Semidwarf Gene d60 Affected by Ubiquitous Gamete Lethal Gene gal Produced Rare Double Dwarf with d30 via Recombination Breaking Repulsion-Phase Linkage on Rice Chromosome 2
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Crosses with Three Testers, d60Gal Line, D60gal Line, and D60Gal Line
2.2. Genotyping Using the Test Crossed F1 Lines
2.3. Linkage Analysis for d60
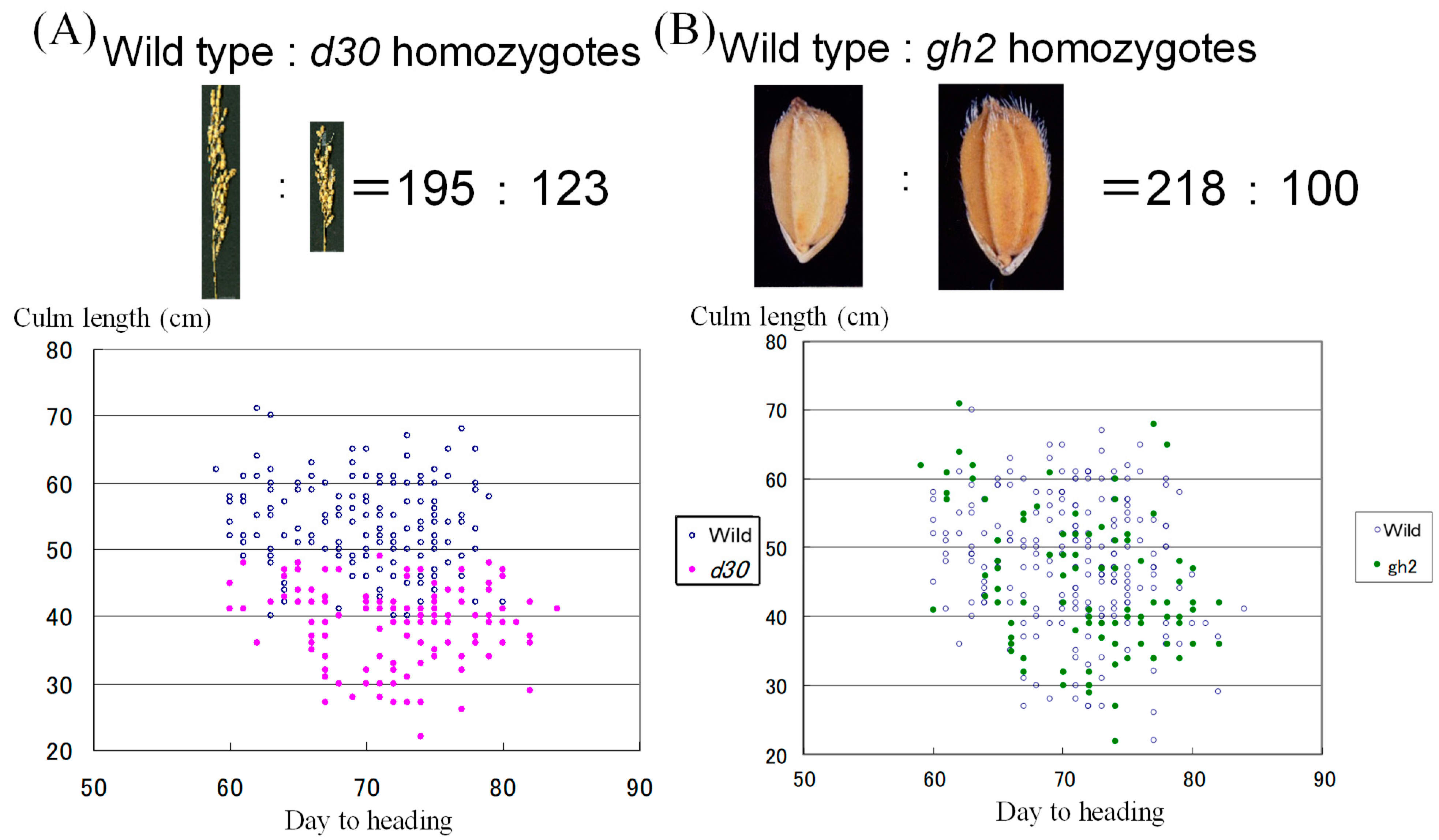
3. Results
3.1. Universal Distribution of Gal and D60 Except for the d60 Donor Hokuriku100
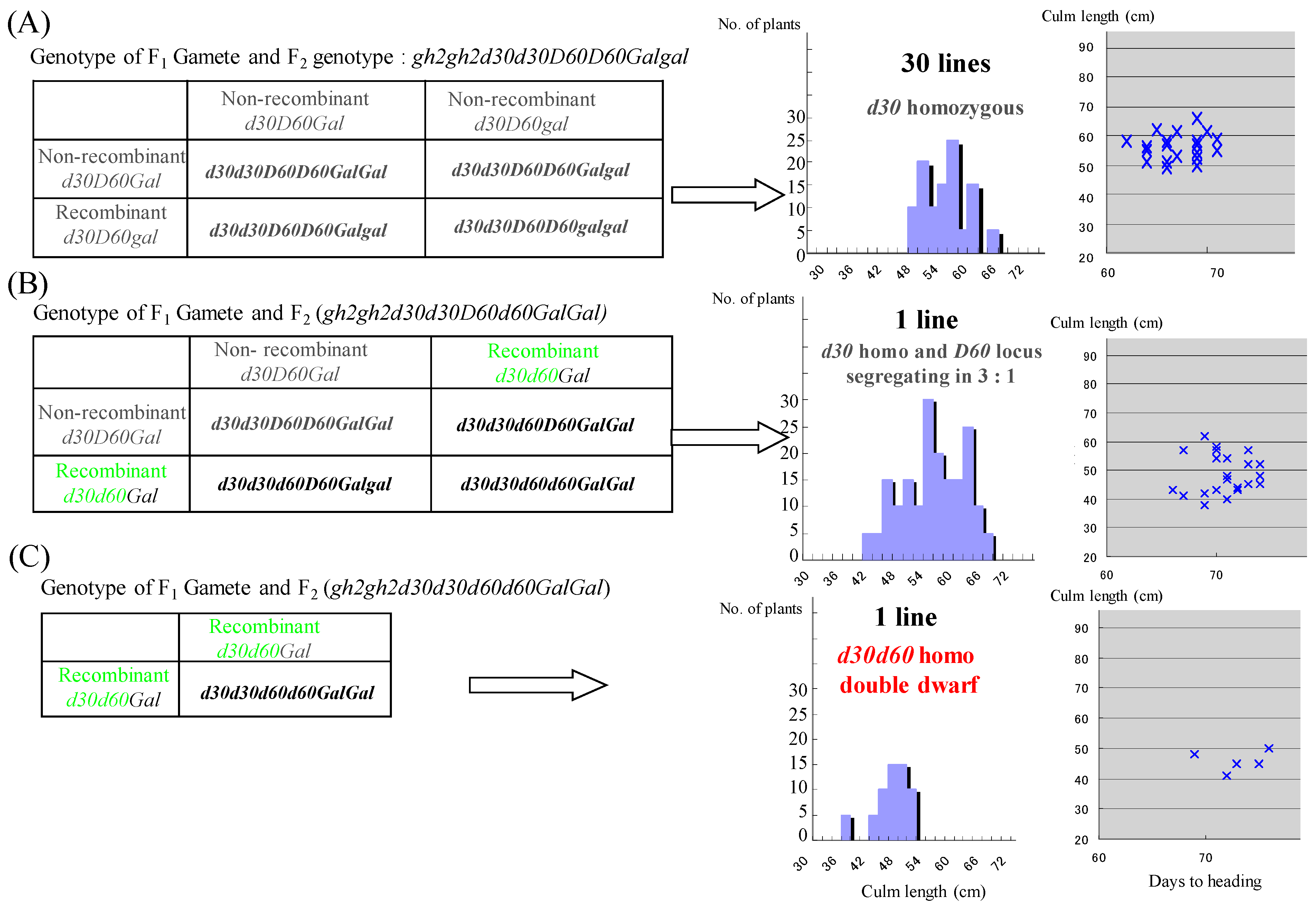
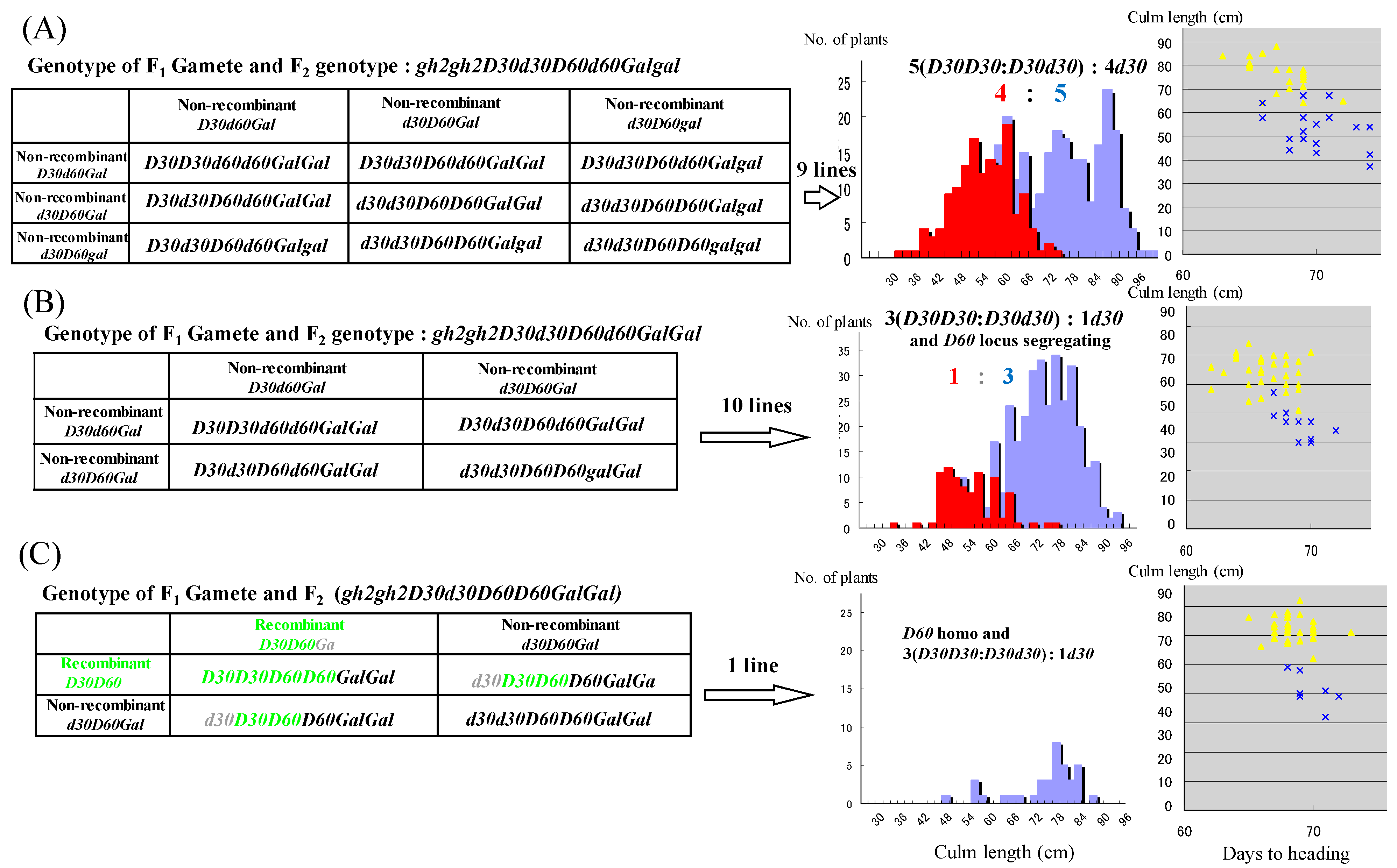
3.2. Double Dwarfness with d30 and d60 Broken by Their Repulsion Linkage on Chromosome 2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khush, G.S. Green revolution: Preparing for the 21st century. Genome 1999, 42, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Ashikari, M.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Swapan, D.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Green revolution: A mutant gibberellin-synthesis gene in rice. Nature 2002, 416, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M. Combining two semidwarfing genes d60 and sd1 for reduced height in ‘Minihikari’, a new rice germplasm in the ‘Koshihikari’ genetic background. Genet. Res. Cambridge 2012, 94, 235–244. [Google Scholar] [CrossRef]
- Tomita, M. Introgression of Green Revolution sd1 gene into isogenic genome of rice super cultivar Koshihikari to create novel semidwarf cultivar ‘Hikarishinseiki’ (Koshihikari-sd1). Field Crops Res. 2009, 114, 173–181. [Google Scholar] [CrossRef]
- Naito, Y.; Tomita, M. Identification of an isogenic semidwarf rice cultivar carrying the Green Revolution sd1 gene using multiplex codominant ASP-PCR and SSR markers. Biochem. Genet. 2013, 51, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Tanisaka, T. The gametic non-lethal gene Gal on chromosome 5 is indispensable for the transmission of co-induced semidwarfing gene d60 in rice. Biology 2019, 8, in press. [Google Scholar]
- Iwata, N.; Satoh, H.; Yoshimura, A. Linkage map for Nishimura’s chromosome 8. Rice Genet. Newsl. 1989, 6, 106–108. [Google Scholar]
- Zhang, K.; Qian, Q.; Huang, Z.; Wang, Y.; Li, M.; Hong, L.; Zeng, D.; Gu, M.; Chu, C.; Cheng, Z. Gold hull and internode2 encodes a primarily multifunctional cinnamyl-alcohol dehydrogenase in rice. Plant Physiol. 2006, 140, 972–983. [Google Scholar] [CrossRef]
- Nakazaki, T.; Okumoto, Y.; Horibata, A.; Yamahira, S.; Teraishi, M.; Nishida, H.; Inoue, H.; Tanisaka, T. Mobilization of a transposon in the rice genome. Nature 2018, 421, 170–172. [Google Scholar] [CrossRef]
- Knutson, T.; Camargo, S.J.; Chan, J.C.L.; Emanuel, K.; Ho, C.H.; Kossin, J.; Mohapatra, M.; Satoh, M.; Sugi, M.; Walsh, K.; et al. Tropical Cyclones and Climate Change Assessment: Part I. Detection and Attribution. Bull. Am. Meteorol. Soc. 2019. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, S.; Chaudhary, C. Lodging: Significance and preventive measures for increasing crop production. Int. J. Chem. Stud. 2018, 6, 700–705. [Google Scholar]
- Hirano, K.; Ordonio, R.L.; Matsuoka, M. Engineering the lodging resistance mechanism of post-Green Revolution rice to meet future demands. Proc. Jpn. Acad. Ser. B 2017, 93, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M. The gametic lethal gene gal: Activated only in the presence of the semidwarfing gene d60 in rice. In Proceedings of the 3rd Rice Genetics Symposium, Manila, Philippines, 16–20 October 1995; pp. 396–403. [Google Scholar]
- Itoh, H.; Tatsumi, T.; Sakamoto, T.; Otomo, K.; Toyomasu, T.; Kitano, H.; Ashikari, M.; Ichihara, S.; Matsuoka, M. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol. Biol. 2004, 54, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi-Tanaka, M.; Fujisawa, Y.; Kobayashi, M.; Ashikari, M.; Iwasaki, Y.; Kitano, H.; Matsuoka, M. Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 2000, 97, 11638–11643. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.I. The mechanism of sterility in the intervarietal hybrid (Phylogenetic differentiation of the cultivated rice plant. VI.). Jpn. J. Breed. 1953, 2, 217–224. [Google Scholar] [CrossRef][Green Version]
- Oka, H.I. Analysis of genes controlling F1 sterility in rice by the use of isogenic lines. Genetics 1974, 77, 521–534. [Google Scholar]
- Kitamura, E. Genetic studies on sterility observed in hybrids between distantly related varieties of rice, Oryza sativa L. Bull. Chugoku Nat. Agric. Exp. Stn. Ser. A 1962, 8, 141–205. [Google Scholar]
- Ikehashi, H.; Araki, H. Varietal screening of compatibility types revealed in F1 fertility of distant crosses in rice. Jpn. J. Breed. 1984, 34, 304–313. [Google Scholar] [CrossRef]
- Ikehashi, H. Theory and application for F1 fertility in remote cross of rice. Agric. Hortic. 1985, 60, 1099–1104. [Google Scholar]
- Ikehashi, H.; Araki, H. Multiple alleles controlling F1 sterility in remote crosses of rice (Oryza sativa). Jpn. J. Breed. 1988, 38, 283–291. [Google Scholar] [CrossRef]
- Ikehashi, H. Genetics of hybrid sterility in wide hybridization in rice. In Biotechnology in Agriculture and Forestry, Vol 14, Rice; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 113–127. [Google Scholar]
- Chen, J.; Ding, J.; Ouyang, Y.; Du, H.; Yang, J.; Cheng, K.; Zhao, J.; Qiu, S.; Zhang, X.; Yao, J.; et al. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica–japonica hybrids in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 11436–11441. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, S.; Kato, H.; Ikehashi, H. A new locus for multiple alleles causing hybrid sterility between an Aus variety and Javanica varieties in rice (Oryza sativa L.). Jpn. J. Breed. 1992, 42, 793–801. [Google Scholar] [CrossRef]
- Wan, J.; Yanagihara, S.; Kato, H.; Ikehashi, H. Multiple alleles at a new locus hybrid sterility between a Korean Indica variety and a Javanica variety in rice (Oryza sativa L.). Jpn. J. Breed. 1993, 43, 507–516. [Google Scholar]
- Wan, J.; Ikehashi, H. Identification of a new locus S-16 causing hybrid sterility in native rice varieties (Oryza sativa L.) from Tai-hu Lake region and Yunnan Province, China. Breed. Sci. 1995, 45, 461–470. [Google Scholar] [CrossRef]
- Wan, J.; Yamaguchi, Y.; Kato, H.; Ikehashi, H. Two new loci for hybrid sterility in cultivated rice (Oryza sativa L.). Theor. Appl. Genet. 1996, 92, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.D.; Wang, J.; Li, H.B.; Xu, C.G.; Liu, A.M.; Li, X.H.; Zhang, Q. A genome-wide analysis of wide compatibility in rice and the precise location of the S5 locus in the molecular map. Theor. Appl. Genet. 1997, 95, 809–814. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.D.; Xu, C.G.; Li, X.H.; Zhang, Q.F. The high level of wide compatibility of variety ‘Dular’ has a complex genetic basis. Theor. Appl. Genet. 1998, 97, 407–412. [Google Scholar] [CrossRef]
- Liu, Y.S.; Zhu, L.H.; Sun, J.S.; Chen, Y. Mapping QTLs for defective female gametophyte development in an inter-subspecific cross in Oryza sativa L. Theor. Appl. Genet. 2001, 102, 1243–1251. [Google Scholar] [CrossRef]
- Song, X.; Qiu, S.Q.; Xu, C.G.; Li, X.H.; Zhang, Q. Genetic dissection of embryo sac fertility, pollen fertility, and their contributions to spikelet fertility of intersubspecific hybrids in rice. Theor. Appl. Genet. 2005, 110, 205–211. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, C.; Zheng, T.; Zhao, Z.; Ikehashi, H.; Wan, J. A new gene located on chromosome 2 causing hybrid sterility in a remote cross of rice. Plant Breed. 2005, 124, 440–445. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, C.; Jiang, L.; Zhu, S.; Ikehashi, H.; Wan, J. Identification of a new hybrid sterility gene in rice (Oryza sativa L.). Euphytica 2006, 151, 331–337. [Google Scholar] [CrossRef]
- Long, Y.; Zhao, L.; Niu, B.; Su, J.; Wu, H.; Chen, Y.; Zhang, Q.; Guo, J.; Zhuang, C.; Mei, M.; et al. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc. Natl. Acad. Sci. USA 2008, 105, 18871–18876. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Chu, Y.E.; Oka, H.I. Genetic studies of speciation in cultivated rice. 1. Genic analysis for the F1 sterility between Oryza sativa L. and Oryza glaberrima Steud. Jpn. J. Genet. 1979, 54, 121–132. [Google Scholar] [CrossRef]
- Sano, Y. A new gene controlling sterility in F1 hybrids of two cultivated rice species. J. Hered. 1983, 74, 435–439. [Google Scholar] [CrossRef]
- Sano, Y. The genetic nature of gamete eliminator in rice. Genetics 1990, 125, 183–191. [Google Scholar] [PubMed]
- Sano, Y. Genetic comparisons of chromosome 6 between wild and cultivated rice. Jpn. J. Breed. 1992, 42, 561–572. [Google Scholar] [CrossRef]
- Sakata, M.; Yamagata, Y.; Doi, K.; Yoshimura, A. Two linked genes on rice chromosome 2 for F1 pollen sterility in a hybrid between Oryza sativa and O. Glumaepatula Breed. Sci. 2014, 64, 309–320. [Google Scholar] [CrossRef]
- Doi, K.; Yoshimura, A.; Iwata, N. RFLP mapping and QTL analysis of heading date and pollen sterility using backcross populations between Oryza sativa L. and Oryza glaberrima Steud. Breed. Sci. 1998, 48, 395–399. [Google Scholar] [CrossRef]

| Variety | a | b | c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dwarf Genotype | d60Gal Line | D60gal Line | D60Gal Line | t-Value between | |||||||
| d60d60GalGal | D60D60galgal | D60D60GalGal | a and c | b and c | |||||||
| F | C | F | C | F | C | F | C | F | C | ||
| Daikoku | d1 | 73.3 | 101.0 | 95.4 | 104.8 | 95.6 | 105.2 | 29.26 ** | 2.21 | 0.15 | 0.25 |
| Ebisu | d2 | 72.4 | 94.2 | 95.1 | 98.3 | 94.8 | 98.2 | 27.56 ** | 2.43 | 0.58 | 0.43 |
| Ebisumochi | d6 | 72.6 | 93.2 | 96.5 | 93.5 | 96.4 | 94.0 | 29.92 ** | 0.73 | 0.14 | 0.18 |
| Kotaketamanishiki | d18 | 72.8 | 102.6 | 96.6 | 104.6 | 96.6 | 106.3 | 28.36 ** | 2.94 | 0.16 | 0.16 |
| Dwarf Kyushu 1 | d29 | 74.6 | 96.6 | 98.7 | 97.9 | 98.5 | 98.1 | 27.38 ** | 0.54 | 0.28 | 0.32 |
| Waiseishirasasa | d30 | 73.9 | 82.3 | 95.9 | 83.7 | 95.6 | 84.1 | 28.24 ** | 1.34 | 0.32 | 0.28 |
| Tanginbozu | d35 | 73.2 | 89.8 | 95.3 | 94.6 | 95.8 | 96.2 | 27.74 ** | 3.18 * | 0.18 | 0.24 |
| Fukei 71 | d50 | 73.8 | 83.6 | 95.6 | 88.6 | 94.9 | 87.4 | 24.82 ** | 1.53 | 0.16 | 0.15 |
| Jukkoku | sd1 | 72.8 | 84.7 | 96.4 | 88.5 | 96.6 | 87.8 | 26.66 ** | 1.33 | 0.22 | 0.18 |
| Shiranui | sd1 | 72.4 | 83.2 | 95.6 | 86.3 | 95.2 | 86.5 | 29.46 ** | 1.48 | 0.52 | 0.56 |
| M101 | sd1 | 73.4 | 74.3 | 95.4 | 79.4 | 95.6 | 78.8 | 26.22 ** | 2.72 | 0.36 | 0.38 |
| Taichung 65 d47 | sd1 | 73.8 | 82.5 | 95.5 | 85.1 | 95.7 | 85.8 | 25.27 ** | 1.73 | 0.20 | 0.22 |
| Kinuhikari | sd1 | 72.9 | 84.6 | 95.9 | 87.8 | 95.8 | 87.9 | 25.13 ** | 1.48 | 0.15 | 0.18 |
| Reimei | sd1 | 72.7 | 75.8 | 95.2 | 78.3 | 95.7 | 78.4 | 25.46 ** | 2.18 | 0.49 | 0.56 |
| HS90 | sd1 | 72.9 | 73.6 | 95.3 | 76.2 | 95.8 | 76.3 | 25.32 ** | 0.67 | 0.18 | 0.20 |
| Isehikari | unknown | 73.2 | 86.1 | 95.8 | 88.3 | 94.7 | 89.0 | 26.77 ** | 1.89 | 0.77 | 0.68 |
| Nipponbare | qCL1 | 73.3 | 75.4 | 94.7 | 78.9 | 94.5 | 79.6 | 27.82 ** | 3.17 * | 0.23 | 0.17 |
| Koganebare | unknown | 74.2 | 80.0 | 95.4 | 88.7 | 94.7 | 85.8 | 27.55 ** | 20.9 | 0.33 | 0.36 |
| Nihonmasari | unknown | 73.4 | 78.6 | 95.6 | 84.6 | 95.1 | 84.7 | 26.82 ** | 3.38 * | 0.24 | 0.28 |
| IM96 | unknown | 73.8 | 93.1 | 96.2 | 93.4 | 95.8 | 92.8 | 25.67 ** | 0.18 | 0.17 | 0.34 |
| IM181 | unknown | 72.5 | 82.3 | 95.6 | 84.4 | 95.5 | 84.2 | 25.46 ** | 1.23 | 0.24 | 0.26 |
| 1M265 | unknown | 73.8 | 81.9 | 96.0 | 82.7 | 95.7 | 82.9 | 28.37 ** | 1.16 | 0.18 | 0.19 |
| Ginbozu | 72.8 | 84.3 | 95.2 | 90.8 | 95.1 | 89.3 | 28.26 ** | 1.88 | 0.34 | 0.36 | |
| EG1 | 72.4 | 88.6 | 95.3 | 87.2 | 95.6 | 86.8 | 27.34 ** | 0.83 | 0.20 | 0.18 | |
| Taichung 65 | 73.1 | 97.1 | 96.3 | 97.8 | 96.2 | 97.2 | 25.68 ** | 1.08 | 0.24 | 0.28 | |
| Inochinoichi | 72.8 | 98.3 | 96.7 | 102.3 | 96.5 | 102.8 | 29.35 ** | 2.28 | 0.78 | 0.68 | |
| Midoriyutaka | 73.4 | 113.6 | 93.9 | 114.2 | 94.6 | 114.3 | 29.65 ** | 0.22 | 0.16 | 0.23 | |
| Yutakakoshihikari | 72.5 | 75.3 | 96.5 | 78.2 | 96.4 | 77.9 | 25.86 ** | 1.34 | 0.27 | 0.22 | |
| Kanto 79 | e1 | 73.5 | 76.7 | 96.9 | 77.0 | 96.8 | 76.8 | 27.25 ** | 0.35 | 0.26 | 0.28 |
| Norin 1 | 72.2 | 96.7 | 96.3 | 96.7 | 97.3 | 95.8 | 30.64 ** | 0.98 | 0.26 | 0.28 | |
| Norin 22 | 73.7 | 75.3 | 95.6 | 77.8 | 95.4 | 78.0 | 28.39 ** | 1.23 | 0.25 | 0.24 | |
| Koshihikari | D60 | 73.6 | 72.7 | 96.2 | 76.6 | 96.6 | 76.4 | 25.64 ** | 1.91 | 0.18 | 0.15 |
| Hokuriku 100 | d60 | 95.6 | 62.8 | 73.4 | 72.8 | 96.5 | 72.6 | 0.15 | 11.26 ** | 26.78 ** | 0.14 |
| Genotype | No. of F3 Lines |
|---|---|
| gh2gh2d30d30D60D60Galgal non-recombinant + non-recombinant gh2gh2d30d30D60D60GalGal non-recombinant + non-recombinant | 30 |
| gh2gh2d30d30d60D60Galgal non-recombinat + recombinant | 0 |
| gh2gh2d30d30d60D60GalGal non-recombinant + recombinant | 1 |
| gh2gh2d30d30d60d60GalGalrecombinant + recombinant | 1 |
| gh2gh2D30d30D60d60Galgal non-recombinant + non-recombinant | 9 |
| gh2gh2D30d30D60d60GalGal non-reconbinant + non-recombinant | 10 |
| gh2gh2d30D30D60D60Galgal non-recombinant + recombinant | 0 |
| gh2gh2d30D30D60D60GalGal non-recombinant + recombinant | 1 |
| gh2gh2D30d30d60d60GalGal non-recombinant + recombinant | 0 |
| gh2gh2D30d30d60D60Galgalrecombinant + recombinant | 0 |
| gh2gh2D30d30d60D60GalGalrecombinant + recombinant | 0 |
| gh2gh2D30D30d60d60GalGal non-recombinant + non-recombinant | 3 |
| gh2gh2D30D30D60d60Galgal non-recombinant + recombinant | 1 |
| gh2gh2D30D30D60d60GalGal non-recombinant + recombinant | 0 |
| gh2gh2D30D30D60d60GalGal non-recombinant+ recombinant | 0 |
| gh2gh2D30D30D60D60GalGalrecombinant + recombinant gh2gh2D30D30D60D60Galgalrecombinant + recombinant | 0 |
| 56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, M.; Tanaka, J. Semidwarf Gene d60 Affected by Ubiquitous Gamete Lethal Gene gal Produced Rare Double Dwarf with d30 via Recombination Breaking Repulsion-Phase Linkage on Rice Chromosome 2. Genes 2019, 10, 874. https://doi.org/10.3390/genes10110874
Tomita M, Tanaka J. Semidwarf Gene d60 Affected by Ubiquitous Gamete Lethal Gene gal Produced Rare Double Dwarf with d30 via Recombination Breaking Repulsion-Phase Linkage on Rice Chromosome 2. Genes. 2019; 10(11):874. https://doi.org/10.3390/genes10110874
Chicago/Turabian StyleTomita, Motonori, and Jun Tanaka. 2019. "Semidwarf Gene d60 Affected by Ubiquitous Gamete Lethal Gene gal Produced Rare Double Dwarf with d30 via Recombination Breaking Repulsion-Phase Linkage on Rice Chromosome 2" Genes 10, no. 11: 874. https://doi.org/10.3390/genes10110874
APA StyleTomita, M., & Tanaka, J. (2019). Semidwarf Gene d60 Affected by Ubiquitous Gamete Lethal Gene gal Produced Rare Double Dwarf with d30 via Recombination Breaking Repulsion-Phase Linkage on Rice Chromosome 2. Genes, 10(11), 874. https://doi.org/10.3390/genes10110874






