Comparative Physiological and Transcriptomic Analyses Reveal Mechanisms of Improved Osmotic Stress Tolerance in Annual Ryegrass by Exogenous Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chitosan Treatments
2.2. Seedling Physiological Analysis
2.3. Measurement of Photosynthetic Characteristics
2.4. RNA Extraction and Transcriptome Sequencing Analysis
2.5. Isolation of Plant RNA and Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. Effects of CTS on Seed Germination under Osmotic Stress
3.2. CTS Improved the Resistance to Osmotic Stress of Annual Ryegrass Seedlings
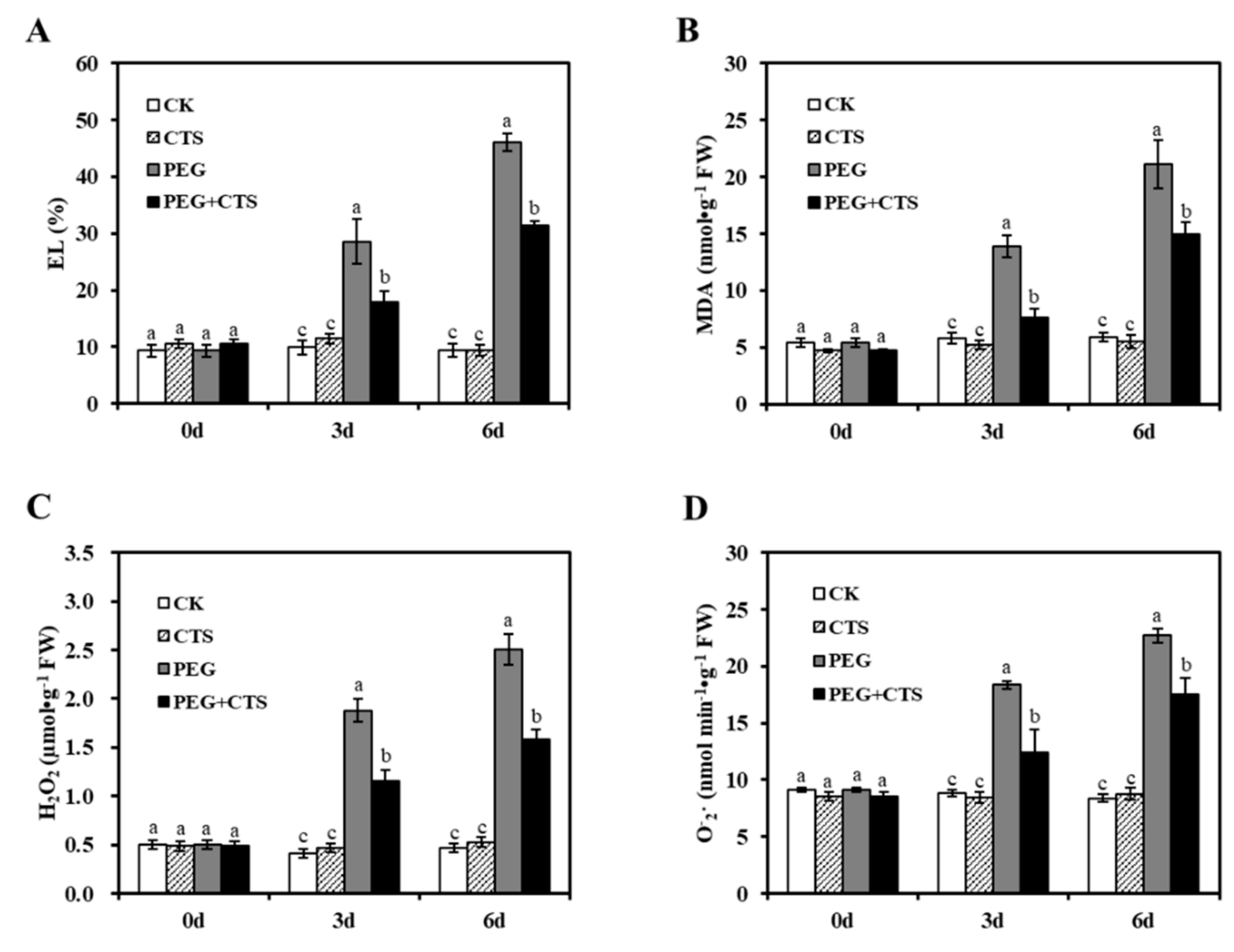
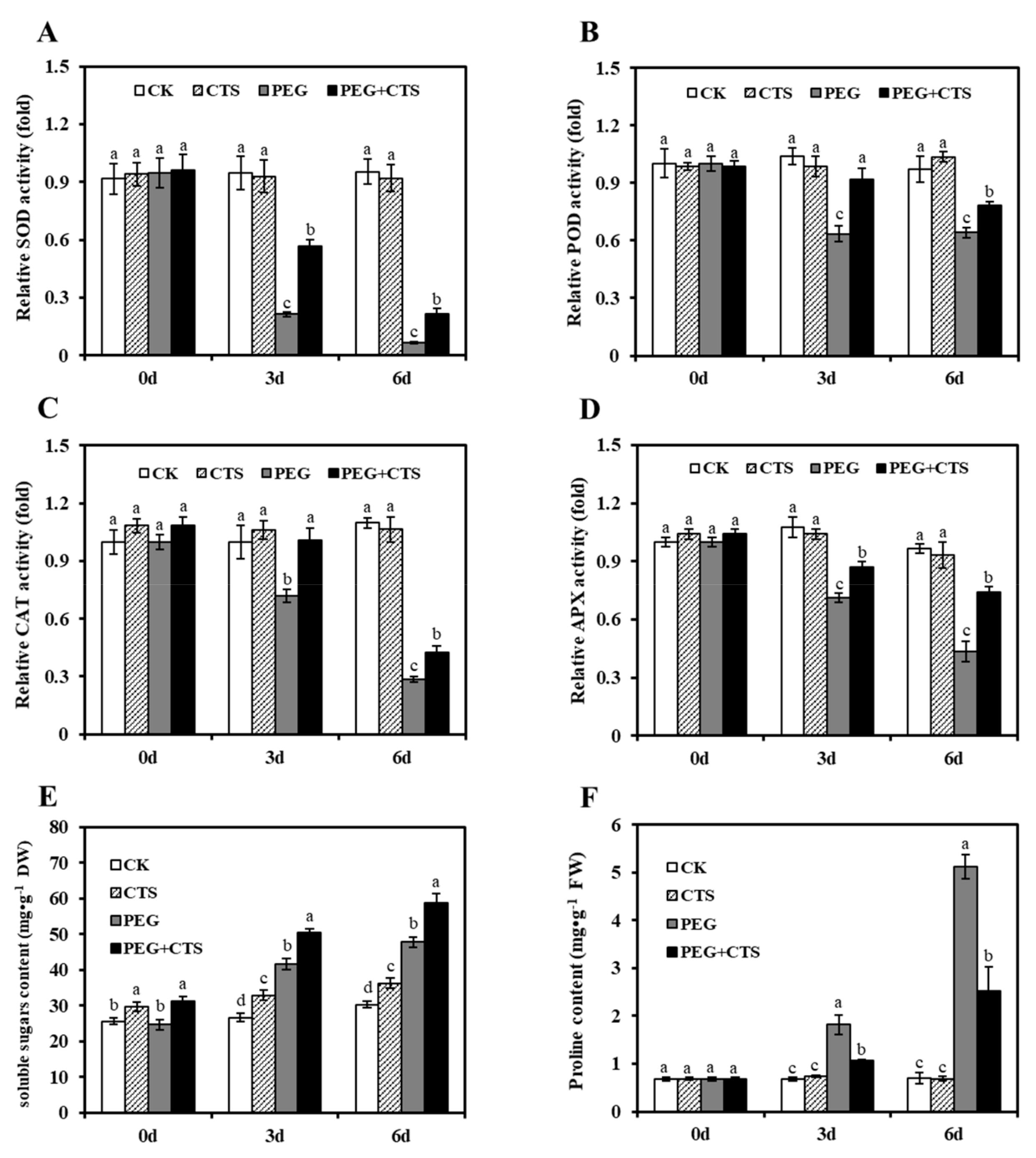
3.3. Application of CTS Positively Contributes to Osmotic Stress Tolerance by Impacting Physiological Traits
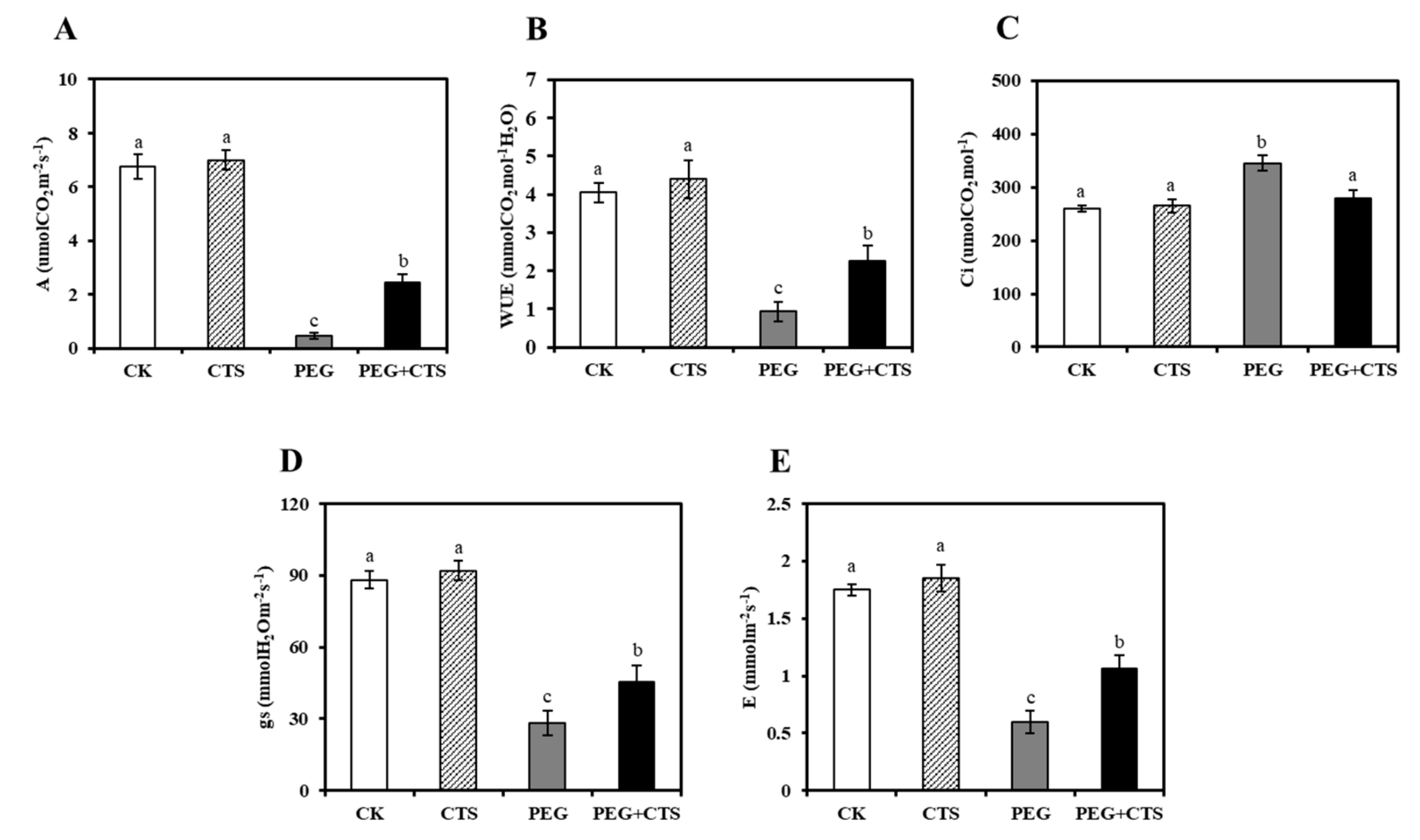
3.4. Application of CTS Mitigates the Effects of Osmotic Stress on Photosynthetic Rates
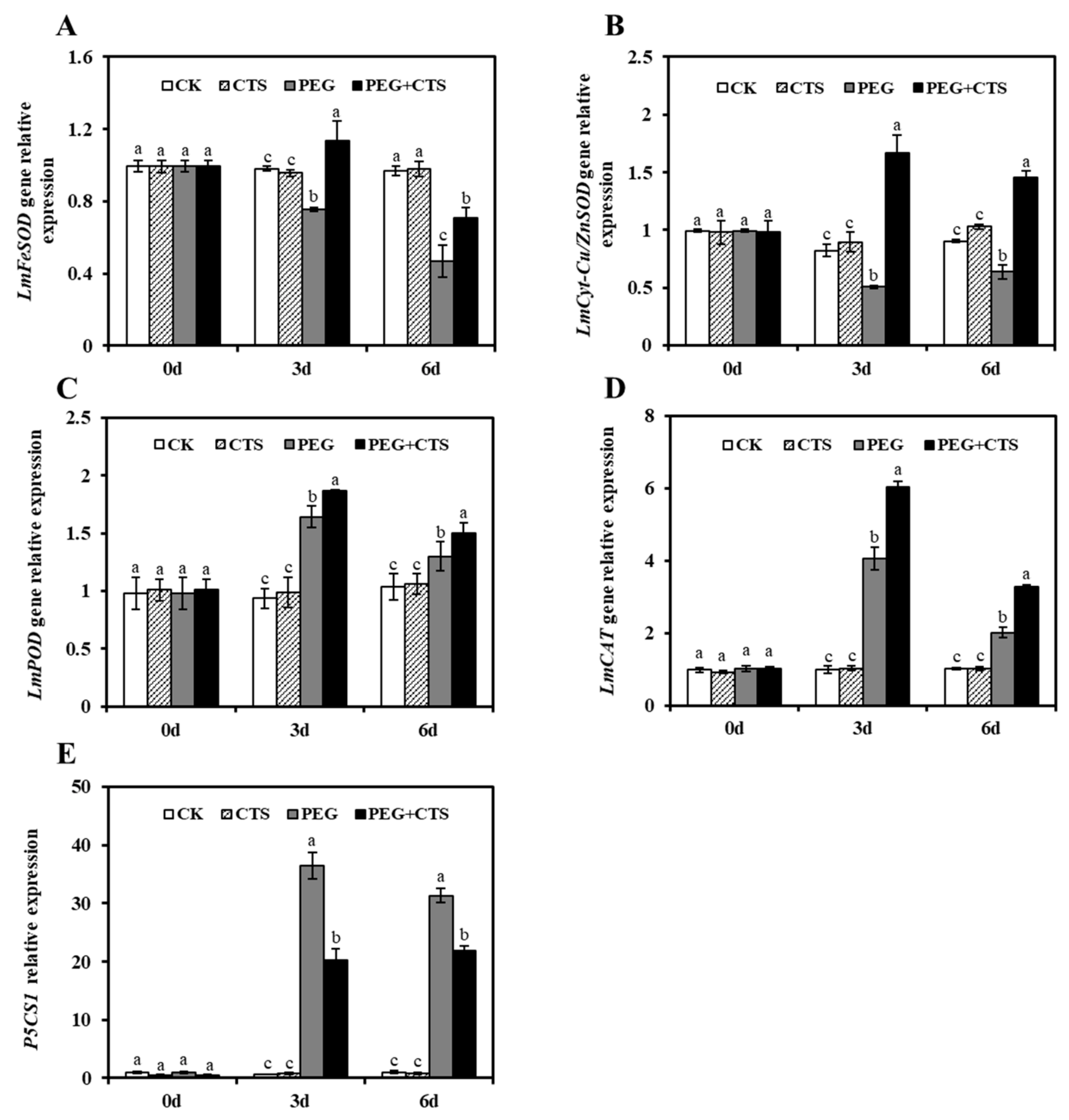
3.5. The Expression of Antioxidant Enzyme-Related Genes and Proline Biosynthetic Gene P5CS1 were Affected by Exogenous CTS Application
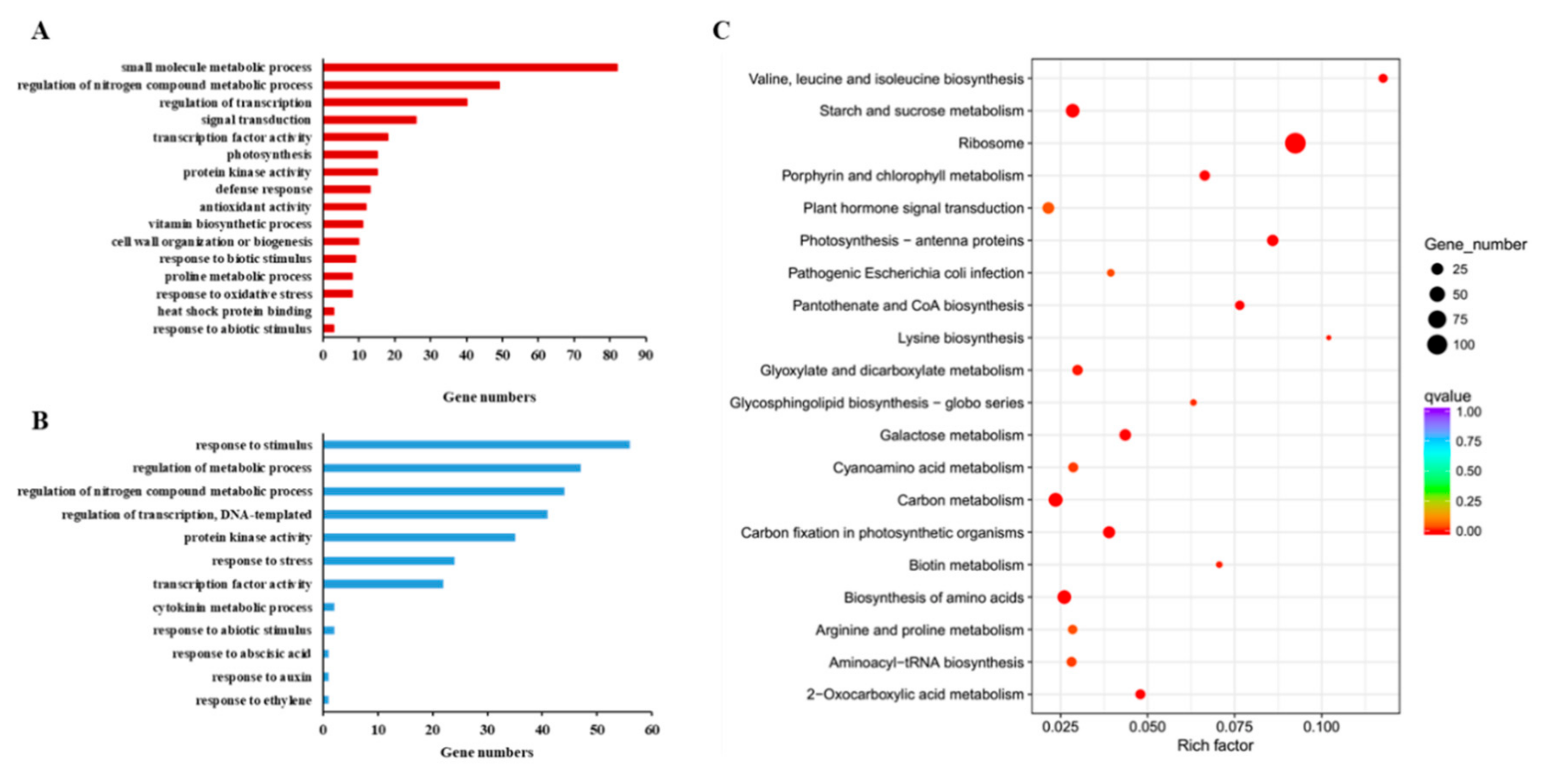
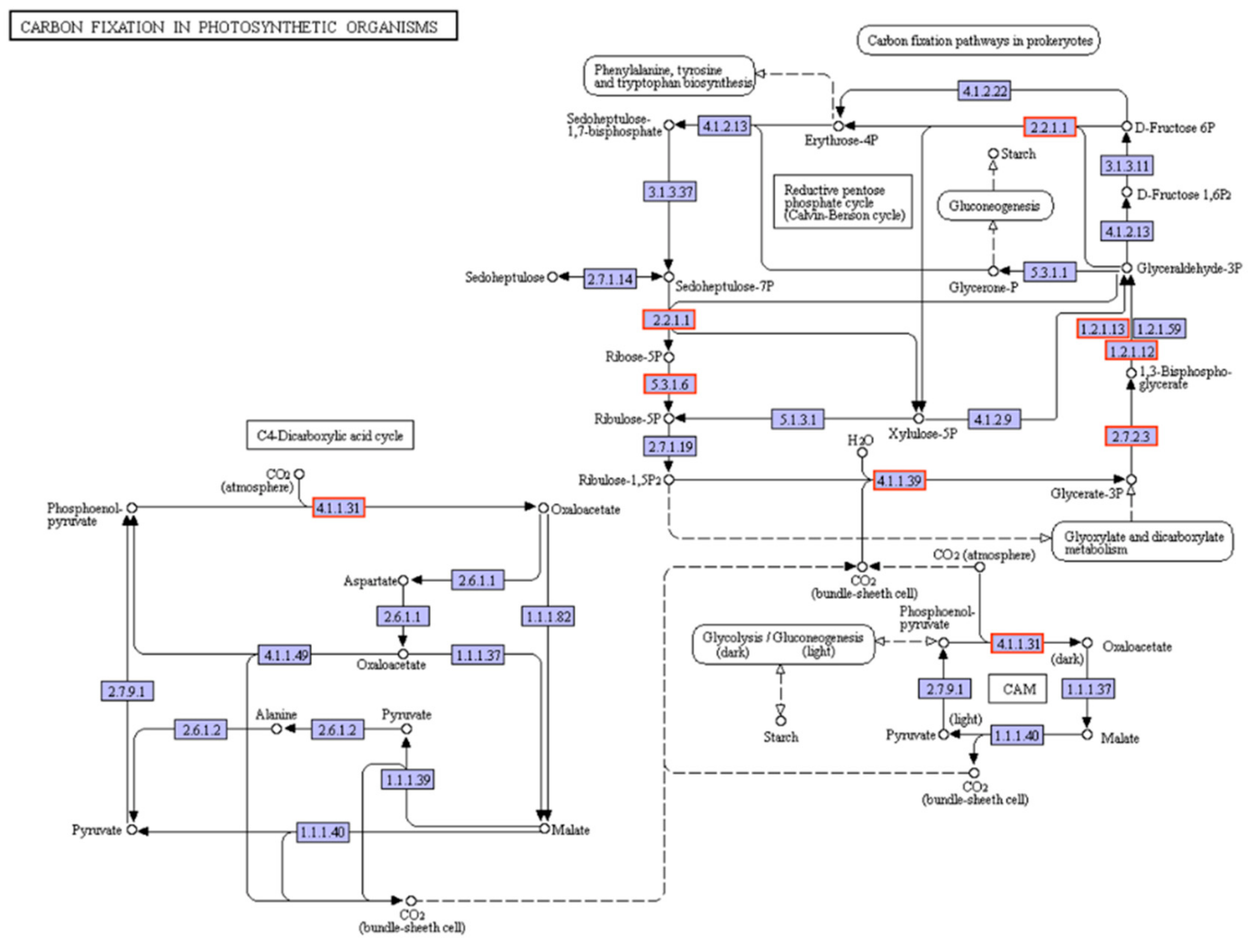
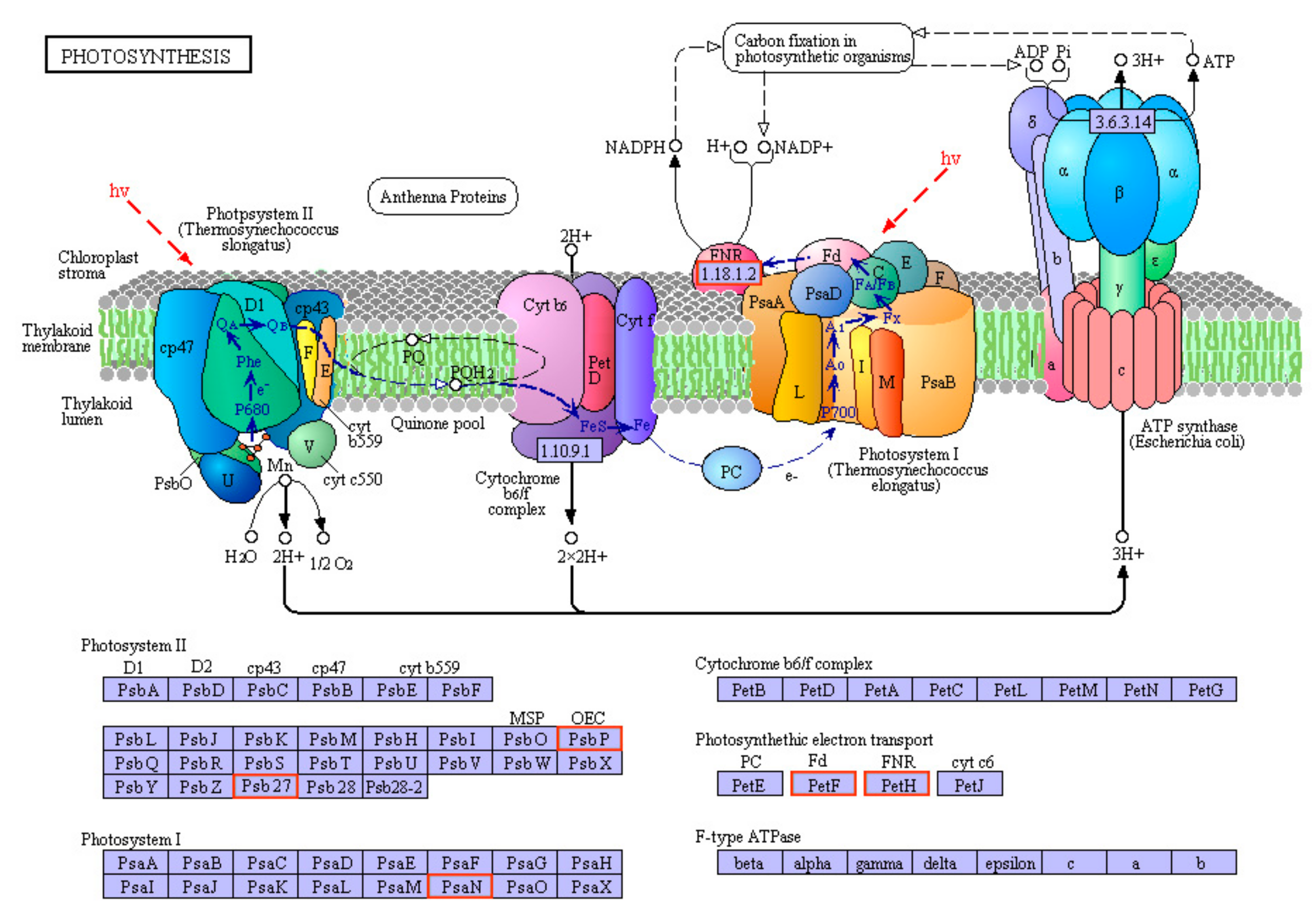
3.6. RNA-Seq and Data Analysis
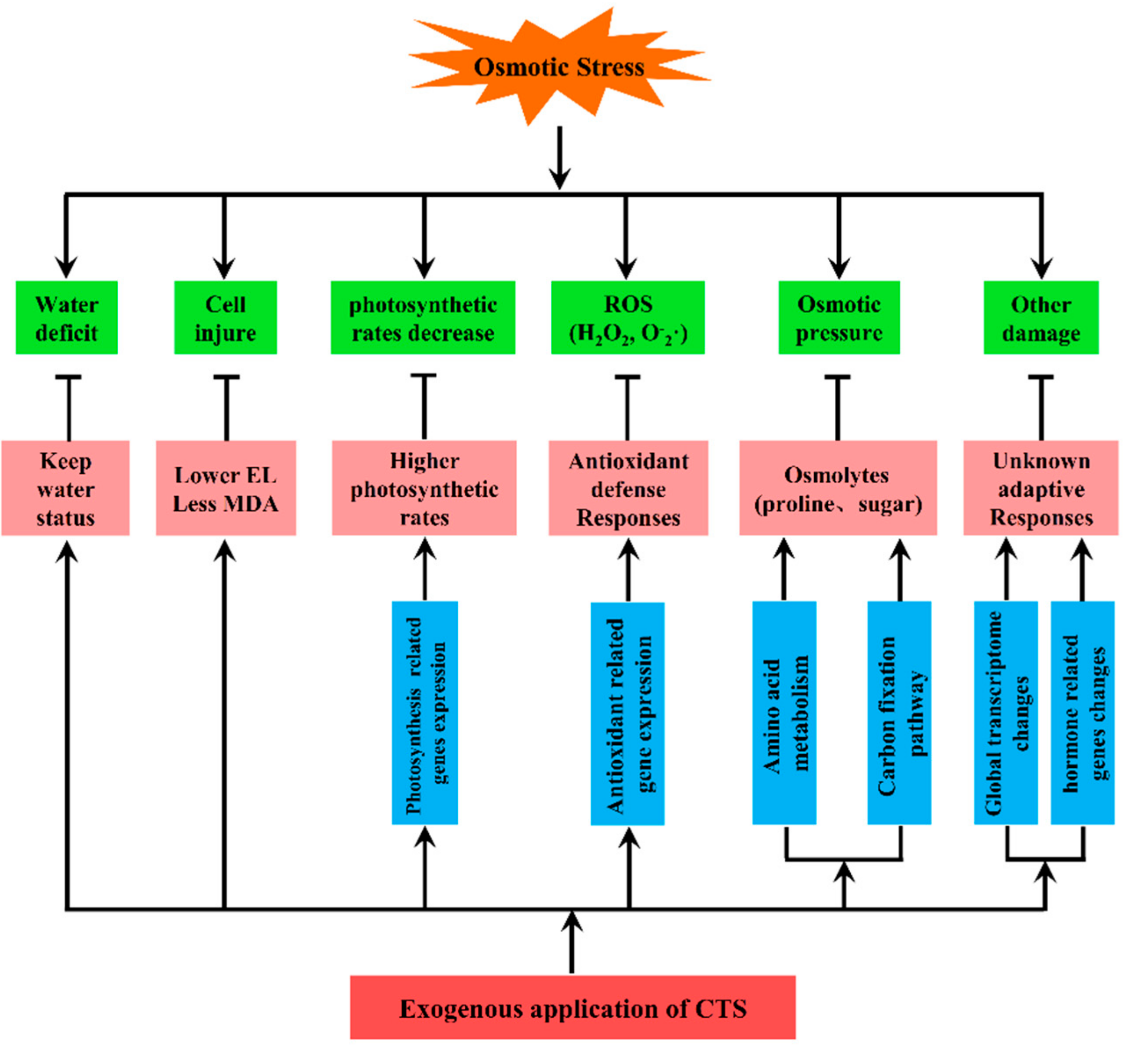
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ.-Sci. B. 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Pongprayoon, W.; Roytrakul, S.; Pichayangkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa l.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Bittelli, M.; Flury, M.; Campbell, G.S.; Nichols, E.J. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 2001, 107, 167–175. [Google Scholar] [CrossRef]
- Yang, F.; Hu, J.J.; Li, J.L.; Wu, X.L.; Qian, Y.R. Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul. 2009, 58, 131–136. [Google Scholar] [CrossRef]
- Li, J.A.; Yan, J.Q.; Wang, J.F.; Zhao, Y.M.; Cao, J.K.; Jiang, W.B. Effects of chitosan coating on oxidative stress in bruised yali pears (pyrus bretschneideri rehd.). Int. J. Food Sci. Technol. 2010, 45, 2149–2154. [Google Scholar] [CrossRef]
- Zhao, T.H.; Wang, J.L.; Wang, Y.; Sun, J.W.; Cao, Y. Effects of reactive oxygen species metabolic system on soybean (Glycine max) under exogenous chitosan to ozone stress. Bull. Environ. Contam. Toxicol. 2010, 85, 59–63. [Google Scholar] [CrossRef]
- El, G.A.; Arul, J.; Ponnampalam, R. Use of chitosan coating to reduce water loss and maintain quality of cucumber and bell pepper fruits. J. Food Process. Preserv. 1991, 15, 359–368. [Google Scholar]
- Chen, H.; Seguin, P.; Archainbault, A.; Constan, L.; Jabaji, S. Gene expression and isoflavone concentrations in soybean sprouts treated with chitosan. Crop Sci. 2009, 49, 224–236. [Google Scholar] [CrossRef]
- Lin, W.L.; Hu, X.Y.; Zhang, W.Q.; Rogers, W.J.; Cai, W.M. Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. J. Plant Physiol. 2005, 162, 937–944. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, X.Q.; Wang, J.P.; Ma, X.; Zhou, M.L.; Huang, L.K.; Nie, G.; Wang, P.X.; Yang, Z.F.; Li, J. Transcriptional profiles of drought-related genes in modulating metabolic processes and antioxidant defenses in Lolium multiflorum. Front. Plant Sci. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.; Loi, A.; Davies, W.J. Selection of annual ryegrass for adaptation to semi-arid conditions. Eur. J. Agron. 1998, 9, 71–78. [Google Scholar] [CrossRef]
- Kemesyte, V.; Statkeviciute, G.; Brazauskas, G. Perennial ryegrass yield performance under abiotic stress. Crop Sci. 2017, 57, 1935–1940. [Google Scholar] [CrossRef]
- Khajeh-Hosseini, M.; Powell, A.A.; Bingham, I.J. The interaction between salinity stress and seed vigour during germination of soybean seeds. Seed Sci. Technol. 2003, 31, 715–725. [Google Scholar] [CrossRef]
- Foito, A.; Byrne, S.L.; Shepherd, T.; Stewart, D.; Barth, S. Transcriptional and metabolic profiles of Lolium perenne L. Genotypes in response to a peg-induced water stress. Plant Biotechnol. J. 2010, 7, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.X.; Li, C.Y.; Liu, R.T.; Rong, Y.P. Drought resistance of annual ryegrass, tall fescue and hybrid festulolium in seed germination stage. Hunan Agric. Sci. 2010, 121–124. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylamine ammonium chloride: A simple assay for sod. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biophys. Biophys 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Enzymol. 1954, 1, 357. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhou, M.; Li, D.; Li, Z.; Hu, Q.; Yang, C.; Zhu, L.; Luo, H. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass. Plant Physiol. 2013, 161, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Moran, Y.; Levin, J.Z.; Thompson, D.A.; Ido, A.; Xian, A.; Lin, F.; Raktima, R.; Qiandong, Z. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 2011, 12, 323. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Markesteijn, L.; Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Kananont, N.; Pichyangkura, R.; Chanprame, S.; Chadchawan, S.; Limpanavech, P. Chitosan specificity for the in vitro seed germination of two dendrobium orchids (asparagales: Orchidaceae). Sci. Hortic. 2010, 124, 239–247. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Anraku, M.; Fujii, T.; Furutani, N.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Tomida, H. Antioxidant effects of a dietary supplement: Reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem. Toxicol. 2009, 47, 104–109. [Google Scholar] [CrossRef]
- Niu, J.; Lin, H.Z.; Jiang, S.G.; Chen, X.; Wu, K.C.; Liu, Y.J.; Wang, S.; Tian, L.X. Comparison of effect of chitin, chitosan, chitosan oligosaccharide and n-acetyl-d-glucosamine on growth performance, antioxidant defenses and oxidative stress status of Penaeus monodon. Aquaculture 2013, 372, 1–8. [Google Scholar] [CrossRef]
- Sun, T.; Xie, W.M.; Xu, P.X. Superoxide anion scavenging activity of graft chitosan derivatives. Carbohydr. Polym. 2004, 58, 379–382. [Google Scholar] [CrossRef]
- Petriccione, M.; Sanctis, F.D.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioproc. Tech. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Xi, Z.M.; Wang, Z.Z.; Fang, Y.L.; Hu, Z.Y.; Hu, Y.; Deng, M.M.; Zhang, Z.W. Effects of 24-epibrassinolide on antioxidation defense and osmoregulation systems of young grapevines (V. vinifera l.) under chilling stress. Plant Growth Regul. 2013, 71, 57–65. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Ludlow, M.M. Who taught plants thermodynamics? The unfulfilled potential of plant water potential. Aust. J. Plant Physiol. 1985, 12, 213–217. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.Q.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.K.; Yan, Y.H. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; DaMatta, F.M.; Chaves, A.R.M.; Fontes, E.P.B.; Loureiro, M.E. Drought tolerance in relation to protection against oxidative stress in clones of Coffea canephora subjected to long-term drought. Plant Sci. 2004, 167, 1307–1314. [Google Scholar] [CrossRef]
- Calderone, V.; Trabucco, M.; Vujičić, A.; Battistutta, R.; Giacometti, G.M.; Andreucci, F.; Barbato, R.; Zanotti, G. Crystal structure of the PsbQ protein of photosystem II from higher plants. EMBO Rep. 2003, 4, 900–905. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, R.; Guo, Z.; Yang, S.; Deng, X. Genome-wide identification and characterization of glyceraldehyde-3-phosphate dehydrogenase genes family in wheat (Triticum aestivum). BMC Genomics 2016, 17, 240. [Google Scholar] [CrossRef]
- Zhang, X.H.; Rao, X.L.; Shi, H.T.; Li, R.J.; Lu, Y.T. Overexpression of a cytosolic glyceraldehyde-3-phosphate dehydrogenase gene OSGAPC3 confers salt tolerance in rice. Plant Cell Tissue Organ Cult. 2011, 107. [Google Scholar] [CrossRef]
- Joshi, R.; Karan, R.; Singla-Pareek, S.L.; Pareek, A. Ectopic expression of Pokkali phosphoglycerate kinase-2 (OsPGK2-P) improves yield in tobacco plants under salinity stress. Plant Cell Rep. 2016, 35, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Harrier, L.A. Isolation and sequence analysis of the arbuscular mycorrhizal fungus Glomus mosseae (Nicol & Gerd.) Gerdemann & Trappe 3-phosphoglycerate kinase (PGK) gene promoter region. DNA Seq. 2001, 11, 463–473. [Google Scholar] [PubMed]
- Wang, Y.; Xu, C.; Zhang, B.; Wu, M.; Chen, G. Physiological and proteomic analysis of rice (Oryza sativa L.) in flag leaf during flowering stage and milk stage under drought stress. Plant Growth Regul. 2017, 82, 201–218. [Google Scholar] [CrossRef]
- Wang, H.; Yan, X.; Aigner, H.; Bracher, A.; Nguyen, N.D.; Hee, W.Y.; Long, B.M.; Price, G.D.; Hartl, F.U.; Hayer-Hartl, M. Rubisco condensate formation by ccmm in β-carboxysome biogenesis. Nature 2019, 566, 131–135. [Google Scholar] [CrossRef]
- Pichersky, E.; Bernatzky, R.; Tanksley, S.D.; Cashmore, A.R. Evidence for selection as a mechanism in the concerted evolution of Lycopersicon esculentum (tomato) genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc. Natl. Acad. Sci. 1986, 83, 3880–3884. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yi, K.; Yu, L.; Li, X.; Jinqing, C. Phosphoenolpyruvate carboxylase in arabidopsis leaves plays a crucial role in carbon and nitrogen metabolism. Plant Physiol. 2015, 167, 671–681. [Google Scholar] [CrossRef]

| CTS (mg/L) | Germination Vigor (%) | Germination Percentage (%) | Shoot Length (cm) | Root Length (cm) | Seedling Fresh Weight (mg·5 Seedling−1) |
|---|---|---|---|---|---|
| mock | 73.54 ± 3.34 a | 91.22 ± 2.33 a | 14.55 ± 2.38 a | 9.47 ± 2.32 a | 1220.27 ± 12.33 a |
| 0 | 22.86 ± 2.86 de | 32.57 ± 4.33 fg | 1.97 ± 0.28 f | 3.43 ± 0.65 f | 83.03 ± 3.86 f |
| 10 | 23.81 ± 4.36 de | 35.42 ± 4.78 fg | 2.34 ± 0.40 de | 4.33 ± 0.45 d | 95.30 ± 3.30 cd |
| 20 | 30.47 ± 3.29 cd | 37.14 ± 5.71 f | 2.64 ± 0.26 cd | 4.97 ± 0.78 c | 96.63 ± 5.20 bc |
| 30 | 32.38 ± 1.64 c | 44.57 ± 3.83 e | 2.89 ± 0.36 bc | 5.00 ± 0.76 c | 98.27 ± 6.84 bc |
| 40 | 33.33 ± 5.95 c | 50.28 ± 1.56 d | 3.08 ± 0.54 b | 5.71 ± 0.60 b | 102.70 ± 6.11 bc |
| 50 | 42.85 ± 5.71 b | 63.42 ± 5.85 b | 3.11 ± 0.35 b | 5.87 ± 0.92 b | 105.13 ± 6.10 b |
| 80 | 37.14 ± 7.56 bc | 57.71 ± 5.11 c | 2.93 ± 0.25 bc | 5.38 ± 0.56 bc | 104.77 ± 4.19 b |
| 100 | 34.29 ± 4.95 c | 57.14 ± 3.49 c | 2.50 ± 0.24 de | 4.18 ± 0.63 d | 87.50 ± 2.85 de |
| 200 | 20.00 ± 2.86 e | 29.52 ± 3.12 g | 2.22 ± 0.45 ef | 3.80 ± 0.61 de | 73.25 ± 2.33 g |
| CTS (mg/L) | RWC (%) | EL (%) | MDA (nmol·g−1 FW) |
|---|---|---|---|
| mock | 94.63 ± 0.83 a | 5.99 ± 0.05 e | 3.48 ± 0.30 e |
| 0 | 47.20 ± 1.32 e | 31.45 ± 0.46 a | 13.22 ± 0.23 a |
| 50 | 55.91 ± 1.24 d | 22.06 ± 2.12 b | 9.70 ± 0.20 b |
| 100 | 57.22 ± 1.08 d | 20.61 ± 0.72 c | 9.55 ± 0.23 b |
| 250 | 60.59 ± 2.43 bc | 18.07 ± 0.70 d | 8.64 ± 0.39 c |
| 500 | 61.27 ± 0.49 b | 17.66 ± 0.37 d | 7.87 ± 0.19 d |
| 1000 | 59.47 ± 0.32 c | 21.53 ± 1.43 bc | 7.93 ± 0.18 d |
| 2000 | 60.65 ± 1.28 bc | 21.67 ± 0.58 bc | 7.83 ± 0.39 d |
| Nucleotides Length (bp) | Transcripts | Unigenes |
|---|---|---|
| 200–500 | 224, 311 | 218, 907 |
| 500–100 | 149, 634 | 146, 798 |
| 1000–2000 | 63, 396 | 16, 843 |
| >2000 | 16, 843 | 16, 819 |
| Total | 454, 184 | 445, 358 |
| Min Length | 201 | 201 |
| Max Length | 16, 197 | 16, 197 |
| Median Length | 506 | 508 |
| Mean Length | 691 | 695 |
| N50 | 855 | 861 |
| N90 | 341 | 343 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Pan, L.; Zhou, M.; Yang, Z.; Meng, Y.; Zhang, X. Comparative Physiological and Transcriptomic Analyses Reveal Mechanisms of Improved Osmotic Stress Tolerance in Annual Ryegrass by Exogenous Chitosan. Genes 2019, 10, 853. https://doi.org/10.3390/genes10110853
Zhao J, Pan L, Zhou M, Yang Z, Meng Y, Zhang X. Comparative Physiological and Transcriptomic Analyses Reveal Mechanisms of Improved Osmotic Stress Tolerance in Annual Ryegrass by Exogenous Chitosan. Genes. 2019; 10(11):853. https://doi.org/10.3390/genes10110853
Chicago/Turabian StyleZhao, Junming, Ling Pan, Man Zhou, Zhongfu Yang, Yu Meng, and Xinquan Zhang. 2019. "Comparative Physiological and Transcriptomic Analyses Reveal Mechanisms of Improved Osmotic Stress Tolerance in Annual Ryegrass by Exogenous Chitosan" Genes 10, no. 11: 853. https://doi.org/10.3390/genes10110853
APA StyleZhao, J., Pan, L., Zhou, M., Yang, Z., Meng, Y., & Zhang, X. (2019). Comparative Physiological and Transcriptomic Analyses Reveal Mechanisms of Improved Osmotic Stress Tolerance in Annual Ryegrass by Exogenous Chitosan. Genes, 10(11), 853. https://doi.org/10.3390/genes10110853




