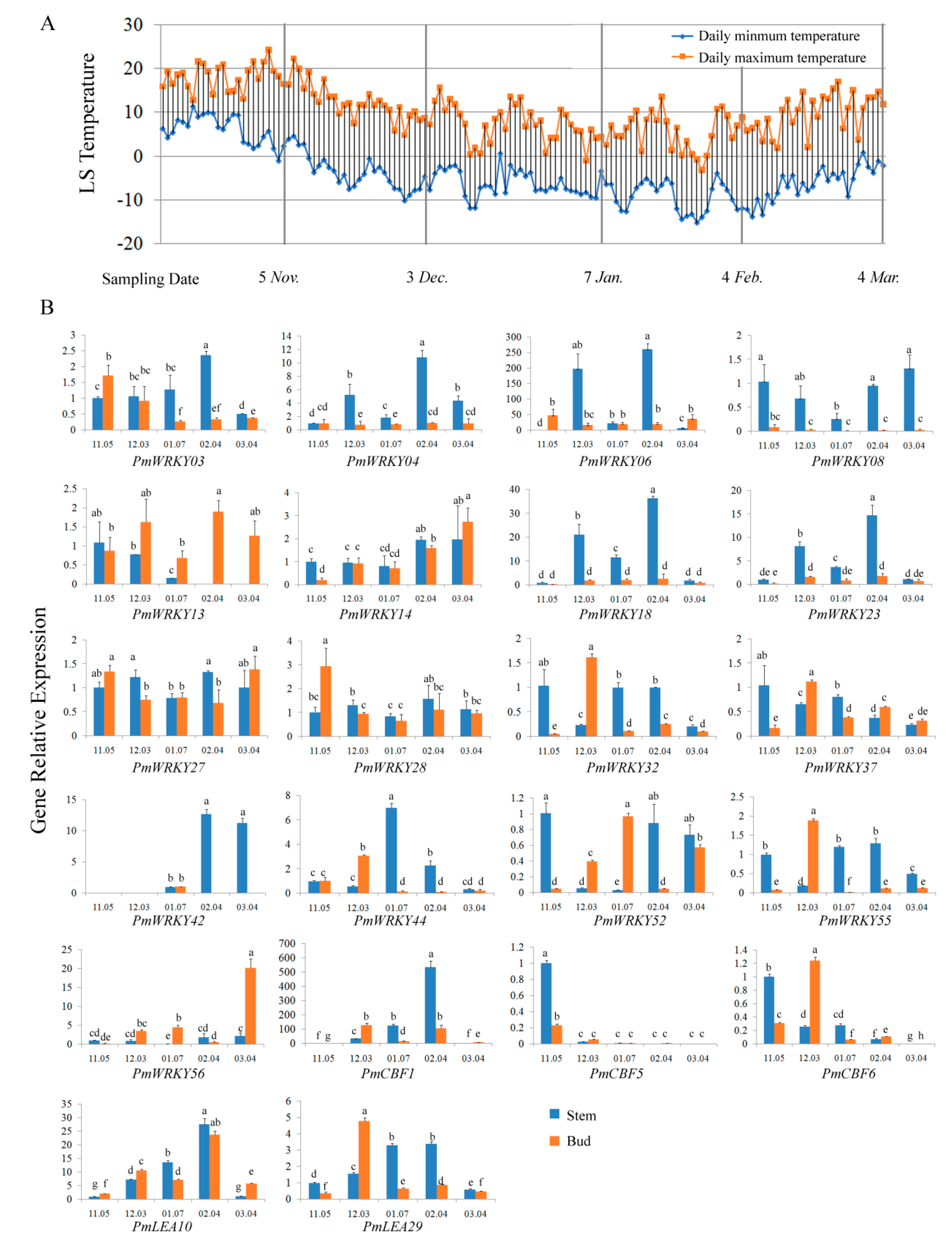
3.7. Expression Analysis of PmWRKY Genes in Winter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of chilling on the structure, function and development of chloroplasts. Front. Plant Sci. 2018, 9, 1715. [Google Scholar] [CrossRef] [PubMed]
- Steponkus, P.L. Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev. Plant Mol. Biol. 1984, 35, 543–584. [Google Scholar] [CrossRef]
- Pearce, R.S. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199. [Google Scholar] [CrossRef]
- Liu, J.J.; Ekramoddoullah, A.K. Identification and characterization of the WRKY transcription factor family in Pinus monticola. Genome 2009, 52, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Genet. Genomics 1994, 244, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Schmelzer, E.; Hahlbrock, K.; Somssich, I.E. Early nuclear events in plant defence signalling: Rapid gene activation by WRKY transcription factors. EMBO J. 1999, 18, 4689–4699. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, Z. A family of dispersed repetitive DNA sequences in tobacco contain clusters of W-box elements recognized by pathogen-induced WRKY DNA-binding proteins. Plant Sci. 2001, 161, 655–664. [Google Scholar] [CrossRef]
- Laloi, C.; Mestresortega, D.; Marco, Y.; Meyer, Y.; Reichheld, J.P. The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol. 2004, 134, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, Y.; Wang, L.; Liu, X.; Liu, Y.; Phillips, J.; Deng, X. A WRKY transcription factor participates in dehydration tolerance in Boea hygrometrica by binding to the W-box elements of the galactinol synthase (BhGolS1) promoter. Planta 2009, 230, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1. [Google Scholar]
- Glöckner, G.; Eichinger, L.; Szafranski, K.; Pachebat, J.A.; Bankier, A.T.; Dear, P.H.; Lehmann, R.; Baumgart, C.; Parra, G.; Abril, J.F.; et al. Sequence and analysis of chromosome 2 of Dictyostelium discoideum. Nature 2002, 418, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tan, Q.; Sun, M.; Li, D.; Fu, X.; Chen, X.; Xiao, W.; Li, L.; Gao, D. Genome-wide identification of WRKY family genes in peach and analysis of WRKY expression during bud dormancy. Mol. Genet. Genomics 2016, 291, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, L.; Li, D.; Wang, F.; Yu, D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Jiang, W.; Yu, D. Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J. Exp. Bot. 2010, 61, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, C.; Zhang, Y.; Meng, X.; Zhou, X.; Chu, C.; Wang, X. OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol. Biol. 2012, 80, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, L.; Wong, D.C.; Wang, Y.; Zhu, Z.; Xu, G.; Wang, Q.; Li, S.; Liang, Z.; Xin, H. The ethylene response factor VaERF092 from Amur grape regulates the transcription factor VaWRKY33, improving cold tolerance. Plant J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Higuera, J.J.; Garrido-Gala, J.; Lekhbou, A.; Arjona-Girona, I.; Amil-Ruiz, F.; Mercado, J.A.; Pliego-Alfaro, F.; Muñoz-Blanco, J.; López-Herrera, C.J.; CABALLERO, J.L. The strawberry FaWRKY1 transcription factor negatively regulates resistance to Colletotrichum acutatum in fruit upon infection. Front. Plant Sci. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, T.; Sun, X.; Wang, Y.; Du, C.; Zhu, Z.; Gichuki, D.K.; Wang, Q.; Li, S.; Xin, H. Overexpression of VaWRKY12, a transcription factor from Vitis amurensis with increased nuclear localization under low temperature, enhances cold tolerance of plants. Plant Mol. Biol. 2019, 100, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, T.; Lin, Z.; Gu, B.; Xing, C.; Zhao, L.; Dong, H.; Gao, J.; Xie, Z.; Zhang, S.; et al. A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Landherr, L.L.; Frohlich, M.W.; Leebensmack, J.; Ma, H.; Depamphilis, C.W. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: Evidence for multiple mechanisms of rapid gene birth. Plant J. 2007, 50, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Tang, H.; Wang, X.; Paterson, A.H. PGDD: A database of gene and genome duplication in plants. Nucleic Acids Res. 2012, 41, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. HemI: A toolkit for illustrating heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, W.; Fang, L.; Sun, X.; Su, L.; Liang, Z.; Wang, N.; Londo, J.P.; Li, S.; Xin, H. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis vinifera. BMC Plant Biol. 2014, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, P.; Nan, Z.; Wang, X. The WRKY transcription factor genes in Lotus japonicus. Int. J. Genomics 2014, 2014, 420128. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, F.; Hou, X.L.; Wang, Z.; Huang, Z.N. Genome-wide fractionation and identification of WRKY transcription factors in Chinese Cabbage (Brassica rapa ssp. pekinensis) reveals collinearity and their expression patterns under abiotic and biotic stresses. Plant Mol. Biol. Rep. 2014, 32, 1–15. [Google Scholar] [CrossRef]
- Holub, E.B. The arms race is ancient history in Arabidopsis, the wildflower. Nat. Rev. Genet. 2001, 2, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, W.; Sun, L.; Zhao, F.; Huang, B.; Yang, W.; Tao, Y.; Wang, J.; Yuan, Z.; Fan, G.; et al. The genome of Prunus mume. Nat. Commun. 2012, 3, 1318. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Liu, Y.; Shen, Q.J. The WRKY gene family in Rice (Oryza sativa). JIPB 2007, 49, 827–842. [Google Scholar] [CrossRef]
- He, H.; Dong, Q.; Shao, Y.; Jiang, H.; Zhu, S.; Cheng, B.; Xiang, Y. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012, 31, 1199–1217. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Atif, R.M.; Azeem, F.; Nadeem, H.; Siddique, M.H.; Ali, M.A. Genome-wide analysis of stress responsive WRKY transcription factors in Arabidopsis thaliana. Turk. J. Agric. Food Sci. Technol. 2016, 4, 279–290. [Google Scholar] [CrossRef]
- Wu, K.L.; Guo, Z.J.; Wang, H.H.; Li, J. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 2005, 12, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Du, D.; An, Y.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Overexpression of Prunus mume dehydrin genes in tobacco enhances tolerance to cold and drought. Front. Plant Sci. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Creelman, R.A.; Zhu, J.K. From laboratory to field: Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiol. 2004, 135, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Jiang, W.; Zhang, Y.; Yu, H.; Mao, Z.; Gu, X.; Huang, S.; Xie, B. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genomics 2011, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012, 10, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lai, Z.; Shi, J.; Xiao, Y.; Chen, Z.; Xu, X. Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol. 2010, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Yan, L.; Liu, Z.Q.; Cao, Z.; Mei, C.; Xin, Q.; Wu, F.Q.; Wang, X.F.; Du, S.Y.; Jiang, T.; et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 2010, 22, 1909–1935. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, C.Z.; Ye, Q.; Wu, W.H.; Chen, Y.F. Arabidopsis WRKY6 transcription factor acts as a positive regulator of abscisic acid signaling during seed germination and early seedling development. PLoS Genet. 2016, 12, e1005833. [Google Scholar] [CrossRef] [PubMed]


| Name | Gene ID | Locus | Protein Length (aa) | MW (kDa) | pI | Localization | EST Number | WRKY Domain | Subgroup | Homolog in P. persica | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conserved Heptapeptide | Zinc Finger Motif | Domain Number | ||||||||||
| PmWRKY01 | LOC103330550 | Pa1:1070280:1072953 | 590 | 64.75 | 6.98 | Nucleus | 1 | WRKYGQK | C2H2 | 2 | I | PpWRKY6 |
| PmWRKY02 | LOC103344588 | Pa1:1909602:1912937 | 740 | 80.02 | 5.65 | Nucleus | 0 | WRKYGQK | C2H2 | 2 | I | PpWRKY5 |
| PmWRKY03 | LOC103331503 | Pa1:13787725:13788689 | 239 | 27.20 | 9.03 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY34 |
| PmWRKY04 | LOC103331584 | Pa1:13972916:13975438 | 197 | 22.17 | 6.20 | Nucleus | 0 | WRKYGKK | C2H2 | 1 | IIc | PpWRKY35 |
| PmWRKY05 | LOC103337527 | Pa1:19841802:19843058 | 326 | 35.77 | 9.60 | Nucleus | 1 | WRKYGQK | C2H2 | 1 | IId | PpWRKY41 |
| PmWRKY06 | LOC103339250 | Pa1:20889375:20891243 | 297 | 33.36 | 5.03 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY36 |
| PmWRKY07 | LOC103340389 | Pa1:21713743:21716122 | 479 | 52.28 | 8.91 | Nucleus | 0 | WRKYGQK | C2H2 | 2 | I | PpWRKY7 |
| PmWRKY08 | LOC103342893 | Pa1:22703478:22705201 | 335 | 37.41 | 5.76 | Nucleus | 0 | WRKYGQK | C2HC | 1 | III | PpWRKY58 |
| PmWRKY09 | LOC103343421 | Pa1:22717809:22719867 | 337 | 38.22 | 5.50 | Nucleus | 0 | WRKYGQK | C2HC | 1 | III | PpWRKY57 |
| PmWRKY10 | LOC103343430 | Pa1:22722643:22724633 | 340 | 37.98 | 5.67 | Nucleus | 0 | WRKYGQK | C2HC | 1 | III | PpWRKY56 |
| PmWRKY11 | LOC103318654 | Pa1:25540026:25541291 | 354 | 40.21 | 9.68 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IId | PpWRKY42 |
| PmWRKY12 | LOC103318792 | Pa1:26409186:26413001 | 733 | 80.43 | 5.88 | Nucleus | 0 | WRKYGQK | C2H2 | 2 | I | PpWRKY9 |
| PmWRKY13 | LOC103319105 | Pa2:1797770:1800086 | 562 | 62.09 | 5.17 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY15 |
| PmWRKY14 | LOC103320106 | Pa2:8913661:8915220 | 342 | 37.88 | 9.48 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IId | PpWRKY39 |
| PmWRKY15 | LOC103320368 | Pa2:10410393:10411854 | 364 | 39.89 | 9.29 | Nucleus | 1 | WRKYGQK | C2H2 | 1 | IId | PpWRKY38 |
| PmWRKY16 | LOC103320597 | Pa2:11611164:11611850 | 162 | 18.82 | 5.35 | Nucleus | 0 | WRKYGKK | C2H2 | 1 | IIc | - |
| PmWRKY17 | LOC103320740 | Pa2:12418544:12419976 | 285 | 31.68 | 8.33 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIa | PpWRKY12 |
| PmWRKY18 | LOC103320741 | Pa2:12424777:12426189 | 323 | 36.40 | 7.62 | Nucleus | 1 | WRKYGQK | C2H2 | 1 | IIa | PpWRKY11/ Prupe.1G071400 |
| PmWRKY19 | LOC103321497 | Pa2:16703139:16705510 | 330 | 36.38 | 6.00 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY24 |
| PmWRKY20 | LOC103321524 | Pa2:16839818:16842349 | 517 | 56.52 | 6.74 | Nucleus | 0 | WRKYGQK | C2H2 | 2 | I | PpWRKY1 |
| PmWRKY21 | LOC103321616 | Pa2:17544191:17547389 | 643 | 70.06 | 6.43 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY14 |
| PmWRKY22 | LOC103322097 | Pa2:20999398:21001199 | 162 | 18.46 | 9.67 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY23 |
| PmWRKY23 | LOC103323186 | Pa2:32805980:32807604 | 390 | 43.02 | 5.82 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY22 |
| PmWRKY24 | LOC103324471 | Pa3:408956:411939 | 591 | 64.44 | 7.06 | Nucleus | 1 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY20 |
| PmWRKY25 | LOC103324889 | Pa3:2860917:2862029 | 280 | 30.55 | 5.45 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIe | PpWRKY47 |
| PmWRKY26 | LOC103325015 | Pa3:3723897:3725432 | 340 | 37.69 | 6.55 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY32 |
| PmWRKY27 | LOC103325306 | Pa3:5613186:5615990 | 515 | 55.88 | 6.07 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIe | PpWRKY48/ Prupe.1G114800 |
| PmWRKY28 | LOC103326420 | Pa3:13640842:13643362 | 547 | 59.91 | 6.13 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY19 |
| PmWRKY29 | LOC103326493 | Pa3:13656379:13658843 | 544 | 59.51 | 6.02 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY19 |
| PmWRKY30 | LOC103326638 | Pa3:15399255:15407416 | 884 | 98.18 | 6.98 | Nucleus | 0 | WRKYGQK | C2H2 | 2 | I | PpWRKY4 |
| PmWRKY31 | LOC103327251 | Pa4:195608:198391 | 616 | 67.23 | 6.42 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY16 |
| PmWRKY32 | LOC103327303 | Pa4:564486:565902 | 321 | 35.77 | 6.51 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY29 |
| PmWRKY33 | LOC103328175 | Pa4:6725543:6727134 | 320 | 35.25 | 8.71 | Nucleus | 1 | WRKYGQK | C2H2 | 1 | IIa | PpWRKY13 |
| PmWRKY34 | LOC103328332 | Pa4:8491309:8494074 | 268 | 29.42 | 5.37 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIe | PpWRKY46/ Purpe.2G185100 |
| PmWRKY35 | LOC103328791 | Pa4:14169139:14171554 | 367 | 41.67 | 7.10 | Nucleus | 1 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY30 |
| PmWRKY36 | LOC103329228 | Pa4:16910810:16913287 | 486 | 53.04 | 5.90 | Nucleus | 0 | WRKYGQK | C2H2 | 2 | I | PpWRKY2 |
| PmWRKY37 | LOC103329304 | Pa4:17519417:17521899 | 649 | 70.74 | 6.11 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY17 |
| PmWRKY38 | LOC103329978 | Pa4:21736698:21738977 | 499 | 54.64 | 6.71 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY18/ Prupe.1G393000 |
| PmWRKY39 | LOC103330053 | Pa4:22207825:22211049 | 533 | 58.44 | 8.45 | Nucleus | 0 | WRKYGQK | C2H2 | 2 | I | PpWRKY3 |
| PmWRKY40 | LOC103331676 | Pa5:13956787:13959777 | 244 | 27.79 | 7.29 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY26 |
| PmWRKY41 | LOC103332060 | Pa5:16864359:16866293 | 221 | 24.84 | 9.24 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY27 |
| PmWRKY42 | LOC103332064 | Pa5:16887019:16890355 | 417 | 46.73 | 7.72 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIe | PpWRKY44 |
| PmWRKY43 | LOC103332261 | Pa5:18120250:18122148 | 357 | 39.80 | 4.90 | Nucleus | 1 | WRKYGQK | C2HC | 1 | III | PpWRKY51 |
| PmWRKY44 | LOC103332696 | Pa5:20443902:20444645 | 209 | 24.04 | 9.05 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY28 |
| PmWRKY45 | LOC103333076 | Pa5:21989132:21991115 | 344 | 37.86 | 5.78 | Nucleus | 0 | WRKYGQK | C2HC | 1 | III | PpWRKY52 |
| PmWRKY46 | LOC103333154 | Pa5:23138449:23140440 | 323 | 36.46 | 5.70 | Nucleus | 0 | WRKYGQK | C2HC | 1 | III | PpWRKY53/ Purpe.2G307400 |
| PmWRKY47 | LOC103333772 | Pa5:25721161:25722357 | 332 | 36.12 | 5.32 | Nucleus | 0 | WRKYGQK | C2HC | 1 | III | PpWRKY54 |
| PmWRKY48 | LOC103333707 | Pa5:25986620:25987499 | 228 | 24.63 | 4.92 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIe | PpWRKY45 |
| PmWRKY49 | LOC103334065 | Pa6:994260:995360 | 242 | 28.23 | 6.02 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIe | PpWRKY50 |
| PmWRKY50 | LOC103334153 | Pa6:1797284:1799454 | 355 | 40.24 | 9.61 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IId | PpWRKY43 |
| PmWRKY51 | LOC103335291 | Pa6:8182262:8186226 | 471 | 52.51 | 7.88 | Chlo, Nucl | 0 | WRKYGQK | C2H2 | 1 | IIx | ppa024204 |
| PmWRKY52 | LOC103337200 | Pa7:5945463:5947021 | 330 | 37.05 | 9.68 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IId | PpWRKY40 |
| PmWRKY53 | LOC103337694 | Pa7:9472026:9473037 | 291 | 32.89 | 5.23 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIe | PpWRKY49 |
| PmWRKY54 | LOC103337660 | Pa7:10206830:10208250 | 356 | 40.00 | 5.41 | Nucleus | 0 | WRKYGQK | C2HC | 1 | III | PpWRKY55 |
| PmWRKY55 | LOC103338090 | Pa7:12882417:12883702 | 170 | 19.52 | 9.35 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIc | PpWRKY33/ Prupe.6G286000 |
| PmWRKY56 | LOC103338408 | Pa7:14366251:14369593 | 683 | 73.58 | 6.45 | Nucleus | 0 | WRKYGQK | C2H2 | 1 | IIb | PpWRKY21 |
| PmWRKY57 | LOC103341266 | Pa8:16767252:16770984 | 537 | 59.39 | 5.47 | Nucleus | 0 | WRKYGQK | C2H2 | 2 | I | PpWRKY10 |
| PmWRKY58 | LOC103342913 | scaffold22:94704:96740 | 536 | 59.89 | 6.82 | Nucleus | 1 | WRKYGQK | C2H2 | 2 | I | PpWRKY8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, F.; Ding, A.; Cheng, T.; Wang, J.; Zhang, Q. Genome-Wide Analysis of Members of the WRKY Gene Family and Their Cold Stress Response in Prunus mume. Genes 2019, 10, 911. https://doi.org/10.3390/genes10110911
Bao F, Ding A, Cheng T, Wang J, Zhang Q. Genome-Wide Analysis of Members of the WRKY Gene Family and Their Cold Stress Response in Prunus mume. Genes. 2019; 10(11):911. https://doi.org/10.3390/genes10110911
Chicago/Turabian StyleBao, Fei, Anqi Ding, Tangren Cheng, Jia Wang, and Qixiang Zhang. 2019. "Genome-Wide Analysis of Members of the WRKY Gene Family and Their Cold Stress Response in Prunus mume" Genes 10, no. 11: 911. https://doi.org/10.3390/genes10110911
APA StyleBao, F., Ding, A., Cheng, T., Wang, J., & Zhang, Q. (2019). Genome-Wide Analysis of Members of the WRKY Gene Family and Their Cold Stress Response in Prunus mume. Genes, 10(11), 911. https://doi.org/10.3390/genes10110911





